Long-Term Effects of Chronic Oral Ritalin Administration on Cognitive and Neural Development in Adolescent Wistar Kyoto Rats
Abstract
:1. Introduction
2. Results
2.1. Experiment 1—Behavioural Effect of Acute Oral MPH Administration
Oral MPH Dose-Response Curves for Locomotor Activity in WKY

2.2. Cognitive-Behavioural Tasks
2.2.1. Locomotor Activity (During Chronic Treatment: 4 Weeks of 2 × 2 mg/kg Oral MPH or dH2O)
2.2.1.1. Locomotor Activity: WKYs

2.2.1.2. Locomotor Activity: SHRs

2.2.2. Delayed Reinforcement Task (DRT, 2 Weeks Following 4 Weeks Chronic Treatment)
2.2.2.1. DRT: WKYs

2.2.2.2. DRT: SHRs

2.2.3. Extinction Task (EXT, 3 Weeks Following Chronic Treatment)
2.2.3.1. EXT: WKYs

2.2.3.2. EXT: SHRs
2.3. TH Immunoreactivity in PFC

2.3.1. Results for WKYs
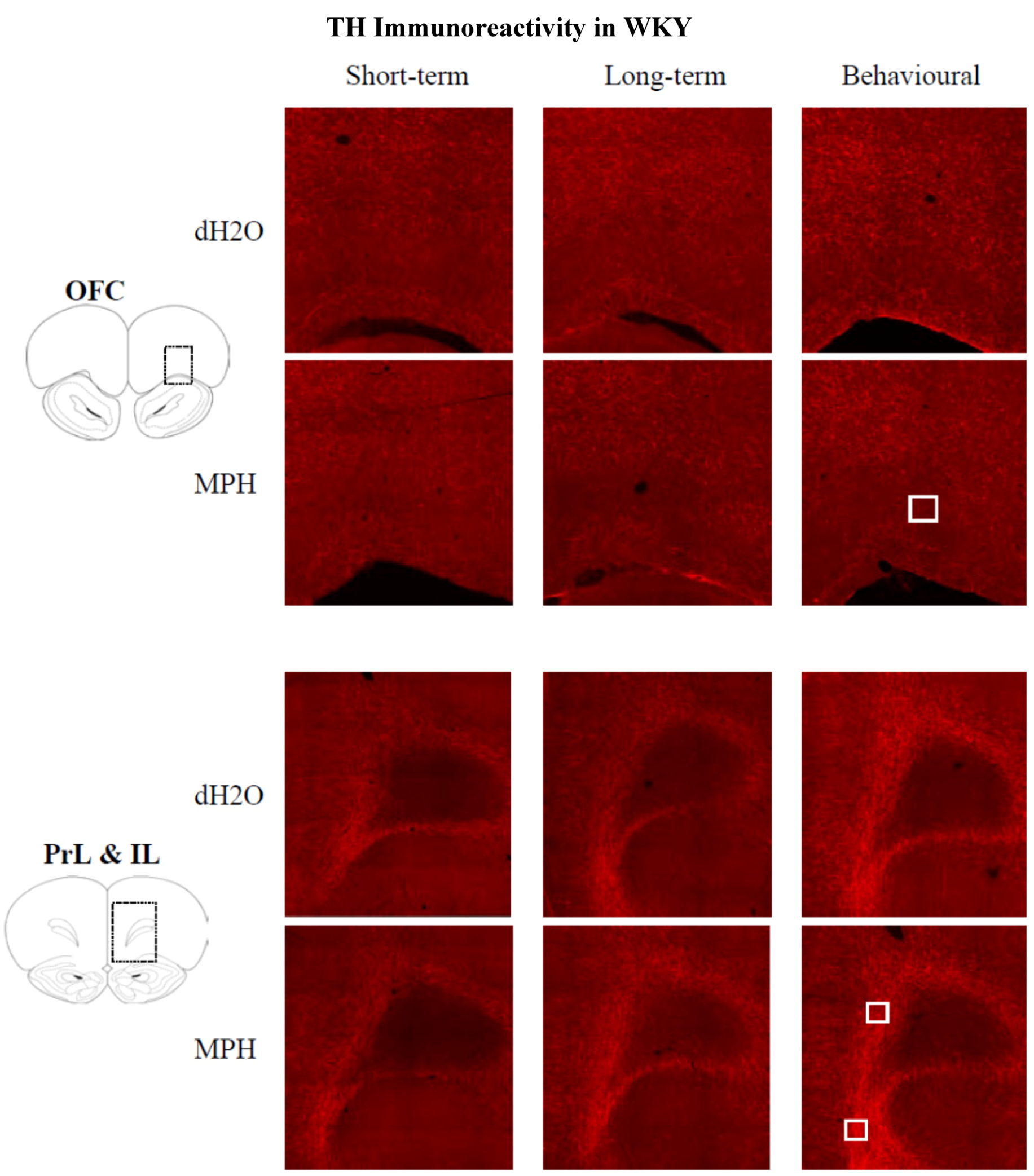

2.3.2. Results for SHRs
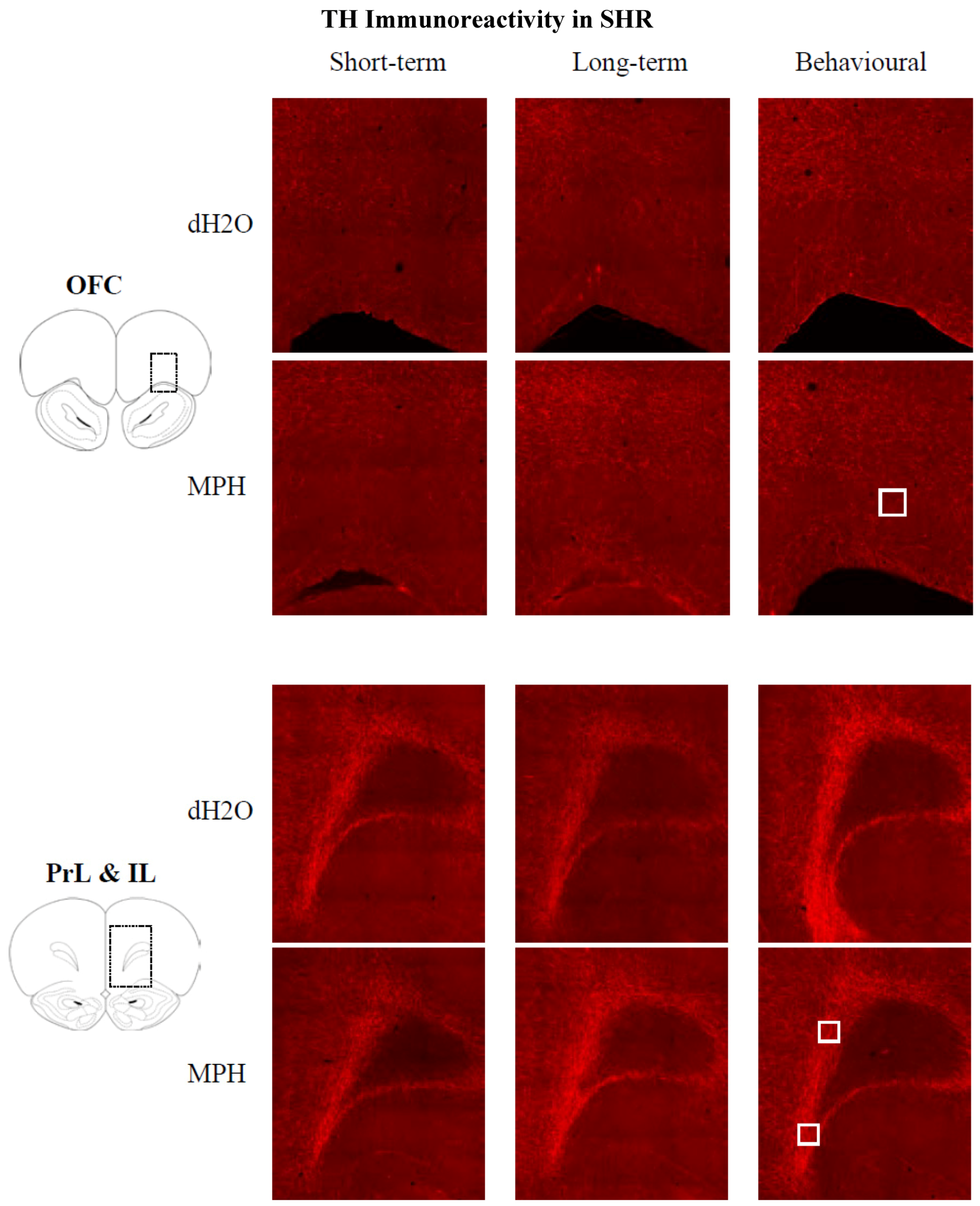
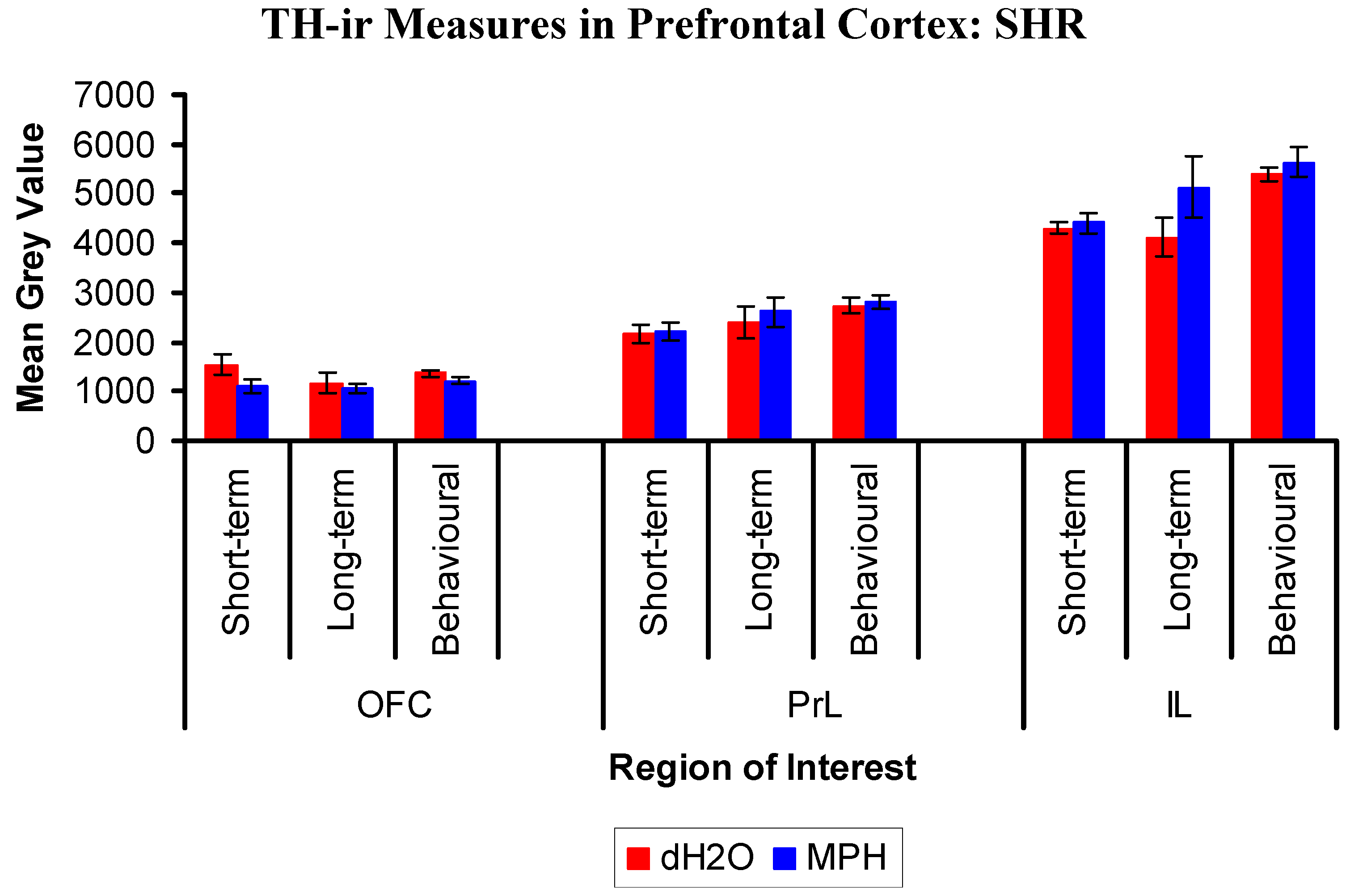
3. Discussion
4. Experimental Section
4.1. Subjects
4.2. Drug Administration Procedure
4.3. Experiment 1: Acute Drug Dose-Response Curve for Locomotor Activity
4.4. Experiment 2: General Chronic Treatment Procedure
4.4.1. Cognitive-Behavioural Tasks
4.4.1.1. Locomotor Activity
4.4.1.2. Delayed Reinforcement (DR) and Extinction (EXT) Tasks
| Test | Delay duration in seconds (each experienced 6 times) |
|---|---|
| 1 | 0, 1, 2, 3, 4 |
| 2 | 0, 2, 4, 6, 8 |
| 3 | 0, 5, 10, 15, 20 |
| 4 | 0, 10, 20, 30, 40 |
| 5 | 0, 10, 20, 40, 60 |
| 6 | 0, 20, 40, 60, 80 |
| 7 | 0, 30, 60, 90, 120 |
4.5. Immunohistochemistry
4.5.1. Procedure
4.5.2. Image Analysis
4.6. Statistical Analyses
4.6.1. Statistical Analyses for Behaviour
4.6.2. Statistical Analyses for Immunohistochemistry
5. Conclusion
Acknowledgments
Conflict of Interest
References
- Biederman, J.; Faraone, S.V. Attention-deficit hyperactivity disorder. Lancet 2005, 366, 237–248. [Google Scholar] [CrossRef]
- Greenhill, L.; Beyer, D.H.; Finkleson, J.; Shaffer, D.; Biederman, J.; Conners, C.K.; Gillberg, C.; Huss, M.; Jensen, P.; Kennedy, J.L. Guidelines and algorithms for the use of methylphenidate in children with attention-deficit/hyperactivity disorder. J. Atten. Disord. 2002, 6, S89–S100. [Google Scholar]
- Solanto, M.V.; Alvir, J. Reliability of DSM-IV symptom ratings of ADHD: Implications for DSM-V. J. Atten. Disord. 2009, 13, 107–116. [Google Scholar] [CrossRef]
