Speed-Interactive Pedaling Training Using Smartphone Virtual Reality Application for Stroke Patients: Single-Blinded, Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Size of the Sample
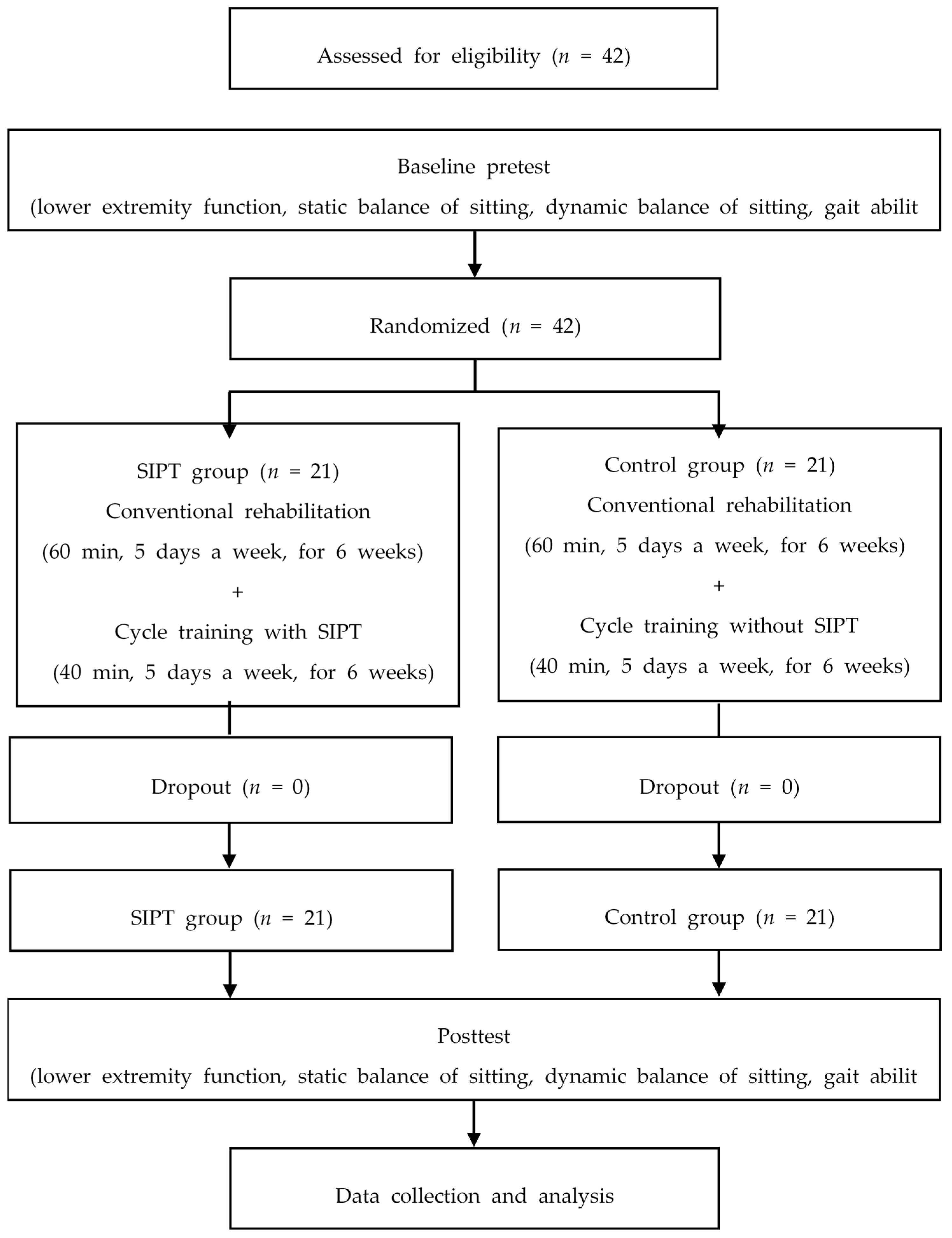
2.3. Procedure
2.4. Speed-Interactive Pedaling Training (SIPT) Equipment and Mirroring Device
2.5. Motion Tracking and SIPT Equipment
2.6. SIPT and Pedaling Training with a Stationary Bike
2.7. Conventional Rehabilitation
2.8. Outcome Measurements
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S. Executive summary: Heart disease and stroke statistics—2014 update: A report from the american heart association. Circulation 2014, 129, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Arene, N.; Hidler, J. Understanding motor impairment in the paretic lower limb after a stroke: A review of the literature. Top. Stroke Rehabil. 2009, 16, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.Y.; Mak, A.F. Development of computer-based environment for simulating the voluntary upper-limb movements of persons with disability. Med. Biol. Eng. Comput. 2001, 39, 414–421. [Google Scholar] [CrossRef]
- Dickstein, R. Rehabilitation of gait speed after stroke: A critical review of intervention approaches. Neurorehabil. Neural Repair 2008, 22, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Hsueh, Y.H.; Yeh, C.Y.; Lo, H.C.; Lan, Y.T. A virtual reality-cycling training system for lower limb balance improvement. BioMed Res. Int. 2016, 2016, 9276508. [Google Scholar] [CrossRef]
- Barbosa, D.; Santos, C.P.; Martins, M. The application of cycling and cycling combined with feedback in the rehabilitation of stroke patients: A review. J. Stroke Cereb. Dis. 2015, 24, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Lee, C.L.; Lin, R.; Hsu, M.J.; Chen, C.H.; Lin, J.H.; Lo, S.K. Effect of biofeedback cycling training on functional recovery and walking ability of lower extremity in patients with stroke. Kaohsiung J. Med. Sci. 2014, 30, 35–42. [Google Scholar] [CrossRef]
- Kautz, S.A.; Brown, D.A. Relationships between timing of muscle excitation and impaired motor performance during cyclical lower extremity movement in post-stroke hemiplegia. Brain 1998, 121, 515–526. [Google Scholar] [CrossRef]
- Raasch, C.C.; Zajac, F.E. Locomotor strategy for pedaling: Muscle groups and biomechanical functions. J. Neurophysiol. 1999, 82, 515–525. [Google Scholar] [CrossRef]
- Lin, S.I.; Lo, C.C.; Lin, P.Y.; Chen, J.J. Biomechanical assessments of the effect of visual feedback on cycling for patients with stroke. J. Electromyogr. Kinesiol. 2012, 22, 582–588. [Google Scholar] [CrossRef]
