The Use of Deep Brain Stimulation in Tourette Syndrome
Abstract
:1. Introduction
2. Methods
3. Literature Review
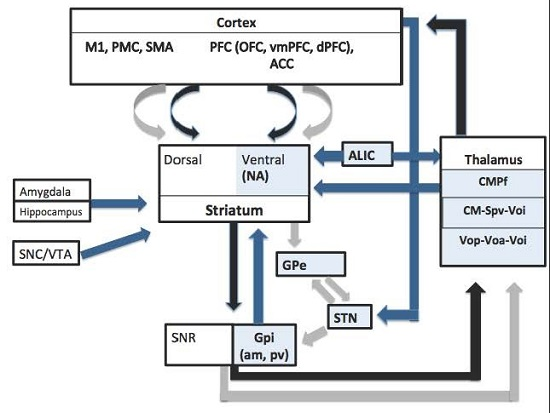
3.1. Thalamic Targets
3.2. Globus Pallidus Internus (GPi)
3.2.1. Posteroventral GPi
3.2.2. Anteromedial GPi
3.3. Other Targets
3.4. Comparative Studies
3.5. Clinical Outcome and Targets
3.6. Inclusion and Exclusion Criteria
3.7. Adverse Effects
3.8. Mechanisms of DBS Action in TS
3.9. Adaptive DBS in TS
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scharf, J.M.; Miller, L.L.; Mathews, C.A.; Ben-Shlomo, Y. Prevalence of Tourette syndrome and chronic tics in the population-based Avon longitudinal study of parents and children cohort. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 192–201. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Leckman, J.F. Tourette’s syndrome. Lancet 2002, 360, 1577–1586. [Google Scholar] [CrossRef]
- Porta, M.; Servello, D.; Sevello, D.; Sassi, M.; Brambilla, A.; Defendi, S.; Priori, A.; Robertson, M. Issues related to deep brain stimulation for treatment-refractory Tourette’s syndrome. Eur. Neurol. 2009, 62, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.H.; Leckman, J.F. Clinical course of Tourette syndrome. J. Psychosom. Res. 2009, 67, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Eapen, V.; Cavanna, A.E.; Robertson, M.M. Comorbidities, social impact, and quality of life in Tourette syndrome. Front. Psychiatry 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Eddy, C.M.; Mitchell, R.; Pall, H.; Mitchell, I.; Zrinzo, L.; Foltynie, T.; Jahanshahi, M.; Limousin, P.; Hariz, M.I.; et al. An approach to deep brain stimulation for severe treatment-refractory Tourette syndrome: The UK perspective. Br. J. Neurosurg. 2011, 25, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Schwabe, K.; Lütjens, G.; Capelle, H.H.; Manu, M.; von Wrangel, C.; Müller-Vahl, K.; Schrader, C.; Scheinichen, D.; Blahak, C.; et al. Comparative characterization of single cell activity in the globus pallidus internus of patients with dystonia or Tourette syndrome. J. Neural Transm. 2015, 122, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.R.; Grosskreutz, J.; Prell, T.; Kaufmann, J.; Bodammer, N.; Peschel, T. Tics are caused by alterations in prefrontal areas, thalamus and putamen, while changes in the cingulate gyrus reflect secondary compensatory mechanisms. BMC Neurosci. 2014, 15, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Makki, M.I.; Govindan, R.M.; Wilson, B.J.; Behen, M.E.; Chugani, H.T. Altered fronto-striato-thalamic connectivity in children with tourette syndrome assessed with diffusion tensor MRI and probabilistic fiber tracking. J. Child Neurol. 2009, 24, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Tinaz, S.; Malone, P.; Hallett, M.; Horovitz, S.G. Role of the right dorsal anterior insula in the urge to tic in tourette syndrome. Mov. Disord. 2015, 30, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Fraint, A.; Pal, G. Deep brain stimulation in Tourette’s syndrome. Front. Neurol. 2015, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Hariz, M.I.; Robertson, M.M. Gilles de la Tourette syndrome and deep brain stimulation. Eur. J. Neurosci. 2010, 32, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J. Am. Acad. Child Adolesc. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Pappert, E.J.; Louis, E.D.; Raman, R.; Leurgans, S. Advantages of a modified scoring method for the Rush video-based tic rating scale. Mov. Disord. 1999, 14, 502–506. [Google Scholar] [CrossRef]
- Goodman, W.K.; Price, L.H.; Rasmussen, S.A.; Mazure, C.; Fleischmann, R.L.; Hill, C.L.; Heninger, G.R.; Charney, D.S. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch. Gen. Psychiatry 1989, 46, 1006–1011. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Schrag, A.; Morley, D.; Orth, M.; Robertson, M.M.; Joyce, E.; Critchley, H.D.; Selai, C. The Gilles de la Tourette syndrome-quality of life scale (GTS-QOL): Development and validation. Neurology 2008, 71, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Mink, J.W. Basal ganglia dysfunction in Tourette’s syndrome: A new hypothesis. Pediatr. Neurol. 2001, 25, 190–198. [Google Scholar] [CrossRef]
- Kaido, T.; Otsuki, T.; Kaneko, Y.; Takahashi, A.; Omori, M.; Okamoto, T. Deep brain stimulation for Tourette syndrome: A prospective pilot study in Japan. Neuromodulation 2011, 14, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, V.; Van Der Linden, C.; Groenewegen, H.J.; Caemaert, J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. Lancet 1999, 353, 724. [Google Scholar] [CrossRef]
- Visser-Vandewalle, V.; Temel, Y.; Boon, P.; Vreeling, F.; Colle, H.; Hoogland, G.; Groenewegen, H.J.; van der Linden, C. Chronic bilateral thalamic stimulation: A new therapeutic approach in intractable Tourette syndrome: Report of three cases. J. Neurosurg. 2003, 99, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Servello, D.; Porta, M.; Sassi, M.; Brambilla, A.; Robertson, M.M. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: The surgery and stimulation. J. Neurol. Neurosurg. Psychiatry 2008, 79, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Servello, D.; Zanaboni, C.; Anasetti, F.; Menghetti, C.; Sassi, M.; Robertson, M.M. Deep brain stimulation for treatment of refractory Tourette syndrome: Long-term follow-up. Acta Neurochir. 2012, 154, 2029–2041. [Google Scholar] [CrossRef] [PubMed]
