Abnormal Gyrus Rectus Asymmetry in Alzheimer’s Disease: An MRI-Based Parcellation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Acquisition
2.3. Volumetric Analysis on MRI
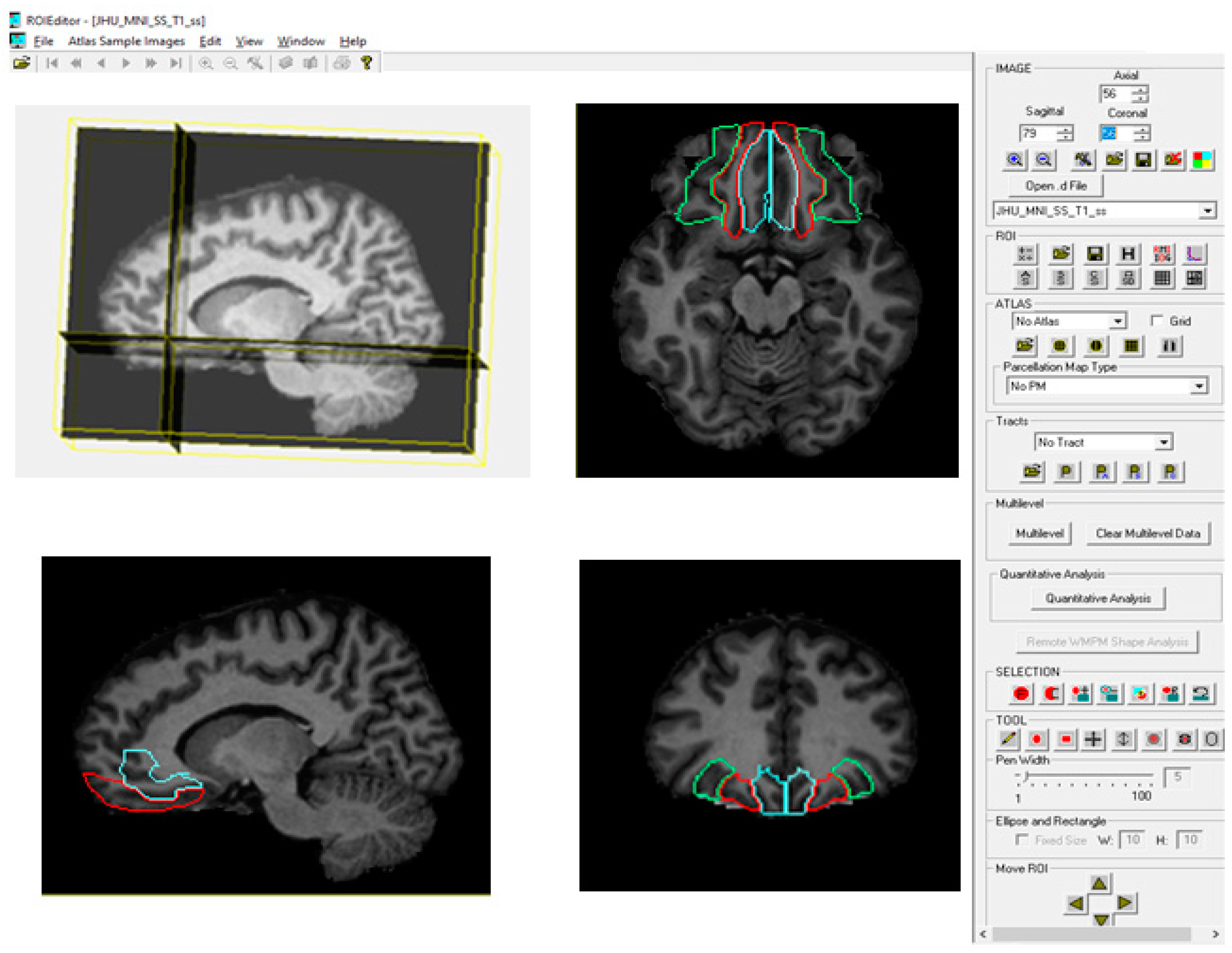
2.3.1. Brain Parcellation with MRIStudio (Atlas-Based Method)
2.3.2. Image Acquisition and Preprocessing
2.3.3. Image Registration and Normalization (DiffeoMap Processing)
2.3.4. Automated Brain Parcellation (RoiEditor Processing)
2.3.5. Asymmetry Index (AI)
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Rajamanickam, K.; Prakash, J.S.; Ramachandran, M. Study on structural atrophy changes and functional connectivity measures in Alzheimer’s disease. J. Med. Imaging 2020, 7, 016002. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Bailey, H.R.; Zacks, J.M.; Hambrick, D.Z.; Zacks, R.T.; Head, D.; Kurby, C.A.; Sargent, J.Q. Medial temporal lobe volume predicts elders’ everyday memory. Psychol. Sci. 2013, 24, 1113–1122. [Google Scholar] [CrossRef]
- Cajanus, A.; Solje, E.; Koikkalainen, J.; Lötjönen, J.; Suhonen, N.M.; Hallikainen, I.; Vanninen, R.; Hartikainen, P.; Marco, M.; Venneri, A.; et al. The association between distinct frontal brain volumes and behavioral symptoms in Mild Cognitive Impairment, Alzheimer’s Disease, and Frontotemporal Dementia. Front. Neurol. 2019, 10, 1059. [Google Scholar] [CrossRef]
- Lindberg, O.; Westman, E.; Karlsson, S.; Östberg, P.; Svensson, L.A.; Simmons, A.; Wahlund, L.O. Is the subcallosal medial prefrontal cortex a common site of atrophy in Alzheimer’s disease and frontotemporal lobar degeneration? Front. Aging Neurosci. 2012, 4, 32. [Google Scholar] [CrossRef]
- Roe, J.M.; Vidal-Piñeiro, D.; Sørensen, Ø.; Brandmaier, A.M.; Düzel, S.; Gonzalez, H.A.; Kievit, R.A.; Knights, E.; Kühn, S.; Lindenberger, U.; et al. Asymmetric thinning of the cerebral cortex across the adult lifespan is accelerated in Alzheimer’s disease. Nat. Commun. 2021, 12, 721. [Google Scholar] [CrossRef]
- Shi, F.; Liu, B.; Zhou, Y.; Yu, C.; Jiang, T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus 2009, 19, 1055–1064. [Google Scholar] [CrossRef]