- Handler, M.W.; DuPaul, G.J. Assessment of ADHD: Differences across psychology specialty areas. J. Atten. Disord. 2005, 9, 402–412. [Google Scholar] [CrossRef]
- Sciutto, M.J.; Eisenberg, M. Evaluating the evidence for and against the overdiagnosis of ADHD. J. Atten. Disord. 2007, 11, 106–113. [Google Scholar] [CrossRef]
- Angold, A.; Erkanli, A.; Egger, H.L.; Costello, E.J. Stimulant treatment for children: A community perspective. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 975–984. [Google Scholar] [CrossRef]
- Sagvolden, T.; Metzger, M.A.; Schiørbeck, H.K.; Rugland, A.L.; Spinnangr, I.; Sagvolden, G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): Changed reactivity to reinforcers and to psychomotor stimulants. Behav. Neural Biol. 1992, 58, 103–112. [Google Scholar] [CrossRef]
- Zito, J.M.; Safer, D.J.; dosReis, S.; Gardner, J.F.; Boles, M.; Lynch, F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000, 283, 1025–1030. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar]
- Gatley, S.J.; Pan, D.; Chen, R.; Chaturvedi, G.; Ding, Y.S. Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sci. 1996, 58, 231–239. [Google Scholar]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Franceschi, D.; Maynard, L.; Ding, Y.S.; Gatley, S.J.; Gifford, A.; Zhu, W.; Swanson, J.M. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: Therapeutic implications. Synapse 2002, 43, 181–187. [Google Scholar] [CrossRef]
- Kuczenski, R.; Segal, D.S. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: Comparison with amphetamine. J. Neurochem. 1997, 68, 2032–2037. [Google Scholar] [CrossRef]
- Berridge, C.W.; Devilbiss, D.M.; Andrzejewski, M.E.; Arnsten, A.F.; Kelley, A.E.; Schmeichel, B.; Hamilton, C.; Spencer, R.C. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry 2006, 60, 1111–1120. [Google Scholar] [CrossRef]
- Berridge, C.W.; Devilbiss, D.M. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol. Psychiatry 2008, 64, 626–635. [Google Scholar] [CrossRef]
- Ongur, D.; Price, J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex 2000, 10, 206–219. [Google Scholar] [CrossRef]
- Cardinal, R.N. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006, 19, 1277–1301. [Google Scholar] [CrossRef]