- Ackermans, L.; Temel, Y.; Cath, D.; van der Linden, C.; Bruggeman, R.; Kleijer, M.; Nederveen, P.; Schruers, K.; Colle, H.; Tijssen, M.A.J.; et al. Deep brain stimulation in Tourette’s syndrome: Two targets? Mov. Disord. 2006, 21, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, R.J.; de Lotbiniere, A.J.; King, R.A.; Jabbari, B.; Quatrano, S.; Kunze, K.; Scahill, L.; Leckman, J.F. Deep brain stimulation in Tourette’s syndrome. Mov. Disord. 2007, 22, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Servello, D.; Sassi, M.; Brambilla, A.; Defendi, S.; Porta, M. Long-term, post-deep brain stimulation management of a series of 36 patients affected with refractory gilles de la tourette syndrome. Neuromodulation 2010, 13, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Idris, Z.; Ghani, A.R.I.; Mar, W.; Bhaskar, S.; Wan Hassan, W.N.; Tharakan, J.; Abdullah, J.M.; Omar, J.; Abass, S.; Hussin, S.; et al. Intracerebral haematomas after deep brain stimulation surgery in a patient with Tourette syndrome and low factor XIIIA activity. J. Clin. Neurosci. 2010, 17, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Ackermans, L.; Duits, A.; Temel, Y.; Winogrodzka, A.; Peeters, F.; Beuls, E.A.M.; Visser-Vandewalle, V. Long-term outcome of thalamic deep brain stimulation in two patients with Tourette syndrome. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Bartsch, C.; Lenartz, D.; Huys, D.; Daumann, J.; Woopen, C.; Hunsche, S.; Maarouf, M.; Klosterkötter, J.; Sturm, V. Clinical effectiveness of unilateral deep brain stimulation in Tourette syndrome. Transl. Psychiatry 2011, 1, e52. [Google Scholar] [CrossRef] [PubMed]
- Duits, A.; Ackermans, L.; Cath, D.; Visser-Vandewalle, V. Unfavourable outcome of deep brain stimulation in a Tourette patient with severe comorbidity. Eur. Child. Adolesc. Psychiatry 2012, 21, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Stead, M.; Mack, K.J.; Lee, K.H.; Klassen, B.T. Deep brain stimulation in Tourette syndrome: A description of 3 patients with excellent outcome. Mayo Clin. Proc. 2012, 87, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.S.; Foote, K.D.; Wu, S.S.; Ward, H.E.; Bowers, D.; Rodriguez, R.L.; Malaty, I.A.; Goodman, W.K.; Gilbert, D.M.; Walker, H.C.; et al. A trial of scheduled deep brain stimulation for Tourette syndrome: Moving away from continuous deep brain stimulation paradigms. JAMA Neurol. 2013, 70, 85. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, M.G.; Smith, M.E.; Landeros-Weisenberger, A.; Kobets, A.J.; King, R.A.; Miravite, J.; de Lotbinière, A.C.J.; Alterman, R.L.; Mogilner, A.Y.; Pourfar, M.H.; et al. Lessons learned from open-label deep brain stimulation for Tourette syndrome: Eight cases over 7 years. Tremor Other Hyperkinetic Mov. 2013, 1, 3. [Google Scholar]
- Huys, D.; Bartsch, C.; Koester, P.; Lenartz, D.; Maarouf, M.; Daumann, J.; Mai, J.K.; Klosterkötter, J.; Hunsche, S.; Visser-Vandewalle, V.; et al. Motor improvement and emotional stabilization in patients with Tourette syndrome after deep brain stimulation of the ventral anterior and ventrolateral motor part of the thalamus. Biol. Psychiatry 2016, 79, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.G.; Lopez, W.O.C.; Dos Santos Ghilardi, M.G.; Barbosa, D.C.; Barbosa, E.R.; Teixeira, M.J.; Fonoff, E.T. Parallel improvement in anxiety and tics after DBS for medically intractable Tourette syndrome: A long-term follow-up. Clin. Neurol. Neurosurg. 2016, 144, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Servello, D.; Zekaj, E.; Saleh, C.; Lange, N.; Porta, M. Deep brain stimulation in Gilles de la Tourette syndrome: What does the future hold? A cohort of 48 patients. Neurosurgery 2016, 78, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Testini, P.; Zhao, C.Z.; Stead, M.; Duffy, P.S.; Klassen, B.T.; Lee, K.H. Centromedian-parafascicular complex deep brain stimulation for Tourette syndrome: A retrospective study. Mayo Clin. Proc. 2016, 91, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zekaj, E.; Saleh, C.; Porta, M.; Servello, D. Temporary deep brain stimulation in Gilles de la Tourette syndrome: A feasible approach? Surg. Neurol. Int. 2015, 6, 122. [Google Scholar]
- Maciunas, R.J.; Maddux, B.N.; Riley, D.E.; Whitney, C.M.; Schoenberg, M.R.; Ogrocki, P.J.; Albert, J.M.; Gould, D.J. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J. Neurosurg. 2007, 107, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Ackermans, L.; Duits, A.; van der Linden, C.; Tijssen, M.; Schruers, K.; Temel, Y.; Kleijer, M.; Nederveen, P.; Bruggeman, R.; Tromp, S.; et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain 2011, 134, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Diederich, N.J.; Kalteis, K.; Stamenkovic, M.; Pieri, V.; Alesch, F. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: A case report. Mov. Disord. 2005, 20, 1496–1499. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, A.W.; Williams, Z.M.; Amirnovin, R.; Kasper, E.; Rauch, S.L.; Cosgrove, G.R.; Eskandar, E.N. Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: Technical case report. Neurosurgery 2005, 57, E403. [Google Scholar] [CrossRef] [PubMed]