- Gour, N.; Felician, O.; Didic, M.; Koric, L.; Gueriot, C.; Chanoine, V.; Confort-Gouny, S.; Guye, M.; Ceccaldi, M.; Ranjeva, J.P. Functional Connectivity Changes Differ in Early and Late-Onset Alzheimer’s Disease. Hum. Brain Mapp. 2014, 35, 2978–2994. [Google Scholar] [CrossRef]
- Henssen, A.; Zilles, K.; Palomero-Gallagher, N.; Schleicher, A.; Mohlberg, H.; Gerboga, F.; Eickhoff, S.B.; Bludau, S.; Amunts, K. Cytoarchitecture and probability maps of the human medial orbitofrontal cortex. Cortex 2016, 75, 87–112. [Google Scholar] [CrossRef] [PubMed]
- Nestor, P.G.; Nakamura, M.; Niznikiewicz, M.; Thompson, E.; Levitt, J.J.; Choate, V.; Shenton, M.E.; McCarley, R.W. In search of the functional neuroanatomy of sociality: MRI subdivisions of orbital frontal cortex and social cognition. Soc. Cogn. Affect. Neurosci. 2013, 8, 460–467. [Google Scholar] [CrossRef]
- Roiz-Santiáñez, R.; Pérez-Iglesias, R.; Ortiz-García de la Foz, V.; Tordesillas-Gutiérrez, D.; Mata, I.; Marco de Lucas, E.; Pazos, A.; Tabarés-Seisdedos, R.; Vázquez-Barquero, J.L.; Crespo-Facorro, B. Straight gyrus morphology in first-episode schizophrenia-spectrum patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.S.; Park, D.S.; Moon, C.T.; Chun, Y.I.; Song, S.W.; Roh, H.G. Relationship between Gyrus Rectus Resection and Cognitive Impairment after Surgery for Ruptured Anterior Communicating Artery Aneurysms. J. Cerebrovasc. Endovasc. Neurosurg. 2016, 18, 223–228. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDSADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; van Zijl, P.C.M.; Kim, J.; Pearlson, G.D.; Mori, S. DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput. Methods Programs Biomed. 2006, 81, 106–116. [Google Scholar] [CrossRef]
- Oishi, K.; Faria, A.; Jiang, H.; Li, X.; Akhter, K.; Zhang, J.; Hsu, J.T.; Miller, M.I.; can Zijl, P.C.M.; Albert, M.; et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: Application to normal elderly and Alzheimer’s disease participants. Neuroimage 2009, 46, 486–499. [Google Scholar] [CrossRef]