- Homayoun, H.; Moghaddam, B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J. Neurosci. 2006, 26, 8025–8039. [Google Scholar] [CrossRef]
- Dalley, J.W.; Mar, A.C.; Economidou, D.; Robbins, T.W. Neurobehavioral mechanisms of impulsivity: Fronto-striatal systems and functional neurochemistry. Pharmacol. Biochem. Behav. 2008, 90, 250–260. [Google Scholar] [CrossRef]
- Mulder, A.B.; Nordquist, R.E.; Orgüt, O.; Pennartz, C.M. Learning-related changes in response patterns of prefrontal neurons during instrumental conditioning. Behav. Brain Res. 2003, 146, 77–88. [Google Scholar] [CrossRef]
- Peters, Y.; O’Donnell, P.; Carelli, R. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse 2005, 56, 74–83. [Google Scholar] [CrossRef]
- Walton, M.E.; Bannerman, D.M.; Alterescu, K.; Rushworth, M.F. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J. Neurosci. 2003, 23, 6475–6479. [Google Scholar]
- Arnsten, A.F.T.; Dudley, A.G. Methylphenidate improves prefrontal cortical cognitive function through α2 adrenoreceptor and dopamine D1 receptor actions: relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav. Brain Funct. 2005, 1, 1–9. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Towards a new understanding of attention-deficit hyperactivity disorder pathophysiology. An important role for prefrontal cortex dysfunction. CNS Drugs 2009, 23, 33–41. [Google Scholar] [CrossRef]
- Adriani, W.; Laviola, G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav. Pharmacol. 2004, 15, 341–352. [Google Scholar] [CrossRef]
- Robinson, T.E.; Kolb, B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 2004, 47, 33–46. [Google Scholar] [CrossRef]
- Kolb, B.; Gibb, R.; Gorny, G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary qualitatively with age and sex. Neurobiol. Learn. Mem. 2003, 79, 1–10. [Google Scholar] [CrossRef]
- LeBlanc-Duchin, D.; Taukulis, H.K. Chronic oral methylphenidate administration to periadolescent rats yields prolonged impairment of memory for objects. Neurobiol. Learn. Mem. 2007, 88, 312–320. [Google Scholar] [CrossRef]
- Adriani, W.; Canese, R.; Podo, F.; Laviola, G. 1H MRS-detectable metabolic brain changes and reduced impulsive behavior in adult rats exposed to methylphenidate during adolescence. Neurotoxicol. Teratol. 2007, 29, 116–125. [Google Scholar] [CrossRef]