- Shields, D.C.; Cheng, M.L.; Flaherty, A.W.; Gale, J.T.; Eskandar, E.N. Microelectrode-guided deep brain stimulation for Tourette syndrome: Within-subject comparison of different stimulation sites. Stereotact. Funct. Neurosurg. 2008, 86, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Lenartz, D.; Mai, J.K.; Huff, W.; Lee, S.-H.; Koulousakis, A.; Klosterkoetter, J.; Sturm, V. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J. Neurol. 2007, 254, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Shahed, J.; Poysky, J.; Kenney, C.; Simpson, R.; Jankovic, J. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology 2007, 68, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Dehning, S.; Mehrkens, J.H.; Müller, N.; Bötzel, K. Therapy-refractory tourette syndrome: Beneficial outcome with globus pallidus internus deep brain stimulation. Mov. Disord. 2008, 23, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Zabek, M.; Sobstyl, M.; Koziara, H.; Dzierzecki, S. Deep brain stimulation of the right nucleus accumbens in a patient with Tourette syndrome. Case report. Neurol. Neurochir. Polska 2008, 42, 554–559. [Google Scholar]
- Neuner, I.; Podoll, K.; Janouschek, H.; Michel, T.M.; Sheldrick, A.J.; Schneider, F. From psychosurgery to neuromodulation: Deep brain stimulation for intractable Tourette syndrome. World J. Biol. Psychiatry 2009, 10, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Dueck, A.; Wolters, A.; Wunsch, K.; Bohne-Suraj, S.; Mueller, J.U.; Haessler, F.; Benecke, R.; Buchmann, J. Deep brain stimulation of globus pallidus internus in a 16-year-old boy with severe Tourette syndrome and mental retardation. Neuropediatrics 2009, 40, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Servello, D.; Sassi, M.; Brambilla, A.; Porta, M.; Haq, I.; Foote, K.D.; Okun, M.S. De novo and rescue DBS leads for refractory Tourette syndrome patients with severe comorbid OCD: A multiple case report. J. Neurol. 2009, 256, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Torres, I.; Hariz, M.I.; Zrinzo, L.; Foltynie, T.; Limousin, P. Improvement of tics after subthalamic nucleus deep brain stimulation. Neurology 2009, 72, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, R.; Zrinzo, L.; Aviles-Olmos, I.; Hariz, M.; Martinez-Torres, I.; Joyce, E.; Jahanshahi, M.; Limousin, P.; Foltynie, T. Deep brain stimulation for Gilles de la Tourette syndrome: A case series targeting subregions of the globus pallidus internus. Mov. Disord. 2011, 26, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Burdick, A.; Foote, K.D.; Goodman, W.; Ward, H.E.; Ricciuti, N.; Murphy, T.; Haq, I.; Okun, M.S. Lack of benefit of accumbens/capsular deep brain stimulation in a patient with both tics and obsessive—Compulsive disorder. Neurocase 2010, 16, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Dehning, S.; Feddersen, B.; Cerovecki, A.; Botzel, K.; Muller, N.; Mehrkens, J.-H. Globus pallidus internus-deep brain stimulation in Tourette’s syndrome: Can clinical symptoms predict response? Mov. Disord. 2011, 26, 2440–2441. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhuang, P.; Zhang, X.-H.; Li, J.-Y.; Li, Y.-J. Unilateral deep brain stimulation of the right globus pallidus internus in patients with Tourette’s syndrome: Two cases with outcomes after 1 year and a brief review of the literature. J. Int. Med. Res. 2012, 40, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Cannon, E.; Silburn, P.; Coyne, T.; O’Maley, K.; Crawford, J.D.; Sachdev, P.S. Deep brain stimulation of anteromedial globus pallidus interna for severe tourette’s syndrome. Am. J. Psychiatry 2012, 169, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, X.; Li, J.; Li, Y. The benefits of low-frequency pallidal deep brain stimulation in a patient with Tourette syndrome. Parkinsonism Relat. Disord. 2014, 20, 1438–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-G.; Ge, Y.; Stead, M.; Zhang, K.; Yan, S.; Hu, W.; Meng, F.-G. Long-term outcome of globus pallidus internus deep brain stimulation in patients with Tourette syndrome. Mayo Clin. Proc. 2014, 89, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Mohan, A.; Cannon, E.; Crawford, J.D.; Silberstein, P.; Cook, R.; Coyne, T.; Silburn, P.A. Deep brain stimulation of the antero-medial globus pallidus interna for Tourette syndrome. PLoS ONE 2014, 9, e104926. [Google Scholar] [CrossRef] [PubMed]
- Huasen, B.; McCreary, R.; Evans, J.; Potter, G.; Silverdale, M. Cervical myelopathy secondary to Tourette’s syndrome managed by urgent deep brain stimulation. Mov. Disord. 2014, 29, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Smeets, A.Y.J.M.; Duits, A.A.; Plantinga, B.R.; Leentjens, A.F.G.; Oosterloo, M.; Visser-Vandewalle, V.; Temel, Y.; Ackermans, L. Deep brain stimulation of the internal globus pallidus in refractory Tourette syndrome. Clin. Neurol. Neurosurg. 2016, 142, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Houeto, J.L.; Karachi, C.; Mallet, L.; Pillon, B.; Yelnik, J.; Mesnage, V.; Welter, M.L.; Navarro, S.; Pelissolo, A.; Damier, P.; et al. Tourette’s syndrome and deep brain stimulation. J. Neurol. Neurosurg. Psychiatry 2005, 76, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Welter, M.-L.; Mallet, L.; Houeto, J.-L.; Karachi, C.; Czernecki, V.; Cornu, P.; Navarro, S.; Pidoux, B.; Dormont, D.; Bardinet, E.; et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch. Neurol. 2008, 65, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Kefalopoulou, Z.; Zrinzo, L.; Jahanshahi, M.; Candelario, J.; Milabo, C.; Beigi, M.; Akram, H.; Hyam, J.; Clayton, J.; Kass-Iliyya, L.; et al. Bilateral globus pallidus stimulation for severe Tourette’s syndrome: A double-blind, randomised crossover trial. Lancet Neurol. 2015, 14, 595–605. [Google Scholar] [CrossRef]
