Design of Linear and Cyclic Mutant Analogues of Dirucotide Peptide (MBP82–98) against Multiple Sclerosis: Conformational and Binding Studies to MHC Class II
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
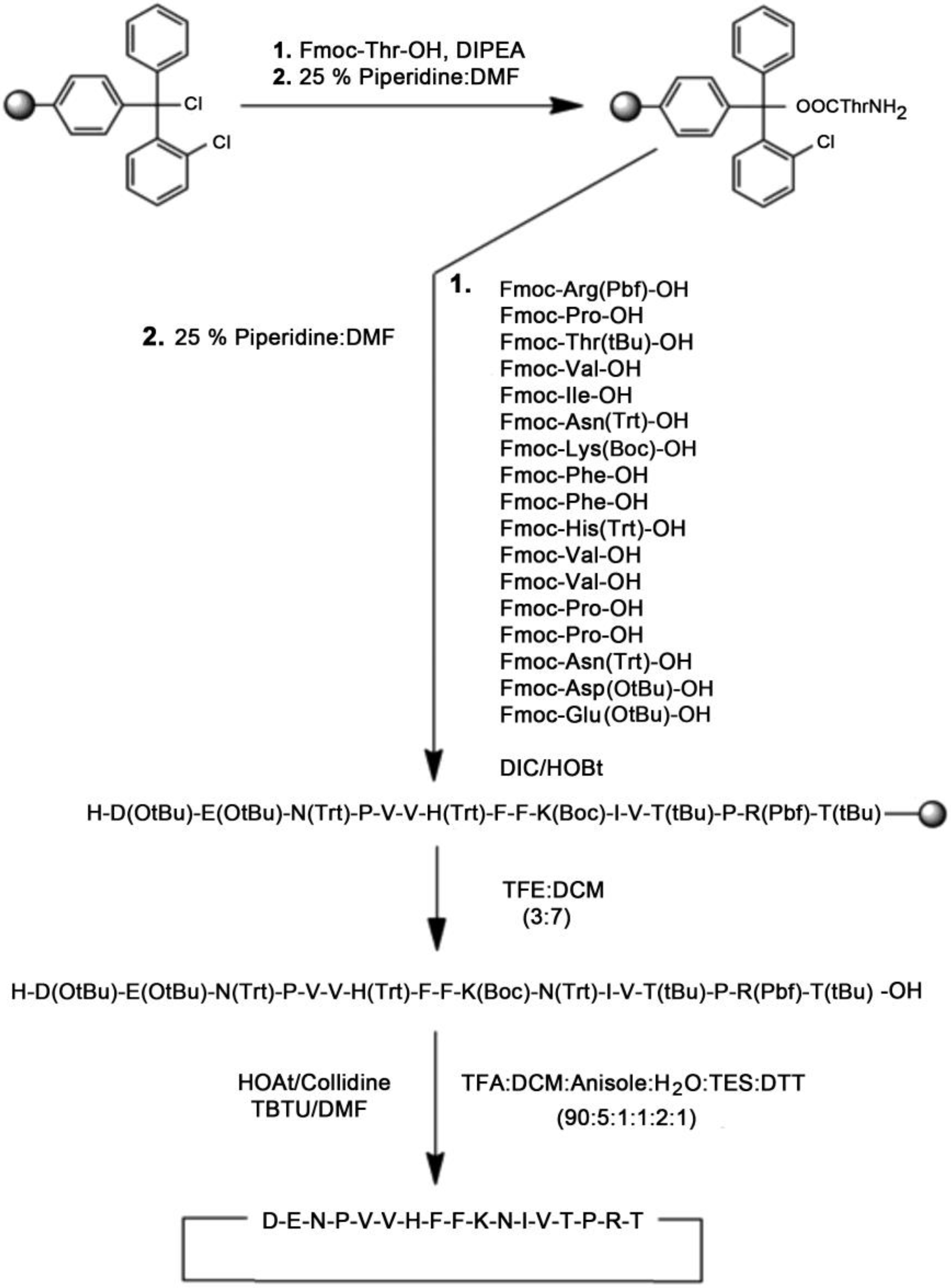
2.1.1. Solid-Phase Peptide Synthesis of Linear and Cyclic-MBP82–98 Analogues
2.1.2. Cyclization Procedure
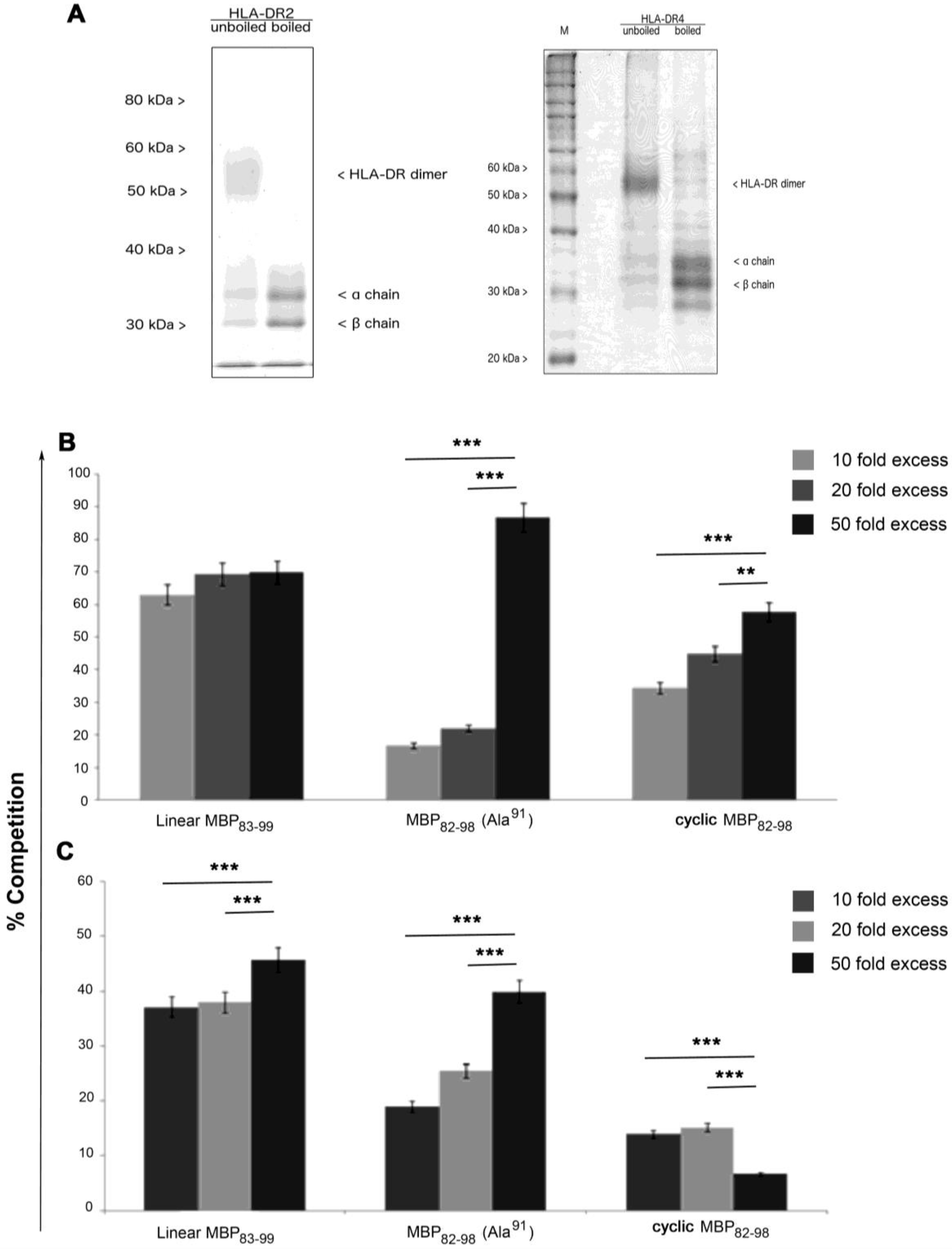
2.2. Competition Binding of Linear and Cyclic MBP82–98 to HLA-DR2 and HLA-DR4
2.3. Structure Elucidation and Conformational Studies
2.3.1. NMR Spectroscopy
2.3.2. Conformational Analysis
2.3.3. Binding Mode of Peptide-HLA-DR Complexes
3. Results and Discussion
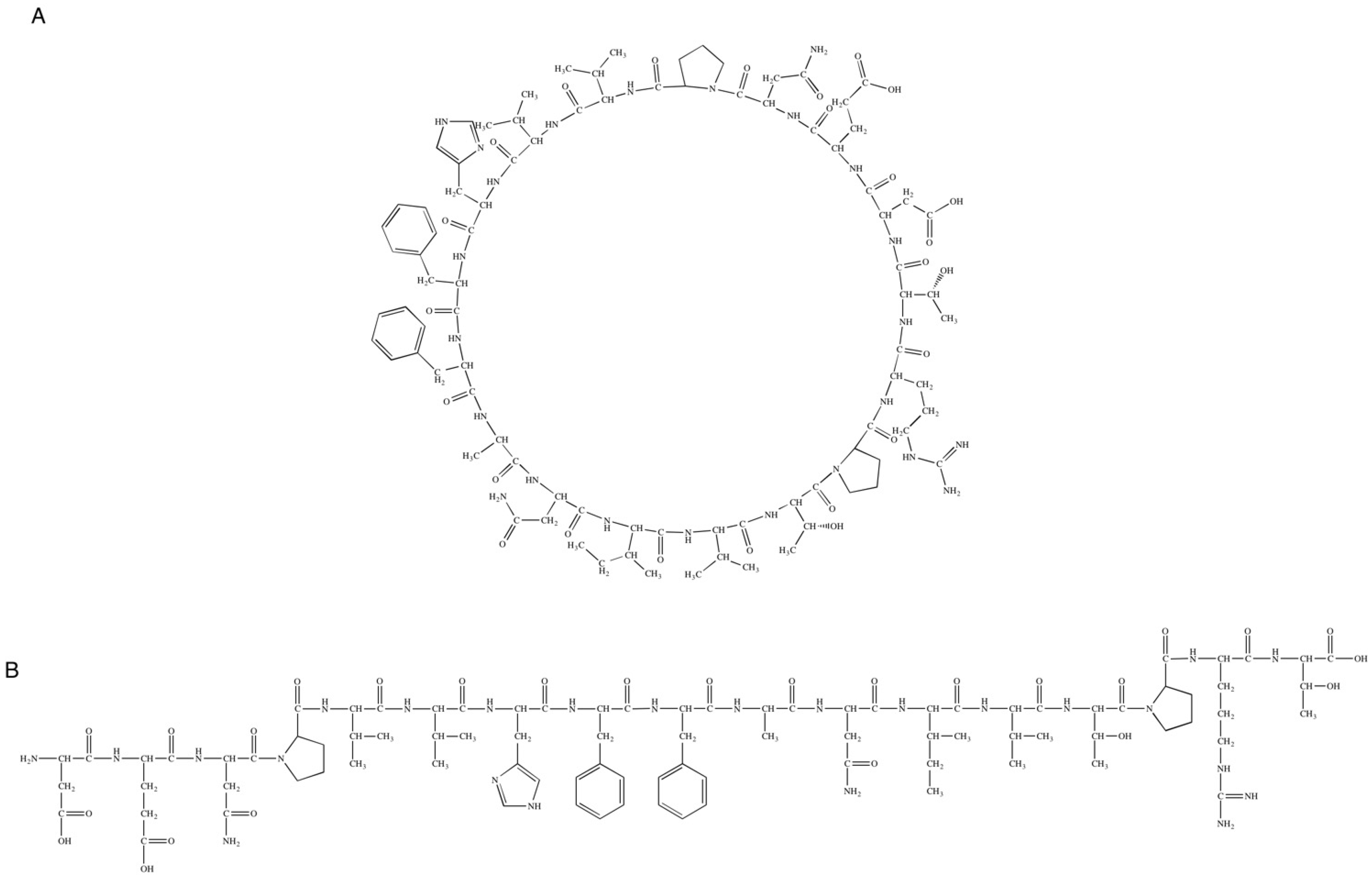
3.1. Synthesis and Purification of Peptide Analogues
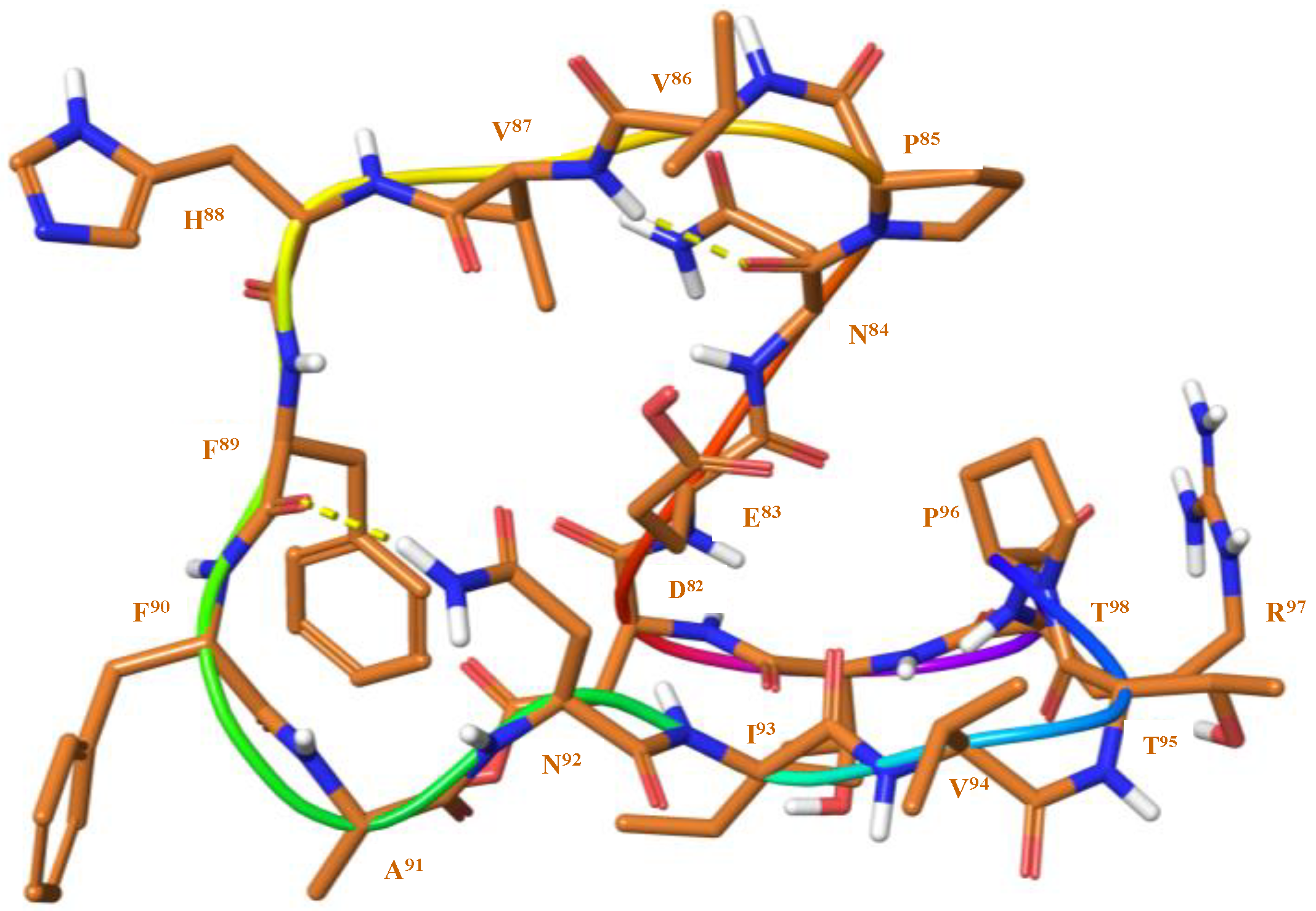
3.2. Structure Elucidation and Conformational Studies of Cyclic and Linear Peptides