- Brown, D.A.; DeBacher, G.A. Bicycle ergometer and electromyographic feedback for treatment of muscle imbalance in patients with spastic hemiparesis. Suggestion from the field. Phys. Ther. 1987, 67, 1715–1719. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Hui-Chan, C.W.; Li, L.S. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: A randomized placebo-controlled trial. Stroke 2005, 36, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Sheng, B.; Zhang, Y.; Meng, W.; Deng, C.; Xie, S. Bilateral robots for upper-limb stroke rehabilitation: State of the art and future prospects. Med. Eng. Phys. 2016, 38, 587–606. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Song, C. Speed-interactive treadmill training using smartphone-based motion tracking technology improves gait in stroke patients. J. Mot. Behav. 2017, 49, 675–685. [Google Scholar] [CrossRef]
- Ustinova, K.I.; Leonard, W.A.; Cassavaugh, N.D.; Ingersoll, C.D. Development of a 3d immersive videogame to improve arm-postural coordination in patients with TBI. J. Neuroeng. Rehabil. 2011, 8, 61. [Google Scholar] [CrossRef]
- Cortes, C.; Ardanza, A.; Molina-Rueda, F.; Cuesta-Gomez, A.; Unzueta, L.; Epelde, G.; Ruiz, O.E.; De Mauro, A.; Florez, J. Upper limb posture estimation in robotic and virtual reality-based rehabilitation. BioMed Res. Int. 2014, 2014, 821908. [Google Scholar] [CrossRef]
- Saposnik, G.; Teasell, R.; Mamdani, M.; Hall, J.; McIlroy, W.; Cheung, D.; Thorpe, K.E.; Cohen, L.G.; Bayley, M. Stroke Outcome Research Canada (SORCan) Working Group. Effectiveness of virtual reality using WII gaming technology in stroke rehabilitation: A pilot randomized clinical trial and proof of principle. Stroke 2010, 41, 1477–1484. [Google Scholar] [CrossRef]
- Cho, K.H.; Lee, K.J.; Song, C.H. Virtual-reality balance training with a video-game system improves dynamic balance in chronic stroke patients. Tohoku J. Exp. Med. 2012, 228, 69–74. [Google Scholar] [CrossRef]
- Lee, D.; Lee, M.; Lee, K.; Song, C. Asymmetric training using virtual reality reflection equipment and the enhancement of upper limb function in stroke patients: A randomized controlled trial. J. Stroke Cereb. Dis. 2014, 23, 1319–1326. [Google Scholar] [CrossRef]
- Weech, S.; Kenny, S.; Barnett-Cowan, M. Presence and cybersickness in virtual reality are negatively related: A review. Front. Psychol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Kim, Y.; Chung, Y.; Hwang, S. Effects of treadmill training with optic flow on balance and gait in individuals following stroke: Randomized controlled trials. Clin. Rehabil. 2012, 26, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Almeida, Q.J.; Bhatt, H. A manipulation of visual feedback during gait training in parkinson’s disease. Parkinson’s Dis. 2012, 2012, 508720. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Propst, M.; Nelson, S.G. Reliability of the fugl-meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys. Ther. 1983, 63, 1606–1610. [Google Scholar] [CrossRef]