- Nair, G.; Evans, A.; Bear, R.E.; Velakoulis, D.; Bittar, R.G. The anteromedial GPi as a new target for deep brain stimulation in obsessive compulsive disorder. J. Clin. Neurosci. 2014, 21, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.; Jimenez-Shahed, J.; Baizabal Carvallo, J.F.; Jankovic, J. Deep brain stimulation for tourette syndrome: Target selection. Stereotact. Funct. Neurosurg. 2012, 90, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Shahed, J. Design challenges for stimulation trials of Tourette’s syndrome. Lancet Neurol. 2015, 14, 563–565. [Google Scholar] [CrossRef]
- Piedimonte, F.; Andreani, J.C.M.; Piedimonte, L.; Graff, P.; Bacaro, V.; Micheli, F.; Vilela Filho, O. Behavioral and motor improvement after deep brain stimulation of the globus pallidus externus in a case of Tourette’s syndrome. Neuromodulation 2013, 16, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Huisman-van Dijk, H.M.; van de Schoot, R.; Rijkeboer, M.M.; Mathews, C.A.; Cath, D.C. The relationship between tics, OC, ADHD and autism symptoms: A cross-disorder symptom analysis in Gilles de la Tourette syndrome patients and family-members. Psychiatry Res. 2016, 237, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, J.C.; Schuller, T.; Huys, D.; Becker, I.; Timmermann, L.; Jessen, F.; Visser-Vandewalle, V.; Kuhn, J. Deep brain stimulation for tourette-syndrome: A systematic review and meta-analysis. Brain Stimul. 2016, 9, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Wårdell, K.; Kefalopoulou, Z.; Diczfalusy, E.; Andersson, M.; Åström, M.; Limousin, P.; Zrinzo, L.; Hariz, M. Deep brain stimulation of the pallidum internum for Gilles de la Tourette syndrome: A patient-specific model-based simulation study of the electric field. Neuromodulation 2015, 18, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Schrock, L.E.; Mink, J.W.; Woods, D.W.; Porta, M.; Servello, D.; Visser-Vandewalle, V.; Silburn, P.A.; Foltynie, T.; Walker, H.C.; Shahed-Jimenez, J.; et al. Tourette syndrome deep brain stimulation: A review and updated recommendations. Mov. Disord. 2015, 30, 448–471. [Google Scholar] [CrossRef] [PubMed]
- Muller-Vahl, K.R.; Cath, D.C.; Cavanna, A.E.; Dehning, S.; Porta, M.; Robertson, M.M.; Visser-Vandewalle, V. European clinical guidelines for Tourette syndrome and other tic disorders. Part IV: Deep brain stimulation. Eur. Child. Adolesc. Psychiatry 2011, 20, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Piedad, J.C.P.; Rickards, H.E.; Cavanna, A.E. What patients with Gilles de la Tourette syndrome should be treated with deep brain stimulation and what is the best target? Neurosurgery 2012, 71, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Servello, D.; Sassi, M.; Gaeta, M.; Ricci, C.; Porta, M. Tourette syndrome (TS) bears a higher rate of inflammatory complications at the implanted hardware in deep brain stimulation (DBS). Acta Neurochir. 2011, 153, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Kawikova, I.; Leckman, J.F.; Kronig, H.; Katsovich, L.; Bessen, D.E.; Ghebremichael, M.; Bothwell, A.L.M. Decreased numbers of regulatory T cells suggest impaired immune tolerance in children with tourette syndrome: A preliminary study. Biol. Psychiatry 2007, 61, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Termine, C.; Franciotta, D.; Castiglioni, E.; Pagani, A.; Lanzi, G.; Marino, F.; Lecchini, S.; Cosentino, M.; Balottin, U. Dopaminergic receptor D5 mRNA expression is increased in circulating lymphocytes of Tourette syndrome patients. J. Psychiatr. Res. 2008, 43, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Vernaleken, I.; Kuhn, J.; Lenartz, D.; Raptis, M.; Huff, W.; Janouschek, H.; Neuner, I.; Schaefer, W.M.; Grunder, G.; Sturm, V. Bithalamical deep brain stimulation in tourette syndrome is associated with reduction in dopaminergic transmission. Biol. Psychiatry 2009, 66, e15–e17. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.; Janouschek, H.; Raptis, M.; Rex, S.; Lenartz, D.; Neuner, I.; Mottaghy, F.M.; Schneider, F.; Schaefer, W.M.; Sturm, V.; et al. In vivo evidence of deep brain stimulation-induced dopaminergic modulation in Tourette’s syndrome. Biol. Psychiatry 2012, 71, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Marsden, C.D. Simple tics in Gilles de la Tourette’ s syndrome are not prefaced by a normal premovement EEG potential. J. Neurol. Neurosurg. Psychiatry 1981, 44, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Karp, B.I.; Hallett, M. Extracorporeal “phantom” tics in Tourette’s syndrome. Neurology 1996, 46, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Van der Salm, S.M.A.; Tijssen, M.A.J.; Koelman, J.H.T.M.; van Rootselaar, A.-F. The bereitschaftspotential in jerky movement disorders. J. Neurol. Neurosurg. Psychiatry 2012, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Marceglia, S.; Servello, D.; Foffani, G.; Porta, M.; Sassi, M.; Mrakic-Sposta, S.; Rosa, M.; Barbieri, S.; Priori, A. Thalamic single-unit and local field potential activity in Tourette syndrome. Mov. Disord. 2010, 25, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Zauber, S.E.; Ahn, S.; Worth, R.M.; Rubchinsky, L.L. Oscillatory neural activity of anteromedial globus pallidus internus in Tourette syndrome. Clin. Neurophysiol. 2014, 125, 1923–1924. [Google Scholar] [CrossRef] [PubMed]
- Barow, E.; Neumann, W.J.; Brucke, C.; Huebl, J.; Horn, A.; Brown, P.; Krauss, J.K.; Schneider, G.H.; Kuhn, A.A. Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain 2014. [Google Scholar] [CrossRef] [PubMed]