- Low, A.; Mak, E.; Malpetti, M.; Chouliaras, L.; Nicastro, N.; Su, L.; Holland, N.; Rittman, T.; Vázquez Rodríguez, P.; Passamonti, L.; et al. Asymmetrical atrophy of thalamic subnuclei in Alzheimer’s disease and amyloid-positive mild cognitive impairment is associated with key clinical features. Alzheimer’s Dement. 2019, 11, 690–699. [Google Scholar] [CrossRef]
- SPSS, Version 16.0; SPSS for Windows; SPSS Inc.: Chicago, IL, USA, 2007. Available online: http://www.unimuenster.de/imperia/md/content/ziv/service/software/spss/handbuecher/englisch/spss_brief_guide_16.0.pdf (accessed on 22 April 2025).
- Burks, J.D.; Conner, A.K.; Bonney, P.A.; Glenn, C.A.; Baker, C.M.; Boettcher, L.B.; Briggs, R.G.; O’Donoghue, D.L.; Wu, D.H.; Sughrue, M.E. Anatomy and white matter connections of the orbitofrontal gyrus. J. Neurosurg. 2018, 128, 1865–1872. [Google Scholar] [CrossRef]
- Ballmaier, M.; Toga, A.W.; Blanton, R.E.; Sowell, E.R.; Lavrersky, H.; Peterson, J.; Pham, D.; Kumar, A. Anterior Cingulate, Gyrus Rectus, and Orbitofrontal Abnormalities in Elderly Depressed Patients: An MRI-Based Parcellation of the Prefrontal Cortex. Am. J. Psychiatry 2004, 161, 99–108. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Du, Z.; Qi, X.; Shu, H.; Liu, D.; Su, F.; Ye, Q.; Liu, X.; Zhou, Z.; et al. Impaired Parahippocampal Gyrus–Orbitofrontal Cortex Circuit Associated with Visuospatial Memory Deficit as a Potential Biomarker and Interventional Approach for Alzheimer Disease. Neurosci. Bull. 2020, 36, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lou, W.; Zhang, W.; Tong, R.K.; Jin, R.; Peng, W. Gyrus rectus asymmetry predicts trait alexithymia, cognitive empathy, and social function in neurotypical adults. Cereb. Cortex 2023, 33, 1941–1954. [Google Scholar] [CrossRef]
- Szatkowska, I.; Grabowska, A.; Szymańska, O. Evidence for the involvement of the ventromedial prefrontal cortex in a short-term storage of visual images. NeuroReport 2001, 12, 1187–1190. [Google Scholar] [CrossRef]