- Guhn, A.; Dresler, T.; Hahn, T.; Mühlberger, A.; Ströhle, A.; Deckert, J.; Herrmann, M.J. Medial prefrontal cortex activity during the extinction of conditioned fear: an investigation using functional near-infrared spectroscopy. Neuropsychobiology 2012, 65, 173–182. [Google Scholar] [CrossRef]
- Maroun, M.; Kavushansky, A.; Holmes, A.; Wellman, C.; Motanis, H. Enhanced extinction of aversive memories by high-frequency stimulation of the rat infralimbic cortex. PloS One 2012, 7, e35853. [Google Scholar]
- Mobini, S.; Body, S.; Ho, M.Y.; Bradshaw, C.M.; Szabadi, E.; Deakin, J.F.; Anderson, I.M. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology 2002, 160, 290–298. [Google Scholar] [CrossRef]
- Evenden, J.L.; Ryan, C.N. The pharmacology of impulsive behaviour in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 1996, 128, 161–170. [Google Scholar] [CrossRef]
- Berger, D.; Sagvolden, T. Sex differences in operant discrimination behaviour in an animal model of Attention-Deficit Hyperactivity Disorder. Behav. Brain Res. 1998, 94, 73–82. [Google Scholar] [CrossRef]
- Sagvolden, T.; Aase, H.; Zeiner, P.; Berger, D. Altered reinforcement mechanisms in Attention-Deficit/Hyperactivity Disorder. Behav. Brain Res. 1998, 94, 61–71. [Google Scholar] [CrossRef]
- Sagvolden, T.; Russell, V.A.; Aase, H.; Johansen, E.B.; Farshbaf, M. Rodent models of Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2005, 57, 1239–1247. [Google Scholar] [CrossRef]
- Levitt, M.; Spector, S.; Sjoerdsma, A.; Udenfriend, S. Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea-pig heart. J. Pharmacol. Exp. Ther. 1965, 148, 1–8. [Google Scholar]
- Gray, J.D.; Punsoni, M.; Tabori, N.E.; Melton, J.T.; Fanslow, V.; Ward, M.J.; Zupan, B.; Menzer, D.; Rice, J.; Drake, C.T.; et al. Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. J. Neurosci. 2007, 27, 7196–7207. [Google Scholar]
- Pickel, V.M.; Joh, T.H.; Field, P.M.; Becker, C.G.; Reis, D.J. Cellular localization of tyrosine hydroxylase by immunohistochemistry. J. Histochem. Cytochem. 1975, 23, 1–12. [Google Scholar] [CrossRef]
- Thanos, P.K.; Ivanov, I.; Robinson, J.K.; Michaelides, M.; Wang, G.J.; Swanson, J.M.; Newcorn, J.H.; Volkow, N.D. Dissociation between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats in baseline performance and methylphenidate response on measures of attention, impulsivity and hyperactivity in a Visual Stimulus Position Discrimination Task. Pharmacol. Biochem. Behav. 2010, 94, 374–379. [Google Scholar] [CrossRef]