- Maling, N.; Hashemiyoon, R.; Foote, K.D.; Okun, M.S.; Sanchez, J.C. Increased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with Tourette’s syndrome. PLoS ONE 2012, 7, e44215. [Google Scholar] [CrossRef]
- McCairn, K.W.; Iriki, A.; Isoda, M. Deep brain stimulation reduces tic-related neural activity via temporal locking with stimulus pulses. J. Neurosci. 2013, 33, 6581–6593. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Giannicola, G.; Rosa, M.; Marceglia, S.; Servello, D.; Sassi, M.; Porta, M. Deep brain electrophysiological recordings provide clues to the pathophysiology of Tourette syndrome. Neurosci. Biobehav. Rev. 2013, 37, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F. Deep brain stimulation for tourette syndrome: Lessons learned and future directions. Biol. Psychiatry 2016, 79, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, A.; Morita, H.; Rossi, P.J.; Allen, W.L.; Alterman, R.L.; Bronte-Stewart, H.; Butson, C.R.; Charles, D.; Deckers, S.; de Hemptinne, C.; et al. Proceedings of the second annual deep brain stimulation think tank: What’s in the pipeline. Int. J. Neurosci. 2015, 7454, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.J.; Lujan, J.L.; Chaturvedi, A.; Goodman, W.K.; Okun, M.S.; McIntyre, C.C.; Haq, I.U. Tractography activation patterns in dorsolateral prefrontal cortex suggest better clinical responses in OCD DBS. Front. Neurosci. 2016, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.S.; Dhatt, H.S.; Ferguson, M.A.; Lopez-Larson, M.; Schrock, L.E.; House, P.A.; Yurgelun-Todd, D. Functional connectivity targeting for deep brain stimulation in essential tremor. Am. J. Neuroradiol. 2011, 32, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Martinez-Ramirez, D.; Rossi, P.J.; Peng, Z.; Gunduz, A.; Okun, M.S. Chasing tics in the human brain: Development of open, scheduled and closed loop responsive approaches to deep brain stimulation for tourette syndrome. J. Clin. Neurol. 2015, 11, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.J.; Gunduz, A.; Judy, J.; Wilson, L.; Machado, A.; Giordano, J.J.; Elias, W.J.; Rossi, M.A.; Butson, C.L.; Fox, M.D.; et al. Proceedings of the third annual deep brain stimulation think tank: A review of emerging issues and technologies. Front. Neurosci. 2016, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Arlotti, M.; Rosa, M.; Marceglia, S.; Barbieri, S.; Priori, A. The adaptive deep brain stimulation challenge. Parkinsonism Relat. Disord. 2016, 28, 12–17. [Google Scholar] [CrossRef] [PubMed]

| Study | Target | Sample Size, Sex (Age, Years) | Follow up (Months) | Stimulation Parameters | Tic outcome (Improvement in YGTSS or MRVRS) | Comoribidity Outcome | Adverse Effects/Comments |
|---|---|---|---|---|---|---|---|
| Vandewalle et al. (1999) [20], Visser-Vandewalle (2003) [21] | CMPf, SPv Voi | Three males (42, 28, 45) | 60, 12, 8 | Bipolar all contacts 100 Hz, 210 µs, 2.4 V right, 2.2 V left; Right double monopolar 65 Hz, 210 µs, 3 V; left monopolar 100 Hz, 210 µs; Right monopolar 2.8 V, 130 Hz, 210 µs; left one monopolar 2.4 V, 100 Hz 200 µs | 90%, 72%, 83% | OCD and SIB disappeared in all 3. | A slight sedative effect in all three; 2 had increased or decreased libido. |
| Diederich et al. (2005) [41] | pvGPi | One male (27) | 14 | Monopolar 2 V, 185 Hz, 60 µs | 73% (previous YGTSS 83) | No apparent change in the mild compulsions of the patient. Significant reduction of anxiety/depression. | Small symptomatic haematoma right pallidum bradykinesia of left extremities |
| Flaherty et al. (2005) [42], Shields et al (2008) [43] | Anterior capsule CMPf, SPv Voi | One female (37), same patient operated at different target | 18, 3 | Bipolar 4.1 V, 185 Hz, 210 µs, 7 V, 90 µs, 185 Hz | YGTSS 25%, 32% | Self-injury stopped (previous retinal detachment led to blindness in one eye), but vision stabilized post DBS | Electrode breakage therefore same patient had stimulation at different target. The AC site caused altered mood and impulse control disturbance. |
| Ackermans et al. 2006 [24] | CMPf, Voi pvGpi | One male (45), one male (27) | 12 | Monopolar 6.4 V, 130 Hz, 120 µs bilaterally; Monopolar 3.1 V, 170 Hz, 210 µs bilaterally | Tics 20 to 3/min, Tics 28 to 2/min | Obssessions and compulsions (measured by the revised Padua inventory) improved by 62% in the ON than OFF condition | CMPf patient is the same as in Vandewalle study- he experienced vertical gaze palsy and decreased libido. Both patients complained of reduced energy. |
| Kuhn et al. 2007 [44] | NAC, internal capsule | One male (26) | 30 | Tetra monopolar 90 µs, 130 Hz, 7 V | 41% with YGTSS, 50% MRVRS (Previous YGTSS 90 and MRVRS 18) | Reduction in SIB, reduction in OCD by 52% based on Y-BOCS | No significant adverse effects |
| Bajwa et al. 2007 [25] | CMPf, SPv Voi | One male (48) | 24 | Bipolar 2 V, 130 Hz and 90 µs | 66% (Previous YGTSS score 35.5 and overall 83) | YBOCS improved 75% | Approximately 14 programming sessions required over 2 years. No serious adverse effects. |
| Shahed et al. 2007 [45] | pvGPi | One male (16) | 6 | Monopolar 5 V, 160 Hz right, 145 Hz left, 90 µs | 84% (YGTSS) | YBOCS improved 69% (only obsessions not compulsions). | No surgical adverse effects |
| Dehning et al. 2008 [46] | pvGPi | One female (44) | 12 | Monopolar 4.2 V, 145 Hz, 210 µs | YGTSS 88% | Patient did have SIB including self-biting and beating but outcome not reported. | Complaints of depression. Vertigo and stomach aches in first few months. No serious adverse effects. |