- Szatkowska, I.; Szymańska, O.; Grabowska, A. The role of the human ventromedial prefrontal cortex in memory for contextual information. Neurosci. Lett. 2004, 364, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Mak, E.; Zhang, L.; Tan, C.H.; Reilhac, A.; Shim, H.Y.; Wen, M.O.Q.; Wong, Z.X.; Chong, E.J.Y.; Xu, X.; Stephenson, M.; et al. Longitudinal associations between β-amyloid and cortical thickness in mild cognitive impairment. Brain Commun. 2023, 5, fcad192. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.W.; Kim, G.H.; Roh, J.H.; Kim, M.J.; Seo, S.W.; Kim, S.T.; Jeon, S.; Lee, J.M.; Heilman, K.M.; et al. Cortical asymmetries in normal, mild cognitive impairment, and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Karaca, O.; Tepe, N.; Ozcan, E. Evaluation of volumetric asymmetry of the dorsolateral prefrontal cortex and medial temporal lobe in Alzheimer’s disease using the atlas-based method. NeuroReport 2023, 34, 592–597. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Xi, Q.; Zhao, X.; Wang, F.; Wang, X.; Men, W. Changes in brain lateralization in patients with mild cognitive impairment and Alzheimer’s Disease: A resting-state functional magnetic resonance study from Alzheimer’s Disease neuroimaging initiative. Front. Neurol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Maillet, D.; Rajah, M.N. Association between prefrontal activity and volume change in prefrontal and medial temporal lobes in aging and dementia: A review. Ageing Res. Rev. 2013, 12, 479–489. [Google Scholar] [CrossRef]
- Palmqvist, S.; Schöll, M.; Strandberg, O.; Mattsson, N.; Stomrud, E.; Zetterberg, H.; Blennow, K.; Landau, S.; Jagust, W.; Hansson, O. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 2017, 8, 1214. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Y.; Cai, Y.; Rong, N.; Li, R.; Shi, R.; Wei, M.; Jiang, J.; Han, Y. Asymmetrical patterns of β-amyloid deposition and cognitive changes in Alzheimer’s disease: The SILCODE study. Cereb. Cortex 2024, 34, bhae485. [Google Scholar] [CrossRef]