- Yang, P.B.; Swann, A.C.; Dafny, N. Acute and chronic methylphenidate dose response assessment on three adolescent male rat strains. Brain Res. Bull. 2006, 71, 301–310. [Google Scholar] [CrossRef]
- Hand, D.J.; Fox, A.T.; Reilly, M.P. Differential effects of d-amphetamine on impulsive choice in spontaneously hypertensive and Wistar-Kyoto rats. Behav. Pharmacol. 2009, 20, 549–553. [Google Scholar] [CrossRef]
- Myers, M.M.; Musty, R.E.; Hendley, E.D. Attenuation of hyperactivity in the Spontaneously Hypertensive rat by amphetamine. Behav. Neural Biol. 1982, 34, 42–54. [Google Scholar] [CrossRef]
- Kuczenski, R.; Segal, D.S. Exposure of adolescent rats to oral methylphenidate: Preferential effects on extracellular noreprinephrine and absence of sensitization and cross-sensitization to methamphetamine. J. Neurosci. 2002, 22, 7264–7271. [Google Scholar]
- Gerasimov, M.; Franceschi, M.; Volkow, N.D.; Gifford, A.; Gatley, S.J.; Marsteller, D.; Molina, P.E.; Dewey, S.L. Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. J. Pharmacol. Exp. Ther. 2000, 295, 51–57. [Google Scholar]
- Russell, V.A.; de Villiers, A.S.; Sagvolden, T.; Lamm, M.C.; Taljaard, J.J. Methylphenidate affects striatal dopamine differently in an animal model for attention-deficit/hyperactivity disorder—The spontaneously hypertensive rat. Brain Res. Bull. 2000, 53, 187–192. [Google Scholar] [CrossRef]
- Hill, J.C.; Herbst, K.; Sanabria, F. Characterizing operant hyperactivity in the Spontaneously Hypertensive Rat. Behav. Brain Funct. 2012, 8, 5–20. [Google Scholar] [CrossRef]
- Andersen, S.L.; Arvanitogiannis, A.; Pliakas, A.M.; LeBlanc, C.; Carlezon, W.A., Jr. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat. Neurosci. 2002, 5, 13–14. [Google Scholar] [CrossRef]
- Yang, P.B.; Ceullar, D.O., III; Swann, A.C.; Dafny, N. Age and genetic strain differences in response to chronic methylphenidate administration. Behav. Brain Res. 2011, 218, 206–217. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Kolb, B.; Forssberg, H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur. J. Neurosci. 2003, 18, 3394–3399. [Google Scholar] [CrossRef]