| Zabek et al. 2008 [47] | Right NAC | One male (31) | 28 | Not reported | 80% (15-minute videotaped exams) | None reported | Unilateral right side only. |
| Neuner et al. 2009 [48] | NAC | One male (38) | 36 | Double monopolar 6 V, 145 Hz, 90 µs | YGTSS 44% mRVTRS 58% | YBOCS 56% | Rapid IPG depletion-2 replacements in 36 months. Incidental finding was that patient no longer wanted to smoke. |
| Dueck 2009 [49] | pvGPi | One male (16) | 12 | Monopolar 4 V, 130 Hz, 120 µs | No improvement | Co-morbidity was severe mental retardation which was not affected | Not reported |
| Servello et al. 2008 [22] | CMPf Voi | 15 males, 3 females (17–47) | 3-18 | Bipolar 2.5–4 V, 90–120 µs, 130 Hz | YGTSS 65% | None reported | Transient stimulation induced vertigo, poor scalp incision healing due to repetitive touching requiring body shield. |
| Servello et al. 2009 [50] | CMPF + ALIC/NA in 3, 1 had only ALIC/NA | Three males, one female (25, 31 ,37, 47) | 10–26 | 3 monopolar, 1 bipolar 4–4.5 V, 130–160 Hz, 150–180 µs | Variable, mostly slight improvement in tics | Sight improvement in OCD | None reported |
| Servello et al. 2010 [26] | Total 79 procedures 36 patients) CMPf/Voi (67) pvGPi (2)ALIC-NA (10) | 25 males, 6 females (17–57). 4 additional patients received leads in multiple targets and one in ALIC/NA | 3–48 | At last follow up 2–5 V, 90–140 µs, 60–180 Hz | YGTSS 47% mean improvement | 17% mean improvement in YBOCS | 2 patients had stimulator switched off, reporting unsatisfactory results, 2 had surgical revision of pulse generator due to infection, and one had hardware failure. |
| Martinez-Torres et al. 2009 [51] | STN | One male (38) | 12 | Monopolar 3 and 3.2 V, 130 Hz, 60 µs | 76% | Not reported | Patient also had Parkinson’s disease which was the indication for DBS in STN |
| Martinez-Fernandez et al. 2011 [52] | pvGPi (2), amGPi (2), pvGPi (1) (then changed to anteromedial region after 18 months) | 4 males (21–60), 1 female (35) | 3–24 | PL GPi: Monopolar 2.5 V, 150 µs, 170 Hz, 2.5 V, 60 µs, 130 Hz; AM GPi: Monopolar 4.2 V, 60 µs, 160 Hz, Monopolar 4 V, 210 µs, 130 Hz; PL then AM GPi: Bipolar 3.6 V, 60 µs, 20 Hz for both | Anteromedial group: MRVRS 54% , Posterolateral group (3 patients, with one experiencing worsening symptoms and responders average 37% improvement). YGTSS 38% improvement versus 20% for amGPi and pvGPi. | YBOCS mean change of 26% at last follow up | Complaints of agitation in 1, anxiety in 2, weight gain in 1, infection in 1 requiring repeat removal of battery. |
| Idris et al. 2010 [27] | CMPf, Voa | 1 male (24) | 2 | 3.5 V, 120 µs, 130 Hz | No scales reported, but tics noted to improve | Not reported | Postoperative bilateral subcortical haematomas attributed to low factor XIIIA |
| Ackermans et al. 2010 [28] | CMPf, SPv, Voi | 2 males (42,45) | 72–120 | Amplitude L1, 8 R1, 5 130 Hz, 90 µs Amplitude L8, 5 R8, 5 100 Hz 150 µs At long-term follow up | Patient 1 (5 years) tic improvement 90.1%, maintained at 10 years (92.6%). In patient 2, after 8 months 82% slightly decreased at 6 years (78%). Video tic rating scale used measuring vocal and motor tics/10minutes. | Not reported, but in one patient “psychopathology“ reported to remain. Compulsions said to have disappeared in both patients. In one patient with depression, only slight decrease after surgery. | Both patients reported reduced energy. Both experienced traction of the lead in neck. One patient experienced a decrease in erectile function whilst the other had increased sexual drive. Both reported some visual blurring. One patient reported decreased verbal fluency and learning. |
| Burdick et al. 2010 [53] | ALIC/NA | One male (33) | 30 | Not reported | YGTSS 15% reduction at 6 months and thereafter until 30 months of last follow up remained unchanged. Initially had mild tics with a pre-operative MRVRS score of 5. | Not reported | No reported adverse effects. |
| Kaido et al. 2011 [19] | CMPf | One male (20) ,two females (19, 21) | 14–21 | Tetrapolar bilateral 2.3 V, 210 µs, 130 Hz; Tetrapolar Left 2.1 V, right 2.3 V, left 210 µs, right 180 µs, 130 Hz; bilaterally Tripolar Right 2.6 V, left 2.5 V, 180 µs, 80 Hz | Tics (52%–71%) Social impairment (56%–71%) | Not reported | No reported side effects. |
| Kuhn et al. 2011 [29] | Vop-Voa-Voi (unilateral) | One male (39), one female (27) | 12 | Bipolar 4.5 V, 130 Hz, 120 µs; Bipolar 3.1 V, 90 Hz, 120 µs | YGTSS 75%–100% MRVRS 77%–100% | BDI-no negative impact | Reduced verbal fluency at one year in both patients. |
| Dehning et al. 2011 [54] (one patient previously reported in Dehning 2008 [46]) | pvGPi | Three females (25–44), one male (38) | 5–13 | 4.2 V, 4 V, 3.8 V; 210 µs, 150 µs, 150 µs, 145 Hz, 130 Hz, 130 Hz (3 females) 3.5 V, 180 Ms, 130 Hz | 2 patients responders (64% and 88% improvement YGTSS) 2 patients non-responders 1 female 2 male | Not reported | Lead revision was done in a non-responder without improvement. |
| Dong et al. 2012 [55] | pvGpi (unilateral R) | Two males (41, 22) | 12 | Quadripolar, 3.5 V, 2.8 V, 90 µs, 160, 130 Hz | YGTSS 53.1%–58.5% | None reported | No apparent adverse effects. |
| Cannon et al. 2012 [56] | amGPi | 8 males (22–50), 3 females (18–34) | 4–30 | Quadripolar, initial stimulation 1 V, 60 µs, 130 Hz, adjusted at follow ups | YGTSS motor 48% vocal 56.5% | Mean YBOCS reduction 59%, HDRS 74%, GTS-QLS 102% | Complications from hardware malfunctions in 3 (due to SIB, MVA, and unknown). 1 patient did not tolerate DBS and switched it off. Anxiety in 2 patients |
