Vestibular Compensation after Vestibular Dysfunction Induced by Arsanilic Acid in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgical Procedures
2.3. Behavioral Observations
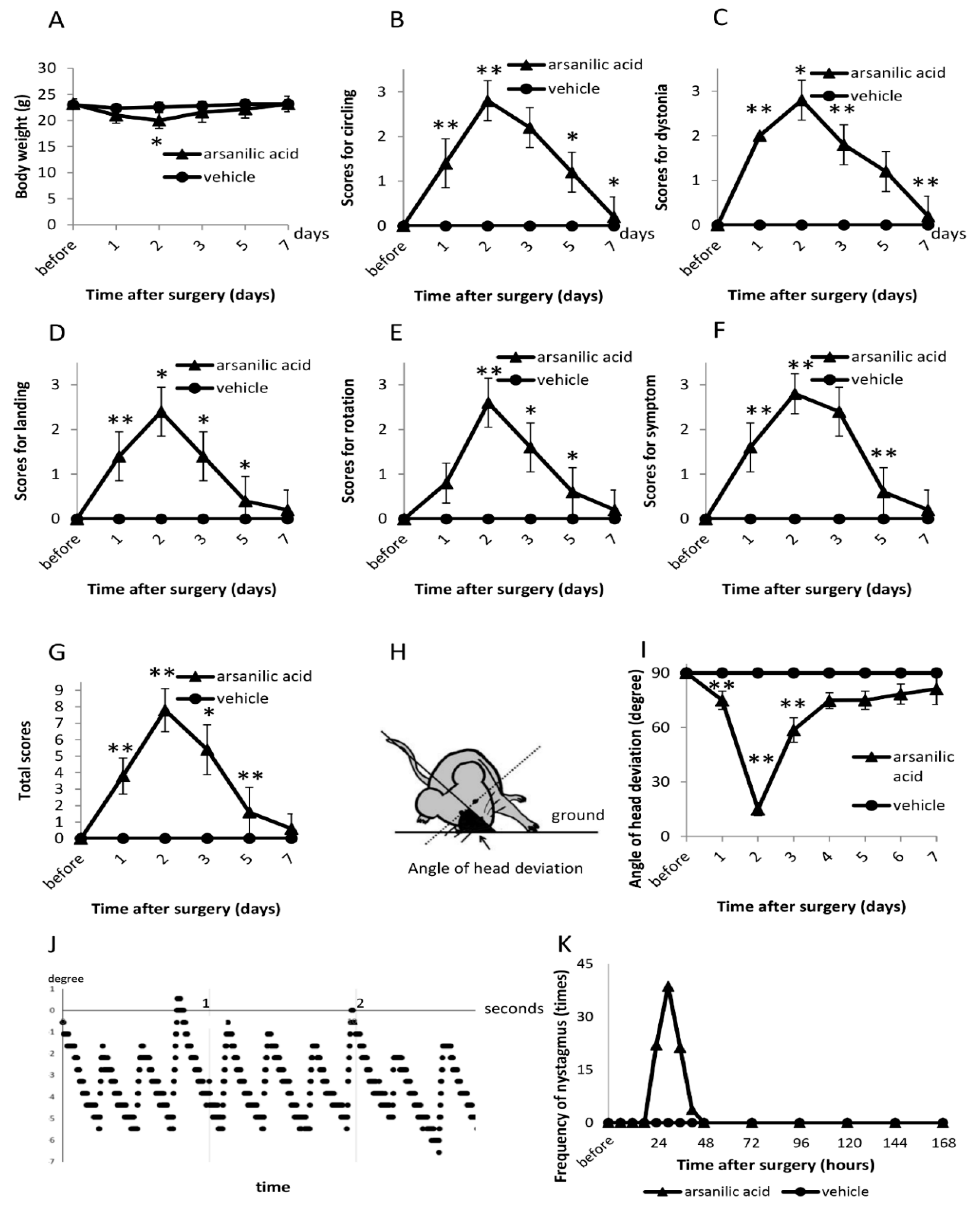
2.4. Vestibular Signs (Open Field)
2.5. Tail-Hanging and Landing Test
2.6. Head Deviation
2.7. Nystagmus
2.8. Histology
2.8.1. Temporal Bone Histology
2.8.2. Silver Staining
2.8.3. c-Fos/Arc/Zif268 Immunohistochemistry
2.9. Cell Counting in the Spinal Vestibular Nucleus (SpVN), Medial Vestibular Nucleus (MVN), and Prepositus Hypoglossal Nucleus (PrHN)
2.10. Statistical Analysis
3. Results
3.1. Behavioral Observations
3.1.1. Vestibular Signs (Open Field)
3.1.2. Tail-Hanging and Landing Test
3.1.3. Head Deviation
3.1.4. Nystagmus
3.2. Temporal Bone Histology
c-Fos/Arc/Zif268 Expression in the Vestibular Nuclei
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UL | unilateral labyrinthectomy |
| IR | immunoreactive |
| IEGs | immediate early genes |
| SpVN | spinal vestibular nucleus |
| MVN | medial vestibular nucleus |
| PrHN | prepositus hypoglossal nucleus |
| NMDA | n-methyl-d-aspartate |
| VOR | vestibule-ocular reflex |
References
- Llinas, R. Vestibular compensation: A distributed property of the central nervous system. Integr. Nerv. Syst. 1979, 145–166. [Google Scholar]
- Precht, W.; Dieringer, N. Neuronal events paralleling functional recovery (compensation) following peripheral vestibular lesions. Rev. Oculomot. Res. 1985, 1, 251–268. [Google Scholar]
- Lacour, M.; Dutheil, S.; Tighilet, B.; Lopez, C.; Borel, L. Tell me your vestibular deficit, and I’ll tell you how you’ll compensate. Ann. N. Y. Acad. Sci. 2009, 1164, 268–278. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Abe, C.; Morita, H. Comparison among ultrasonic, electrical apparatus, and toxic chemicals for vestibular lesion in mice. J. Neurosci. Methods 2018, 295, 58–67. [Google Scholar] [CrossRef]
- Cassel, R.; Bordiga, P.; Pericat, D.; Hautefort, C.; Tighilet, B.; Chabbert, C. New mouse model for inducing and evaluating unilateral vestibular deafferentation syndrome. J. Neurosci. Methods 2018, 293, 128–135. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.H.; Jin, Y.Z.; Kry, D.; Park, B.R. Temporal changes of cFos-like protein expression in medial vestibular nuclei following arsanilate-induced unilateral labyrinthectomy in rats. Neurosci. Lett. 2002, 319, 9–12. [Google Scholar] [CrossRef]
- Imai, T.; Takimoto, Y.; Takeda, N.; Uno, A.; Inohara, H.; Shimada, S. High-Speed Video-Oculography for Measuring Three-Dimensional Rotation Vectors of Eye Movements in Mice. PLoS ONE 2016, 11, e0152307. [Google Scholar] [CrossRef]