3.3. Cyclic Peptides Bind to HLA-DR2 and HLA-DR4
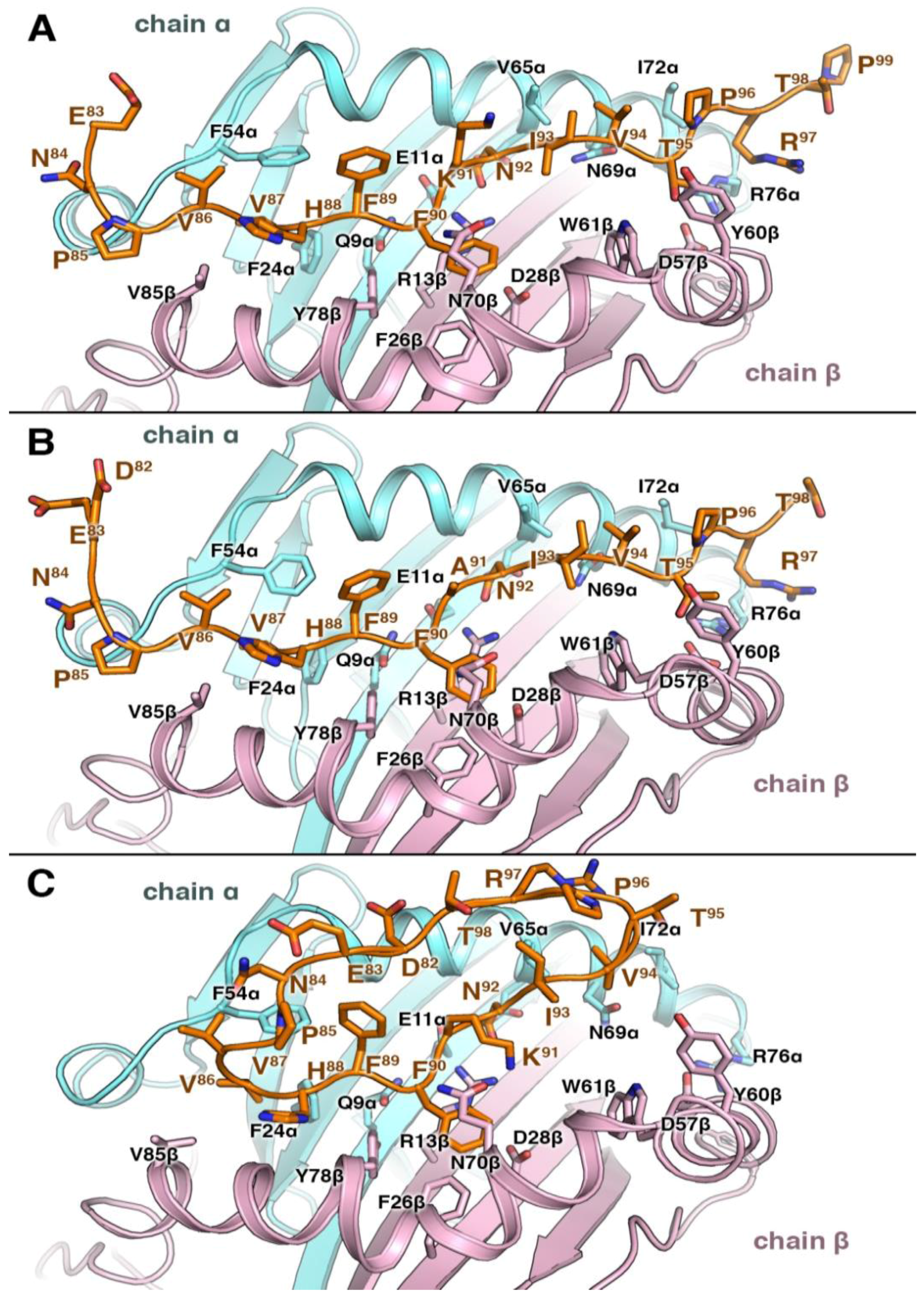
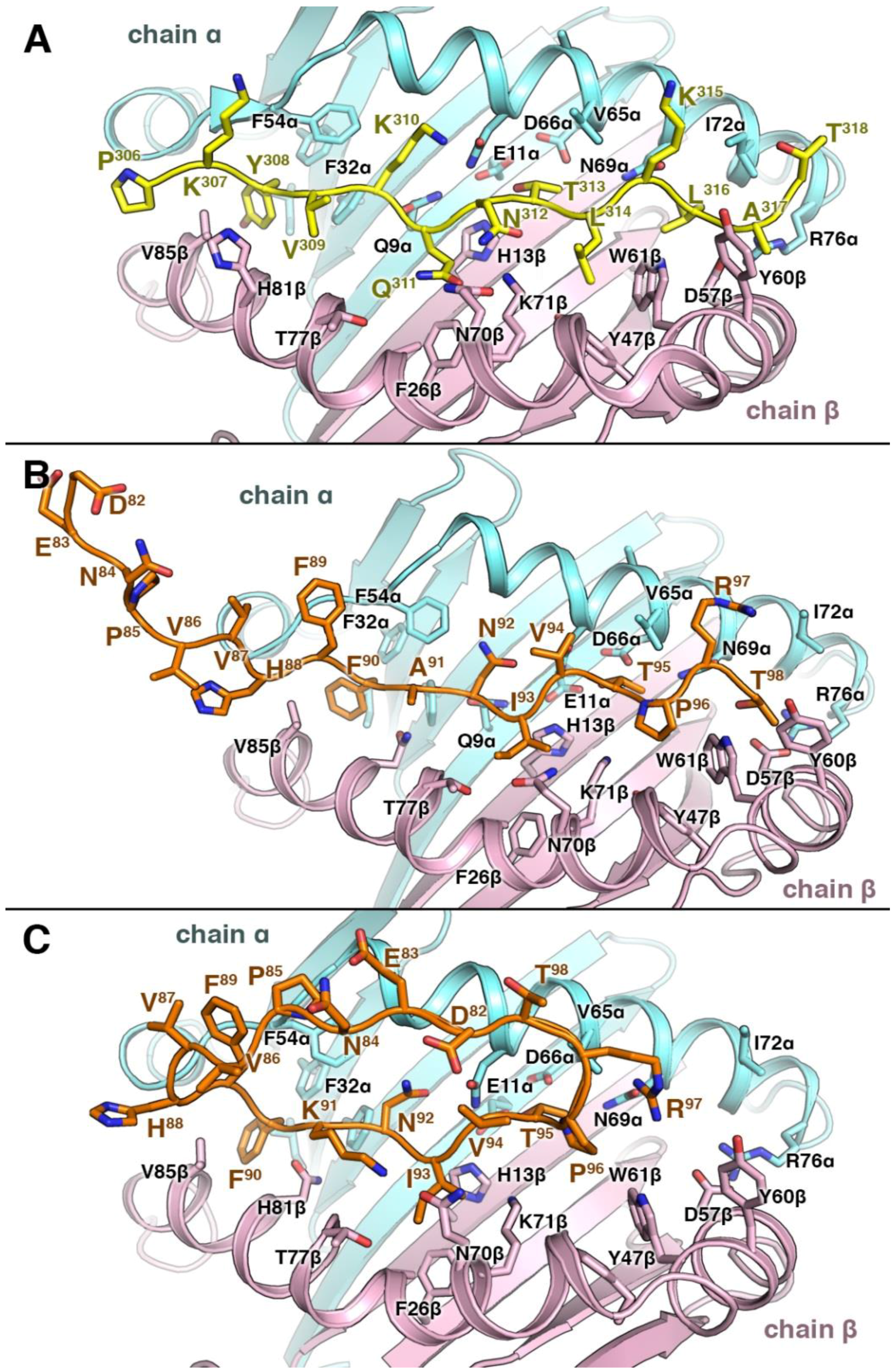
3.4. Structural Elements of Different Peptides Binding to HLA Alleles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Day, S.; Tselios, T.; Androutsou, M.E.; Tapeinou, A.; Frilligou, I.; Stojanovska, L.; Matsoukas, J.; Apostolopoulos, V. Mannosylated linear and cyclic single amino acid mutant peptides using a small 10 amino acid linker constitute promising candidates against multiple sclerosis. Front. Immunol. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Deraos, G.; Rodi, M.; Kalbacher, H.; Chatzantoni, K.; Karagiannis, F.; Synodinos, L.; Plotas, P.; Papalois, A.; Dimisianos, N.; Papathanasopoulos, P.; et al. Properties of myelin altered peptide ligand cyclo (87–99) (ala91, ala96) MBP87–99 render it a promising drug lead for immunotherapy of multiple