- Swanson, J.M.; Volkow, N.D. Pharmacokinetic and Pharmacodynamic Properties of Methylphenidate in Humans. In Stimulant Drugs and ADHD: Basic and Clinical Neuroscience; Solanto, M.V., Arnsten, A.F.T., Castellanos, F.X., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 259–282. [Google Scholar]
- Schoenbaum, G.; Saddoris, M.P.; Ramus, S.J.; Shaham, Y.; Setlow, B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur. J. Neurosci. 2004, 19, 1997–2002. [Google Scholar] [CrossRef]
- Jenstch, J.D.; Olausson, P.; de La Garza, R., II; Taylor, J.R. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology 2002, 26, 183–190. [Google Scholar]
- Jentsch, J.D.; Taylor, J.R. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behaviour by reward-related stimuli. Psychopharmacology 1999, 146, 373–390. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology 2006, 31, 2376–2383. [Google Scholar] [CrossRef]
- Chudasama, Y.; Passetti, F.; Rhodes, S.E.; Lopian, D.; Desai, A.; Robbins, T.W. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: Differential effects on selectivity, impulsivity and compulsivity. Behav. Brain Res. 2003, 146, 105–119. [Google Scholar] [CrossRef]
- Rosenberg, D.R.; Lewis, D.A. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: A tyrosine hydroxylase immunohistochemical analysis. J. Comp. Neurol. 1995, 358, 383–400. [Google Scholar] [CrossRef]
- Huttenlocher, P.R. Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Res. 1979, 163, 195–205. [Google Scholar] [CrossRef]
- Joseph, R. Environmental influences on neural plasticity, the limbic system, emotional development and attachment: A review. Child Psychiatry Hum. Dev. 1999, 29, 189–208. [Google Scholar] [CrossRef]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Neural consequences of environmental enrichment. Nature 2000, 1, 191–198. [Google Scholar]
- Kolb, B.; Gorny, G.; Li, Y.; Samaha, A.N.; Robinson, T.E. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc. Natl. Acad. Sci. USA 2003, 100, 10523–10528. [Google Scholar] [CrossRef]
- Pierce, R.C.; Kalivas, P.W. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Rev. 1997, 25, 192–216. [Google Scholar] [CrossRef]
- Floresco, S.B.; Whelan, J.M. Perturbations in different forms of cost/benefit decision making induced by repeated amphetamine exposure. Psychopharmacology 2009, 205, 189–201. [Google Scholar] [CrossRef]