- Saxon, D.W.; Beitz, A.J. Cerebellar injury induces NOS in Purkinje cells and cerebellar afferent neurons. NeuroReport 1994, 5, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Gunther, L.; Beck, R.; Xiong, G.; Potschka, H.; Jahn, K.; Bartenstein, P.; Brandt, T.; Dutia, M.; Dieterich, M.; Strupp, M.; et al. N-acetyl-L-leucine accelerates vestibular compensation after unilateral labyrinthectomy by action in the cerebellum and thalamus. PLoS ONE 2015, 10, e0120891. [Google Scholar] [CrossRef] [PubMed]
- De Olmos, J.S.; Beltramino, C.A.; De Lorenzo, S.D.O. Use of an amino-cupric-silver technique for the detection of early and semiacute neuronal degeneration caused by neurotoxicants, hypoxia, and physical trauma. Neurotoxicol. Teratol. 1994, 16, 545–561. [Google Scholar] [CrossRef]
- Tatsumi, K.; Takebayashi, H.; Manabe, T.; Tanaka, K.F.; Makinodan, M.; Yamauchi, T.; Makinodan, E.; Matsuyoshi, H.; Okuda, H.; Ikenaka, K.; et al. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J. Neurosci. Res. 2008, 86, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Gundersen, H.J. Estimators of the number of objects per area unbiased by edge effects. Microsc. Acta 1978, 81, 107–117. [Google Scholar] [PubMed]
- Kitahara, T.; Saika, T.; Takeda, N.; Kiyama, H.; Kubo, T. Changes in Fos and Jun expression in the rat brainstem in the process of vestibular compensation. Acta Oto-Laryngol. 1995, 115 Pt 520, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Darlington, C.L.; Lawlor, P.; Smith, P.F.; Dragunow, M. Temporal relationship between the expression of fos, jun and krox-24 in the guinea pig vestibular nuclei during the development of vestibular compensation for unilateral vestibular deafferentation. Brain Res. 1996, 735, 173–176. [Google Scholar] [CrossRef]
- Sato, T.; Tokuyama, W.; Miyashita, Y.; Okuno, H. Temporal and spatial dissociation of expression patterns between Zif268 and c-Fos in rat inferior olive during vestibular compensation. NeuroReport 1997, 8, 1891–1895. [Google Scholar] [CrossRef]
- Takumida, M.; Anniko, M. Localization of endotoxin in the inner ear following inoculation into the middle ear. Acta Oto-Laryngol. 2004, 124, 772–777. [Google Scholar] [CrossRef]
- Vignaux, G.; Chabbert, C.; Gaboyard-Niay, S.; Travo, C.; Machado, M.L.; Denise, P.; Comoz, F.; Hitier, M.; Landemore, G.; Philoxène, B.; et al. Evaluation of the chemical model of vestibular lesions induced by arsanilate in rats. Toxicol. Appl. Pharmacol. 2012, 258, 61–71. [Google Scholar] [CrossRef]
- Cirelli, C.; Pompeiano, M.; D’Ascanio, P.; Arrighi, P.; Pompeiano, O. c-fos Expression in the rat brain after unilateral labyrinthectomy and its relation to the uncompensated and compensated stages. Neuroscience 1996, 70, 515–546. [Google Scholar] [CrossRef]
- Kaufman, G.D.; Anderson, J.H.; Beitz, A.J. Brainstem Fos expression following acute unilateral labyrinthectomy in the rat. NeuroReport 1992, 3, 829–832. [Google Scholar] [CrossRef]
- Kitahara, T.; Takeda, N.; Kiyama, H.; Kubo, T. Molecular mechanisms of vestibular compensation in the central vestibular system-review. Acta Oto-Laryngol. 1998, 539, 19–27. [Google Scholar] [CrossRef]