sclerosis. Eur. J. Med. Chem. 2015, 101, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. Design of novel cyclic altered peptide ligands of myelin basic protein MBP83–99 that modulate immune responses in SJL/J mice. J. Med. Chem. 2008, 51, 3971–3978. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Deraos, G.; Tselios, T.; Matsoukas, M.T.; Friligou, I.; Matsoukas, J.; Apostolopoulos, V. Design and synthesis of a cyclic double mutant peptide (cyclo (87–99) [A91, A96] MBP87–99) induces altered responses in mice after conjugation to mannan: Implications in the immunotherapy of multiple sclerosis. J. Med. Chem. 2009, 52, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. A double mutation of MBP(83–99) peptide induces IL-4 responses and antagonizes IFN-gamma responses. J. Neuroimmunol. 2008, 200, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. Mannosylation of mutated MBP83–99 peptides diverts immune responses from Th1 to Th2. Mol. Immunol. 2008, 45, 3661–3670. [Google Scholar] [CrossRef]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Tselios, T.; Deraos, G.; Lourbopoulos, A.; Grigoriadis, N.; Matsoukas, J.; Apostolopoulos, V. Altered peptide ligands of myelin basic protein (MBP87–99) conjugated to reduced mannan modulate immune responses in mice. Immunology 2009, 128, 521–533. [Google Scholar] [CrossRef] [Green Version]
- Laimou, D.; Lazoura, E.; Troganis, A.N.; Matsoukas, M.T.; Deraos, S.N.; Katsara, M.; Matsoukas, J.; Apostolopoulos, V.; Tselios, T.V. Conformational studies of immunodominant myelin basic protein 1–11 analogues using NMR and molecular modeling. J. Comput. Aided Mol. Des. 2011, 25, 1019–1032. [Google Scholar] [CrossRef]