- Era, P.; Sainio, P.; Koskinen, S.; Haavisto, P.; Vaara, M.; Aromaa, A. Postural balance in a random sample of 7979 subjects aged 30 years and over. Gerontology 2006, 52, 204–213. [Google Scholar] [CrossRef]
- Katz-Leurer, M.; Fisher, I.; Neeb, M.; Schwartz, I.; Carmeli, E. Reliability and validity of the modified functional reach test at the sub-acute stage post-stroke. Disabil. Rehabil. 2009, 31, 243–248. [Google Scholar] [CrossRef]
- Verheyden, G.; Nieuwboer, A.; Mertin, J.; Preger, R.; Kiekens, C.; De Weerdt, W. The trunk impairment scale: A new tool to measure motor impairment of the trunk after stroke. Clin. Rehabil. 2004, 18, 326–334. [Google Scholar] [CrossRef]
- Shen, C.; Liu, F.; Yao, L.; Li, Z.; Qiu, L.; Fang, S. Effects of motomed movement therapy on the mobility and activities of daily living of stroke patients with hemiplegia: A systematic review and meta-analysis. Clin. Rehabil. 2018, 32, 1569–1580. [Google Scholar] [CrossRef]
- Bernhardt, J.; Langhorne, P.; Lindley, R.I.; Thrift, A.G.; Ellery, F.; Collier, J.; Churilov, L.; Moodie, M.; Dewey, H.; Donnan, G. Efficacy and safety of very early mobilisation within 24 h of stroke onset (avert): A randomised controlled trial. Lancet 2015, 386, 46–55. [Google Scholar]
- Rao, N.; Zielke, D.; Keller, S.; Burns, M.; Sharma, A.; Krieger, R.; Aruin, A.S. Pregait balance rehabilitation in acute stroke patients. Int. J. Rehabil. Res. 2013, 36, 112–117. [Google Scholar] [CrossRef]

| SIPT Group (n = 21) | Control Group (n = 21) | χ2/t | p | |
|---|---|---|---|---|
| Age (year) | 61.67 ± 8.42 | 64.24 ± 10.83 | 0.859 | 0.395 |
| Height (cm) | 165.62 ± 7.05 | 162.29 ± 9.32 | 1.307 | 0.199 |
| Weight (kg) | 62.20 ± 7.04 | 61.35 ± 9.15 | 0.334 | 0.740 |
| BMI (point) | 22.67 ± 2.19 | 23.20 ± 2.05 | 0.817 | 0.419 |
| Duration of stroke (month) | 14.81 ± 7.30 | 16.48 ± 7.13 | 0.748 | 0.459 |
| MMSE | 25.81 ± 1.29 | 25.76 ± 0.94 | 0.137 | 0.892 |
| Gender (male/female) | 14/7 | 13/8 | 0.747 | 0.104 |
| Paretic side (right/left) | 12/9 | 10/11 | 0.537 | 0.382 |
| Stroke type (infarction/hemorrhage) | 15/6 | 13/8 | 0.513 | 0.429 |
| Variables | SIPT Group (n = 21) | Control Group (n = 21) | Significance of Change Scores | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Post | Change Score | Baseline | Post | Change Score | t | |
| Lower extremity function | |||||||
| FMA-LE (point) | 16.91 ± 3.62 | 19.49 ± 3.56 | 2.58 ± 0.63 * | 17.15 ± 3.13 | 18.59 ± 2.72 | 1.44 ± 2.41 * | 2.347 † |
| Static sitting balance ability | |||||||
| EO-MLS (mm/s) | 3.95 ± 1.27 | 3.00 ± 0.82 | −0.95 ± 0.88 * | 3.75 ± 1.21 | 3.34 ± 1.03 | −0.41 ± 0.92 * | 2.461 † |
| EO-APS (mm/s) | 5.85 ± 1.41 | 4.60 ± 1.38 | −1.25 ± 0.81 * | 5.89 ± 1.18 | 5.20 ± 1.30 | −0.69 ± 0.64 * | 2.282 † |