- Li, Y.; Kauer, J.A. Repeated exposure to amphetamine disrupts dopaminergic modulation of excitatory synaptic plasticity and neurotransmission in nucleus accumbens. Synapse 2004, 51, 1–10. [Google Scholar] [CrossRef]
- Renaud, B.; Joh, T.H.; Reis, D.J. An abnormal regulation of tyrosine hydroxylase restricted to one catecholamine nucleus in the brain stem of spontaneously hypertensive rats. Brain Res. 1979, 173, 164–167. [Google Scholar] [CrossRef]
- Alsop, B. Problems with spontaneously hypertensive rats (SHR) as a model of Attention-Deficit/Hyperactivity Disorder (AD/HD). J. Neurosci. Methods 2007, 162, 42–48. [Google Scholar] [CrossRef]
- Van den Bergh, F.S.; Bloemarts, E.; Chan, J.S.; Groenink, L.; Olivier, B.; Oosting, R.S. Spontaneously hypertensive rats do not predict symptoms of Attention-Deficit Hyperactivity Disorder. Pharmacol. Biochem. Behav. 2006, 83, 380–390. [Google Scholar] [CrossRef]
- Drolet, G.; Proulx, K.; Pearson, D.; Rochford, J.; Deschepper, C.F. Comparisons of behavioral and neurochemical characteristics between WKY, WKHA, and Wistar Rat Strains. Neuropsychopharmacology 2002, 27, 400–409. [Google Scholar] [CrossRef]
- Pardey, M.C.; Homewood, J.; Taylor, A.; Cornish, J.L. Re-evaluation of an animal model for ADHD using a free-operant choice task. J. Neurosci. Methods 2009, 176, 166–171. [Google Scholar] [CrossRef]
- Berbatis, C.G.; Sunderland, V.B.; Bulsara, M. Licit psychostimulant consumption in Australia, 1984–2000: International and jurisdictional comparison. Med. J. Aust. 2002, 177, 539–543. [Google Scholar]
- Preen, D.B.; Calver, J.; Sanfilippo, F.M.; Bulsara, M.; Holman, C.D. Patterns of psychostimulant prescribing to children with ADHD in Western Australia: Variations in age, gender, medication type and dose prescribed. Aust. N. Z. J. Public Health 2007, 31, 120–126. [Google Scholar] [CrossRef]
- Prosser, B.; Reid, R. Psychostimulant use for children with ADHD in Australia. J. Emot. Behav. Disord. 1999, 7, 110–117. [Google Scholar] [CrossRef]
- Prosser, B.; Reid, R. Changes in use of psychostimulant medication for ADHD in South Australia (1990–2006). Aust. N. Z. J. Psychiatry 2009, 43, 340–347. [Google Scholar] [CrossRef]
- National Health and Medical Research Council (NHMRC), Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 7th ed; NHMRC: Canberra, Australia, 2004.