- Matsoukas, J.; Apostolopoulos, V.; Kalbacher, H.; Papini, A.M.; Tselios, T.; Chatzantoni, K.; Biagioli, T.; Lolli, F.; Deraos, S.; Papathanassopoulos, P.; et al. Design and synthesis of a novel potent myelin basic protein epitope 87–99 cyclic analogue: Enhanced stability and biological properties of mimics render them a potentially new class of immunomodulators. J. Med. Chem. 2005, 48, 1470–1480. [Google Scholar] [CrossRef]
- Tselios, T.; Apostolopoulos, V.; Daliani, I.; Deraos, S.; Grdadolnik, S.; Mavromoustakos, T.; Melachrinou, M.; Thymianou, S.; Probert, L.; Mouzaki, A.; et al. Antagonistic effects of human cyclic MBP(87–99) altered peptide ligands in experimental allergic encephalomyelitis and human T-cell proliferation. J. Med. Chem. 2002, 45, 275–283. [Google Scholar] [CrossRef]
- Barcellos, L.F.; Oksenberg, J.R.; Begovich, A.B.; Martin, E.R.; Schmidt, S.; Vittinghoff, E.; Goodin, D.S.; Pelletier, D.; Lincoln, R.R.; Bucher, P.; et al. HLA-DR2 dose effect on susceptibility to multiple sclerosis and influence on disease course. Am. J. Hum. Genet. 2003, 72, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Tselios, T.; Deraos, S.; Deraos, G.; Matsoukas, M.T.; Lazoura, E.; Matsoukas, J.; Apostolopoulos, V. Round and round we go: Cyclic peptides in disease. Curr. Med. Chem. 2006, 13, 2221–2232. [Google Scholar]
- Gilon, C.; Halle, D.; Chorev, M.; Selinger, Z.; Byk, G. Backbone cyclization: A new method for conferring conformational constraint on peptides. Biopolymers 1991, 31, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H. Cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther. 2012, 20, 19–26. [Google Scholar] [CrossRef]
- Molecule of the Month. Dirucotide. Drug News Perspect. 2008, 21, 572. Available online: https://journals.prous.com/journals/servlet/xmlxsl/pk_journals.xml_summary_pr?p_JournalId=3&p_RefId=4319&p_IsPs=Y (accessed on 20 August 2018).