| Duits et al. 2012 [30] | CM-Spv-Voi | One male (21) | 23 | Not reported | YGTSS- worse with DBS Pre-op-42 Stim OFF-12 Stim on-39 | Y-BOCS Pre-op: 20 Stim OFF-8 Stim ON-7 | Severe post-operative complications including psychogenic paroxysmal hypertonia.The patient may have had a somatoform disorder, that may contra-indicate DBS. |
| Savica et al. 2012 [31] | CMPf | 2 males, one female (17, 17, 35) | 1 | 17 years old males: Bipolar 3.7 V, 120 µs, 117 Hz; Monopolar 2.5 V, 90 µs, 130 Hz. 35 years old female: Bipolar 4.1 V, 120 µs, 107 Hz | YGTSS 70% mean improvement | No formal assessment, but co-morbid symptoms appeared stable or slightly improved. | Adverse effects related to stimulation such as mild paraesthesias but corrected with programming changes. |
| Okun et al. 2013 [32] | CM (scheduled stimulation) | 3 females, 2 males (28–39) | 6 | Not reported | YGTSS 19% mean improvement mRVRS 36% mean improvement | YBOCS, HDRS, QOLAS did not improve. | No serious side effects. |
| Porta et al. 2012 [23] | Cm-Pfc-Voa | 15 male, 3 females (17–47) | 5–6 years | 2.5–4 V, 60–120 µs, 130 Hz | YGTSS 73% | YBOCS 42% (p = 0.003), STAI-46%, BDI−55% | One patient developed poor healing of scalp scar due to compulsion to touch, the other developed abdominal haematoma where pulse generator was located. Majority had some minor side effects if voltage> 4 such as vertigo or blurring of vision. |
| Motlagh et al. 2013 [33] | Midline thalamic | 4 males (16–44) | 6–95 | 0.1–5 V, 60–120 µs, 60–200 Hz (4 bipolar one tripolar) | YGTSS- Greater improvement in the 2 younger patients (67%–85%), compared to older patients (7%–20%). | YBOCS-100% improvement in on patient but minimal change or worsening in others, HDRS and HARS-no change | 44 year old male picked compulsively at chest and cranial incisions so DBS was removed due to infection. 42 year old male had DBS system removed due to lack of therapeutic effect. |
| Dong et al. 2014 [57] | pvGpi | 1 male (33) | 39 | Monopolar 2.8 V, 90 µs, 130 Hz (frequency then reduced to 65 Hz 33 months) | YGTSS 92.9% at 33 and 39 months | YBOCS went from 18 to 0HAS and HDS markedly reduced | Supports that low frequency stimulation may be an optional therapeutic strategy in some patients |
| Zhang et al. 2014 [58] | pvGpi | 12 males (16–34), 1 female (21) | 13–80 | Not reported | YGTSS Mean 52.1% (13–80 months). | GTS-QOL improved by a mean of 45.7% (range, 11.0%–77.2%). | Not reported |
| Sachdev et al. 2014 [59] | amGPi | 14 males, 3 females (17–51) | 8–46 | Mean at follow up: 4.14 V, 95.2 µs, 139.4 Hz | Overall 48.3% reduction motor tics, 41.3% in phonic, 1 month 70.6% of patients had >50% reduction in YGTSS | YBOCS average 62% improvement (p = 0.001), 39% improvement in GTS-QOL (p < 0.001) | Lead breakage in 4 patients, one patient had infection, 2 transient anxiety, 1 dizziness, 1 poor balance. |
| Huasen et al. 2014 [60] | amGPi | One female (19) | 12 | Bipolar 2.9 V, 180 Hz, 180 µs | YGTSS 55% improvement | Not formally assessed | Patient had a cervical cord contusion secondary to violent cervical tics, with DBS used to preserve limb function and led to improvement in her neck extension tics |
| Zekaj et al. 2015 [38] | CMPf, Vo | 1 male (17) | 84 | Not reported | At 12 months YGTSS 58.2% | Not reported | 5 years post surgery DBS removed, and patient had stabilized despite stimulation. 2 year later continues stable. Symptoms may have resolved spontaneously. However, supports DBS in younger patients. |
| Huys et al. 2016 [34] | Ventro- anterior and ventrolateral motor parts of thalamus | Three female, five male (19–56) | 12 | 1.3–3.7 V, 80–130 Hz, 60–150 µs | YGTSS and mRVRS at last follow up average 58% | In 5 no OCD at baseline, 2 mild and 1 severe. No significant effect on OCD comorbidity. Significant effect on quality of life improvement. | One patient had mild infection of subcutaneous pulse generator one patient had disturbance of eye motility, tremor of lower jaw. 1 patient had suicidal thoughts, 1 dysarthria. |
| Smeets et al. 2016 [61] | Anterior GPi | Three males and two females (35–57) | 5–38 | Tetrapolar 2.2–5.6 V, 180-360 µs, 100–180 Hz | YGTSS 71.5% average | In the three patients with baseline OCB (12%, 35% and 100%). | Two males had previous CMPf stimulation but due to side effects such as gaze disturbance, switched to GPi after 6 and 8 years after infection with IPG replacement. Other adverse effects included apathy in 2 patients, weight loss and agitation in 1 patient |
| Cury et al. 2016 [35] | CMPf | One male (23) | 18 | Not reported | YGTSS 70.5% Subscore impairment 60% | Patient did not have OCD 53% improvement on hospital anxiety scale (HAS) | None reported |
| Servello et al. 2016 [36], Testini et al. 2016 [37] | Vo-Cm-Pf-30, Vo-Cm-Pf and NA-ALIC-2, NA-ALIC (in 3 patients as single target, 3 as rescue therapy) amGPi (1 patient and 2 as rescue) CMPf | 14 females, 34 males (total 48), 37 included in final analysis (17–57) 18 males, 3 females (17–46) | Up to 4 years 2–91 | 130 Hz initally in all patients, 60–120 µs, 2.5–4.5 V Bilateral quadripolar, specific parameters not reported | For remaining 37 patients Mean postoperative decrease in YGTSS 63% Reduction of more than 50% seen in 78.4% patients Average YGTSS 54% (46% motor, 52% vocal and 59% impairment). All but two patients reported marked reduction in tic severity and quality of life | 35 OCD, 25 both OCD and depressive disorder. In 4 patients with moderate/severe depression, BDI improved by 45%, in the other 2 only slightly or inconsistently. All patients had psychiatric co-morbidities but outcome not reported. | In 11 patients the device was removed due to inflammatory complications or poor compliance and these were not included in final analysis. NA-ALIC was the single target in 3 patients, joint target in another 3, and rescue for further 3 patients. PvGPi targeted in one patient who then required rescue surgery in NA-ALIC. 12 patients had skin erosions requiring surgical intervention. 1 patient underwent wound revision due to scalp erosion and wound infection. Three years before, one patient had pallidal DBS with no apparent benefit. Postsurgical adverse effects reported on neuropsychological evaluation included occipital headache, and memory loss (including temporary anterograde amnesia) |