| EO-VM (mm/s2) | 5.06 ± 2.18 | 3.71 ± 1.68 | −1.35 ± 0.97 * | 4.74 ± 2.04 | 4.12 ± 1.93 | −0.62 ± 1.07 * | 2.313 † |
| EC-MLS (mm/s) | 3.95 ± 1.27 | 2.85 ± 0.66 | −1.10 ± 0.98 * | 3.75 ± 1.21 | 3.24 ± 0.93 | −0.50 ± 0.84 * | 2.098 † |
| EC-APS (mm/s) | 5.85 ± 1.41 | 4.73 ± 1.43 | −1.12 ± 0.71 * | 5.89 ± 1.18 | 5.20 ± 1.30 | −0.69 ± 0.64 * | 2.059 † |
| EC-VM (mm/s2) | 4.18 ± 1.30 | 2.79 ± 1.32 | −1.39 ± 0.76 * | 4.03 ± 1.13 | 3.27 ± 1.29 | −0.76 ± 1.06 * | 2.227 † |
| Dynamic sitting balance ability | |||||||
| mFRT-forward (mm) | 302.27 ± 113.40 | 328.41 ± 108.52 | 26.14 ± 22.12 * | 274.97 ± 122.87 | 279.15 ± 126.13 | 4.18 ± 6.11 * | 4.384 † |
| mFRT-non-affected (mm) | 175.23 ± 48.60 | 197.89 ± 54.79 | 22.66 ± 20.57 * | 158.75 ± 61.74 | 161.13 ± 63.61 | 2.38 ± 5.07 * | 4.388 † |
| mFRT-affected (mm) | 88.72 ± 24.24 | 108.07 ± 33.26 | 19.35 ± 14.96 * | 84.31 ± 37.48 | 85.62 ± 38.88 | 1.68 ± 3.07 * | 5.302 † |
| TIS (score) | 12.33 ± 1.59 | 14.38 ± 2.09 | 2.05 ± 1.20 * | 12.24 ± 1.89 | 13.14 ± 0.48 | 0.90 ± 1.70 * | 2.515 † |
| Gait ability | |||||||
| Temporal gait parameter | |||||||
| Velocity (cm/s) | 0.46 ± 0.15 | 0.56 ± 0.18 | 0.10 ± 0.04 * | 0.41 ± 0.22 | 0.42 ± 0.22 | 0.01 ± 0.03 | 8.135 † |
| Cadence (step/min) | 76.26 ± 14.43 | 83.34 ± 16.11 | 7.08 ± 3.38 * | 74.56 ± 15.77 | 75.40 ± 18.05 | 0.84 ± 4.09 | 5.389 † |
| Stride time (sec) | 1.63 ± 0.30 | 1.49 ± 0.28 | −0.13 ± 0.07 * | 1.67 ± 0.29 | 1.67 ± 0.33 | 0.00 ± 0.09 | 5.427 † |
| Step time (sec) | 0.81 ± 0.15 | 0.74 ± 0.13 | −0.07 ± 0.04 * | 0.83 ± 0.15 | 0.83 ± 0.17 | 0.00 ± 0.04 | 5.247 † |
| Spatial parameter | |||||||
| Stride length (cm) | 71.66 ± 18.57 | 80.44 ± 20.00 | 8.77 ± 3.75 * | 63.59 ± 21.67 | 65.63 ± 20.97 | 2.04 ± 5.81 | 4.461 † |
| Step length (cm) | 35.87 ± 9.22 | 40.24 ± 9.96 | 4.37 ± 1.88 * | 31.61 ± 10.40 | 32.82 ± 10.48 | 1.20 ± 3.09 | 4.016 † |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K. Speed-Interactive Pedaling Training Using Smartphone Virtual Reality Application for Stroke Patients: Single-Blinded, Randomized Clinical Trial. Brain Sci. 2019, 9, 295. https://doi.org/10.3390/brainsci9110295
Lee K. Speed-Interactive Pedaling Training Using Smartphone Virtual Reality Application for Stroke Patients: Single-Blinded, Randomized Clinical Trial. Brain Sciences. 2019; 9(11):295. https://doi.org/10.3390/brainsci9110295
Chicago/Turabian StyleLee, Kyeongjin. 2019. "Speed-Interactive Pedaling Training Using Smartphone Virtual Reality Application for Stroke Patients: Single-Blinded, Randomized Clinical Trial" Brain Sciences 9, no. 11: 295. https://doi.org/10.3390/brainsci9110295
APA StyleLee, K. (2019). Speed-Interactive Pedaling Training Using Smartphone Virtual Reality Application for Stroke Patients: Single-Blinded, Randomized Clinical Trial. Brain Sciences, 9(11), 295. https://doi.org/10.3390/brainsci9110295