- Warren, K.G.; Catz, I.; Ferenczi, L.Z.; Krantz, M.J. Intravenous synthetic peptide MBP8298 delayed disease progression in an HLA class II-defined cohort of patients with progressive multiple sclerosis: Results of a 24-month double-blind placebo-controlled clinical trial and 5 years of follow-up treatment. Eur. J. Neurol. 2006, 13, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Bar-Or, A.; Oger, J.; Traboulsee, A.; Patry, D.; Young, C.; Olsson, T.; Li, D.; Hartung, H.P.; Krantz, M.; et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology 2011, 77, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Loo, E.W.; Krantz, M.J.; Agrawal, B. High dose antigen treatment with a peptide epitope of myelin basic protein modulates T cells in multiple sclerosis patients. Cell. Immunol. 2012, 280, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Tseveleki, V.; Tselios, T.; Kanistras, I.; Koutsoni, O.; Karamita, M.; Vamvakas, S.S.; Apostolopoulos, V.; Dotsika, E.; Matsoukas, J.; Lassmann, H.; et al. Mannan-conjugated myelin peptides prime non-pathogenic Th1 and Th17 cells and ameliorate experimental autoimmune encephalomyelitis. Exp. Neurol. 2015, 267, 254–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsoukas, J.M.; Panagiotopoulos, D.; Keramida, M.; Mavromoustakos, T.; Yamdagni, R.; Wu, Q.; Moore, G.J.; Saifeddine, M.; Hollenberg, M.D. Synthesis and contractile activities of cyclic thrombin receptor-derived peptide analogues with a Phe-Leu-Leu-Arg motif: Importance of the Phe/Arg relative conformation and the primary amino group for activity. J. Med. Chem. 1996, 39, 3585–3591. [Google Scholar] [CrossRef]
- Max, H.; Halder, T.; Kalbus, M.; Gnau, V.; Jung, G.; Kalbacher, H. A 16mer peptide of the human autoantigen calreticulin is a most prominent HLA-DR4DW4-associated self-peptide. Human Immunol. 1994, 41, 39–45. [Google Scholar] [CrossRef]
- Max, H.; Halder, T.; Kropshofer, H.; Kalbus, M.; Muller, C.A.; Kalbacher, H. Characterization of peptides bound to extracellular and intracellular HLA-DR1 molecules. Human Immunol. 1993, 38, 193–200. [Google Scholar] [CrossRef]
- Scally, S.W.; Petersen, J.; Law, S.C.; Dudek, N.L.; Nel, H.J.; Loh, K.L.; Wijeyewickrema, L.C.; Eckle, S.B.; van Heemst, J.; Pike, R.N.; et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J. Exp. Med. 2013, 210, 2569–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsara, M.; Minigo, G.; Plebanski, M.; Apostolopoulos, V. The good, the bad and the ugly: How altered peptide ligands modulate immunity. Expert Opin. Biol. Ther. 2008, 8, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Yuriev, E.; Ramsland, P.A.; Halton, J.; Osinski, C.; Li, W.; Plebanski, M.; Paulsen, H.; McKenzie, I.F. A glycopeptide in complex with MHC class I uses the GaINAc residue as an anchor. Proc. Natl. Acad. Sci. USA 2003, 100, 15029–15034. [Google Scholar] [CrossRef] [PubMed]
- Lazoura, E.; Lodding, J.; Farrugia, W.; Ramsland, P.A.; Stevens, J.; Wilson, I.A.; Pietersz, G.A.; Apostolopoulos, V. Enhanced major histocompatibility complex class I binding and immune responses through anchor modification of the non-canonical tumour-associated mucin 1–8 peptide. Immunology 2006, 119, 306–316. [Google Scholar] [CrossRef] [PubMed]
- de Haan, E.C.; Moret, E.E.; Wagenaar-Hilbers, J.P.; Liskamp, R.M.; Wauben, M.H. Possibilities and limitations in the rational design of modified peptides for T cell mediated immunotherapy. Mol. Immunol. 2005, 42, 365–373. [Google Scholar] [CrossRef]
- Karin, N.; Mitchell, D.J.; Brocke, S.; Ling, N.; Steinman, L. Reversal of experimental autoimmune encephalomyelitis by a soluble peptide variant of a myelin basic protein epitope: T cell receptor antagonism and reduction of interferon gamma and tumor necrosis factor alpha production. J. Exp. Med. 1994, 180, 2227–2237. [Google Scholar] [CrossRef] [Green Version]
- Lourbopoulos, A.; Deraos, G.; Matsoukas, M.T.; Touloumi, O.; Giannakopoulou, A.; Kalbacher, H.; Grigoriadis, N.; Apostolopoulos, V.; Matsoukas, J. Cyclic MOG35–55 ameliorates clinical and neuropathological features of experimental autoimmune encephalomyelitis. Bioorg. Med. Chem. 2017, 25, 4163–4174. [Google Scholar] [CrossRef]
- Lourbopoulos, A.; Matsoukas, M.T.; Katsara, M.; Deraos, G.; Giannakopoulou, A.; Lagoudaki, R.; Grigoriadis, N.; Matsoukas, J.; Apostolopoulos, V. Cyclization of PLP139–151 peptide reduces its encephalitogenic potential in experimental autoimmune encephalomyelitis. Bioorg. Med. Chem. 2018, 26, 2221–2228. [Google Scholar] [CrossRef]