| Study | Target | Sample Size, Sex (Age, Years) | Follow up | Stimulation Parameters | Effect on Tics Severity/YGTSS or MRVRS | Effect on Comorbidity | Adverse Effects/Comments |
|---|---|---|---|---|---|---|---|
| Maciunas et al. 2007 [39] | CMPf, Voi | Five males (18–34) | 3 | Variable polarity 3.5–3.6 V, 90–210 µs,130–180 Hz | Double blind comparison during first 4 weeks showed a 17% improvement. At 3 months 44% (mean) Non-responders with 4.3%–260% tic exacerbation | Mean score improvements: YBOCS 44%, BDI-2 60%, Hamilton anxiety scale, (HAS) 51% | One patient experienced acute psychosis during randomised period which was successfully treated. Overall three responders and two non-responders |
| Houeto et al. 2005 [62], Welter et al. 2008 [63] | CMPF and anteromedial GPi | Two females, one male (36, 30, 30) | 60, 27, 20 | CMPf: double monopolar 1.5–1.7 V, 60 µs, 130 Hz, Gpi: Single or double monopolar 1.5–3.5 V, 60 µs, 130 Hz | YGTSS cross-over period, (a)-AmGPi: (65%, 96%, 74%, (b)-CMPf: 30%, 40%, 64%, (c)-Gpi and CMPF 43%, 60%, 76% (after 60 months) | One patient previously had major depressive disorder and self-injurious behaviours and impulsiveness. Depressive mood, anxiety and impulsiveness tended to decrease with thalamic and pallidal stimulation but not paillidal sitmulation alone. None of the patients had OCD. | Reduced libido in one patient having thalamic stimulation. Lethargy, anxiety reported under pallidal stimulation and vertigo under higher intensity stimulation; arm paraesthesia under thalamic. Pallidal better than thalamic stimulation and both better than sham stimulation. |
| Ackermans et al. 2011 [40] | CM-Spv-Voi | 6 males (completed full trial) (35–48 years) | 36 | 1.3–7 V, 60–210 µs, 70–130 Hz. Monopolar stimulation in three patients and bipolar stimulation in the other three patients. | YGTSS at blinded ON compared to OFF stimulation was significantly lower (37%). After one year 49% and MRVRS 35%. | No significant difference found between the behavioural disorders and mood in the ON and OFF stimulation conditions. | One small haemorrhage ventral to electrode, one infection of pulse generator, subjective gaze disturbances which resolved after 6 months; all patient reported reduced energy levels. All patients when further questioned had subtle changes in oculomotor function, from visual disturbance to blurred vision and fixation problems, with no objective abnormalities detected with investigations. One younger patient with very severe SIB and life-threatening tics developed hypertonia, mutism and repeated fainting which needed extensive diagnostic evaluation- she was not randomised and considered loss to follow up. Only two patients completed the full 3 months on and of stimulation periods. |
| Kefalopoulou et al. 2015 [64] | AmGPi (13 patients), pvGPi (2 patients due to dystonic features). | 11 males 4 females (25–55 years), 14 randomly assigned 13 completed assessments in both blinded periods. All 15 received stimulation in open-label phase | 20–60 | In blinded phase, 9 patients had monopolar and double monopolar in 4 | YGTSS from off to on stimulation during blinded crossover period was 15.3%. Increased to 40.1% in open label phase | YBOCS-modest non-significant improvements Significant improvement in BDI GTS-QOL 38.9% | 2 patients experienced infection of hardware requiring removal of leads and pulse generator with antibiotic treatment who were re-implanted 22 months later. One patient experienced deterioration of tics and hypomanic behaviour during on-stimulation periods, requiring stimulation parameter alterations and benzodiazepine treatment. 23 non-serious adverse events occurred, 15 of which resolved. During blinded phase, 6 patients had no clear benefit (<10% improvement in YGTSS) but most of these had more significant improvement in open label phase when parameters could be optimized. In open-label phase, 4 patients had less than 20% improvement n YGTSS. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbarian-Tefaghi, L.; Zrinzo, L.; Foltynie, T. The Use of Deep Brain Stimulation in Tourette Syndrome. Brain Sci. 2016, 6, 35. https://doi.org/10.3390/brainsci6030035
Akbarian-Tefaghi L, Zrinzo L, Foltynie T. The Use of Deep Brain Stimulation in Tourette Syndrome. Brain Sciences. 2016; 6(3):35. https://doi.org/10.3390/brainsci6030035
Chicago/Turabian StyleAkbarian-Tefaghi, Ladan, Ludvic Zrinzo, and Thomas Foltynie. 2016. "The Use of Deep Brain Stimulation in Tourette Syndrome" Brain Sciences 6, no. 3: 35. https://doi.org/10.3390/brainsci6030035
APA StyleAkbarian-Tefaghi, L., Zrinzo, L., & Foltynie, T. (2016). The Use of Deep Brain Stimulation in Tourette Syndrome. Brain Sciences, 6(3), 35. https://doi.org/10.3390/brainsci6030035