- Caldwell, J.Z.K.; Zhuang, X.; Leavitt, M.J.; Banks, S.J.; Cummings, J.; Cordes, D. Sex moderates amyloid and Apolipoprotein ε4 effects on default mode network connectivity at rest. Front. Neurol. 2019, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Wang, Z.; Yang, Y.; Jia, X.; Li, K. Functional disconnection and compensation in mild cognitive impairment: Evidence from DLPFC connectivity using resting-state fMRI. PLoS ONE 2011, 6, e22153. [Google Scholar] [CrossRef] [PubMed]
- Dolcos, F.; Rice, H.J.; Roberto Cabeza, R. Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neurosci. Biobehav. Rev. 2002, 26, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Browndyke, J.N.; Giovanello, K.; Petrella, J.; Hayden, K.; Chiba-Falek, O.; Tucker, K.A.; Burke, J.R.; Welsh-Bohmer, K.A. Phenotypic regional fMRI activation patterns during memory encoding in MCI and AD. Alzheimer’s Dement. 2013, 9, 284–294. [Google Scholar] [CrossRef]
- Anderson, M.C.; Bunce, J.M.; Barbas, H. Prefrontal–hippocampal pathways underlying inhibitory control over memory. Neurobiol. Learn. Mem. 2016, 134, 145–161. [Google Scholar] [CrossRef]
- Haj, M. Memory suppression in Alzheimer’s disease. Neurol. Sci. 2016, 37, 337–343. [Google Scholar] [CrossRef]
- Cheng, S.; Qiu, X.; Li, S.; Mo, L.; Xu, F.; Zhang, D. Different Roles of the Left and Right Ventrolateral Prefrontal Cortex in Cognitive Reappraisal: An Online Transcranial Magnetic Stimulation Study. Front. Hum. Neurosci. 2022, 16, 928077. [Google Scholar] [CrossRef]
- Lu, F.Y.; Yang, W.; Wei, D.T.; Sun, J.Z.; Zhang, Q.; Qiu, J. Superior frontal gyrus and middle temporal gyrus connectivity mediates the relationship between neuroticism and thought suppression. Brain Imaging Behav. 2022, 16, 1400–1409. [Google Scholar] [CrossRef]
| AD Patients | Controls | F | p | |
|---|---|---|---|---|
| No. of subjects | 25 | 25 | - | |
| Age (yr) | 69.20 ± 7.7 (56–80) | 62.16 ± 8.2 (52–80) | 0.059 | 0.003 |
| Women/men | (20/5) | (18/7) | - | |
| MMSE score | 18.08 ± 5.7 | 29.48 ± 0.5 | 23.701 | 0.001 |
| Brain Regions | Volume (cm3) (Mean ± SD) | |||||
|---|---|---|---|---|---|---|
| Controls | AD Patients | |||||