- Gerstner, C.; Dubnovitsky, A.; Sandin, C.; Kozhukh, G.; Uchtenhagen, H.; James, E.A.; Ronnelid, J.; Ytterberg, A.J.; Pieper, J.; Reed, E.; et al. Functional and structural characterization of a novel HLA-DRB1*04:01-restricted α-enolase T cell epitope in rheumatoid arthritis. Front. Immunology 2016, 7, 494. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Pyrdol, J.; Gauthier, L.; Wiley, D.C.; Wucherpfennig, K.W. Crystal structure of HLA-DR2 (DRA* 0101, DRB1* 1501) complexed with a peptide from human myelin basic protein. J. Exp. Med. 1998, 188, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Deraos, G.; Chatzantoni, K.; Matsoukas, M.T.; Tselios, T.; Deraos, S.; Katsara, M.; Papathanasopoulos, P.; Vynios, D.; Apostolopoulos, V.; Mouzaki, A.; et al. Citrullination of linear and cyclic altered peptide ligands from myelin basic protein (MBP(87–99)) epitope elicits a Th1 polarized response by T cells isolated from multiple sclerosis patients: Implications in triggering disease. J. Med. Chem. 2008, 51, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Hennecke, J.; Wiley, D.C. Structure of a complex of the human α/β T cell receptor (TCR) HA1.7, influenza hemagglutinin peptide, and major histocompatibility complex class II molecule, HLA-DR4 (DRA0101 and DRB10401) insight into TCR cross-restriction and alloreactivity. J. Exp. Med. 2002, 195, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Martin, R.; Mariuzza, R.A. Structural basis for the binding of an immunodominant peptide from myelin basic protein in different registers by two HLA-DR2 proteins. J. Mol. Biol. 2000, 304, 177–188. [Google Scholar] [CrossRef] [PubMed]


| Peptides | Peptide Analogue of MBP82–98 | Sequence |
|---|---|---|
| P1 | MBP83–99 | ENPVVHFFK91NIVTPRTP |
| P2 | MBP82–98 (Ala91) | DENPVVHFFA91NIVTPRT |
| P3 | cyclic MBP82–98 | cycloDENPVVHFFK91NIVTPRT |
| P4 | cyclic MBP82–98 (Ala91) | cycloDENPVVHFFA91NIVTPRT |
| Crucial NOEs | Distance (Å) | +10% (Å) | −10% (Å) | Conformer 237.40 kJ mol−1 | |
|---|---|---|---|---|---|
| D82 β′H | E83 β/β′H | 2.22 | 2.44 | 1.99 | 2.62 |
| D82 β′H | T98 γH CH3 | 2.92 | 3.21 | 2.62 | 3.21 |
| T95 γH CH3 | P96 δH | 2.91 | 3.20 | 2.61 | 3.28 |
| N84 NH | V87 NH | 3.07 | 3.37 | 2.76 | 3.38 |
| H88 2,4Ar. | F89 NH | 3.08 | 3.39 | 2.78 | 2.84 |
| Crucial NOEs | Distance (Å) | +10% (Å) | −10% (Å) | Conformer 1 248.52 kJ mol−1 | Conformer 2 192.93 kJ mol−1 | |
|---|---|---|---|---|---|---|
| V86 NH | V87 NH | 2.41 | 2.65 | 2.17 | 2.35 | 2.60 |
| I93 NH | V94 NH | 2.38 | 2.62 | 2.14 | 2.71 | 2.40 |
| R97 NH | T98 NH | 2.81 | 3.09 | 2.53 | 2.45 | 2.52 |
| N92 NH | I93 NH | 3.31 | 3.64 | 2.98 | 2.97 | 2.94 |
| F90 NH | A91 NH | 3.24 | 3.56 | 2.91 | 3.24 | 3.49 |
| E83 NH | N84 NH | 3.15 | 3.47 | 2.84 | 2.65 | 2.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deraos, G.; Kritsi, E.; Matsoukas, M.-T.; Christopoulou, K.; Kalbacher, H.; Zoumpoulakis, P.; Apostolopoulos, V.; Matsoukas, J. Design of Linear and Cyclic Mutant Analogues of Dirucotide Peptide (MBP82–98) against Multiple Sclerosis: Conformational and Binding Studies to MHC Class II. Brain Sci. 2018, 8, 213. https://doi.org/10.3390/brainsci8120213
Deraos G, Kritsi E, Matsoukas M-T, Christopoulou K, Kalbacher H, Zoumpoulakis P, Apostolopoulos V, Matsoukas J. Design of Linear and Cyclic Mutant Analogues of Dirucotide Peptide (MBP82–98) against Multiple Sclerosis: Conformational and Binding Studies to MHC Class II. Brain Sciences. 2018; 8(12):213. https://doi.org/10.3390/brainsci8120213
Chicago/Turabian StyleDeraos, George, Eftichia Kritsi, Minos-Timotheos Matsoukas, Konstantina Christopoulou, Hubert Kalbacher, Panagiotis Zoumpoulakis, Vasso Apostolopoulos, and John Matsoukas. 2018. "Design of Linear and Cyclic Mutant Analogues of Dirucotide Peptide (MBP82–98) against Multiple Sclerosis: Conformational and Binding Studies to MHC Class II" Brain Sciences 8, no. 12: 213. https://doi.org/10.3390/brainsci8120213
APA StyleDeraos, G., Kritsi, E., Matsoukas, M.-T., Christopoulou, K., Kalbacher, H., Zoumpoulakis, P., Apostolopoulos, V., & Matsoukas, J. (2018). Design of Linear and Cyclic Mutant Analogues of Dirucotide Peptide (MBP82–98) against Multiple Sclerosis: Conformational and Binding Studies to MHC Class II. Brain Sciences, 8(12), 213. https://doi.org/10.3390/brainsci8120213