- Laviola, G.; Macri, S.; Morley-Fletcher, S.; Adriani, W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 2003, 27, 19–31. [Google Scholar] [CrossRef]
- Martins, S.; Tramontina, S.; Polanczyk, G.; Eizirik, M.; Swanson, J.M.; Rohde, L.A. Weekend holidays during methylphenidate use in ADHD children: A randomized clinical trial. J. Child Adolesc. Psychopharmacol. 2004, 14, 195–206. [Google Scholar] [CrossRef]
- Johansen, E.B.; Sagvolden, T.; Kvande, G. Effects of delayed reinforcers on the behavior of an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD). Behav. Brain Res. 2005, 162, 47–61. [Google Scholar] [CrossRef]
- McGregor, I.S. Using Strawberry Tree Workbench Mac and Workbench PC for data acquisition and control in the animal learning laboratory. Behav. Res. Methods 1996, 28, 38–48. [Google Scholar] [CrossRef]
- Adriani, W.; Laviola, G. Elevated levels of impulsivity and reduced place conditioning with d-Amphetamine: Two behavioral features of adolescence in mice. Behav. Neurosci. 2003, 117, 695–703. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pardey, M.C.; Kumar, N.N.; Goodchild, A.K.; Clemens, K.J.; Homewood, J.; Cornish, J.L. Long-Term Effects of Chronic Oral Ritalin Administration on Cognitive and Neural Development in Adolescent Wistar Kyoto Rats. Brain Sci. 2012, 2, 375-404. https://doi.org/10.3390/brainsci2030375
Pardey MC, Kumar NN, Goodchild AK, Clemens KJ, Homewood J, Cornish JL. Long-Term Effects of Chronic Oral Ritalin Administration on Cognitive and Neural Development in Adolescent Wistar Kyoto Rats. Brain Sciences. 2012; 2(3):375-404. https://doi.org/10.3390/brainsci2030375
Chicago/Turabian StylePardey, Margery C., Natasha N. Kumar, Ann K. Goodchild, Kelly J. Clemens, Judi Homewood, and Jennifer L. Cornish. 2012. "Long-Term Effects of Chronic Oral Ritalin Administration on Cognitive and Neural Development in Adolescent Wistar Kyoto Rats" Brain Sciences 2, no. 3: 375-404. https://doi.org/10.3390/brainsci2030375
APA StylePardey, M. C., Kumar, N. N., Goodchild, A. K., Clemens, K. J., Homewood, J., & Cornish, J. L. (2012). Long-Term Effects of Chronic Oral Ritalin Administration on Cognitive and Neural Development in Adolescent Wistar Kyoto Rats. Brain Sciences, 2(3), 375-404. https://doi.org/10.3390/brainsci2030375