| Left | Right | AI (L-R) | Left | Right | AI (L-R) | |
| Hemisphere | 545.1 ± 66 | 551.8 ± 69 | −1.15 ± 2.5 | 517.3 ± 50 | 525.5 ± 50 | −1.59 ± 3.0 |
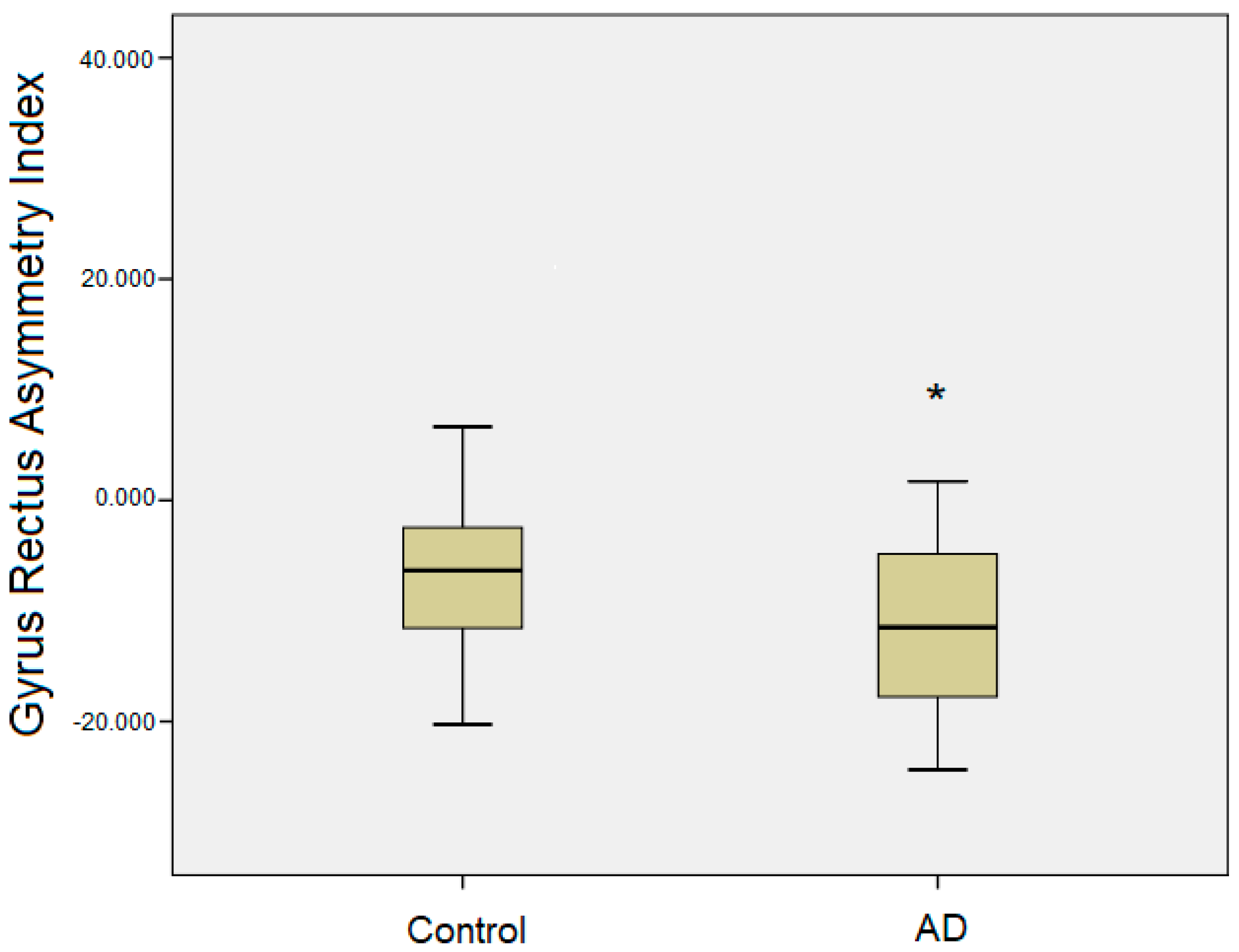
| Gyrus Rectus | 6.07 ± 1.6 | 6.47 ± 1.8 | −4.26 ± 11 | 6.00 ± 1.6 | 6.78 ± 1.8 | −11.82 ± 7.7 * |
| Lateral OFC | 6.12 ± 1.8 | 6.87 ± 2.1 | −7.66 ± 21 | 6.35 ± 1.5 | 7.23 ± 1.6 | −12.71 ± 12 |
| Medial OFC | 5.13 ± 1.6 | 4.64 ± 1.4 | 12.8 ± 22 | 5.16 ± 1.3 | 4.68 ± 1.1 | 7.7 ± 13 |
| Correlation to MMSE | |||||
|---|---|---|---|---|---|
| Left | r | p | Right | r | p |
| Hemisphere | 0.621 | 0.001 * | Hemisphere | 0.530 | 0.006 * |
| Gyrus Rectus | 0.398 | 0.049 * | Gyrus Rectus | 0.410 | 0.042 * |
| Lateral OFC | 0.302 | 0.143 | Lateral OFC | 0.274 | 0.185 |
| Medial OFC | 0.257 | 0.214 | Medial OFC | 0.329 | 0.108 |
| Brain Regions | Left Lateral OFC | Right Lateral OFC | Left Medial OFC | Right Medial OFC | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Left Gyrus Rectus | 0.775 | 0.000 * | 0.933 | 0.000 * | 0.894 | 0.000 * | 0.920 | 0.000 * |
| Right Gyrus Rectus | 0.823 | 0.000 * | 0.919 | 0.000 * | 0.889 | 0.000 * | 0.941 | 0.000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaca, Ö.; Kibar, A.A.; Aslantekin, B.; Tepe, N. Abnormal Gyrus Rectus Asymmetry in Alzheimer’s Disease: An MRI-Based Parcellation Method. Brain Sci. 2025, 15, 452. https://doi.org/10.3390/brainsci15050452
Karaca Ö, Kibar AA, Aslantekin B, Tepe N. Abnormal Gyrus Rectus Asymmetry in Alzheimer’s Disease: An MRI-Based Parcellation Method. Brain Sciences. 2025; 15(5):452. https://doi.org/10.3390/brainsci15050452
Chicago/Turabian StyleKaraca, Ömür, Ahmet Arman Kibar, Burcu Aslantekin, and Nermin Tepe. 2025. "Abnormal Gyrus Rectus Asymmetry in Alzheimer’s Disease: An MRI-Based Parcellation Method" Brain Sciences 15, no. 5: 452. https://doi.org/10.3390/brainsci15050452
APA StyleKaraca, Ö., Kibar, A. A., Aslantekin, B., & Tepe, N. (2025). Abnormal Gyrus Rectus Asymmetry in Alzheimer’s Disease: An MRI-Based Parcellation Method. Brain Sciences, 15(5), 452. https://doi.org/10.3390/brainsci15050452