- Fosnaugh, J.S.; Bhat, R.V.; Yamagata, K.; Worley, P.F.; Baraban, J.M. Activation of arc, a putative “effector” immediate early gene, by cocaine in rat brain. J. Neurochem. 1995, 64, 2377–2380. [Google Scholar] [CrossRef] [PubMed]
- Veyrac, A.; Besnard, A.; Caboche, J.; Davis, S.; Laroche, S. The transcription factor Zif268/Egr1, brain plasticity, and memory. Prog. Mol. Biol. Transl. Sci. 2014, 122, 89–129. [Google Scholar] [PubMed]
- Link, W.; Konietzko, U.; Kauselmann, G.; Krug, M.; Schwanke, B.; Frey, U.; Kuhl, D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc. Natl. Acad. Sci. USA 1995, 92, 5734–5738. [Google Scholar] [CrossRef] [PubMed]
- Lyford, G.L.; Yamagata, K.; Kaufmann, W.E.; Barnes, C.A.; Sanders, L.K.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Lanahan, A.A.; Worley, P.F. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 1995, 14, 433–445. [Google Scholar] [CrossRef]
- Kitahara, T.; Takeda, N.; Saika, T.; Kubo, T.; Kiyama, H. Effects of MK801 on Fos expression in the rat brainstem after unilateral labyrinthectomy. Brain Res. 1995, 700, 182–190. [Google Scholar] [CrossRef]
- Kitahara, T.; Takeda, N.; Saika, T.; Kubo, T.; Kiyama, H. Role of the flocculus in the development of vestibular compensation: Immunohistochemical studies with retrograde tracing and flocculectomy using Fos expression as a marker in the rat brainstem. Neuroscience 1997, 76, 571–580. [Google Scholar] [CrossRef]
- Tighilet, B.; Mourre, C.; Lacour, M. Plasticity of the histamine H3 receptors after acute vestibular lesion in the adult cat. Front. Integr. Neurosci. 2014, 7, 87. [Google Scholar] [CrossRef]
- Murdin, L.; Schilder, A.G. Epidemiology of balance symptoms and disorders in the community: A systematic review. Otol. Neurotol. 2015, 36, 387–392. [Google Scholar] [CrossRef]
- Lacour, M. Restoration of vestibular function: Basic aspects and practical advances for rehabilitation. Curr. Med. Res. Opin. 2006, 22, 1651–1659. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Halmagyi, G.M. Vestibular compensation. Adv. Oto-Rhino-Laryngol. 1999, 55, 82–110. [Google Scholar]
- Tjernstrom, F.; Zur, O.; Jahn, K. Current concepts and future approaches to vestibular rehabilitation. J. Neurol. 2016, 263 (Suppl. 1), S65–S70. [Google Scholar] [CrossRef]
- Lehnen, N.; Kellerer, S.; Knorr, A.G.; Schlick, C.; Jahn, K.; Schneider, E.; Heuberger, M.; Ramaioli, C. Head-Movement-Emphasized Rehabilitation in Bilateral Vestibulopathy. Front. Neurol. 2018, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, T.; Horii, A.; Kubo, T.; Shin-ichi, O. Vestibular Compensation after Vestibular Neuronitis in Elderly Patients. Equilib. Res. 2008, 67, 506–511. [Google Scholar] [CrossRef]
- Funabiki, K.; Mishina, M.; Hirano, T. Retarded vestibular compensation in mutant mice deficient in delta 2 glutamate receptor subunit. NeuroReport 1995, 7, 189–192. [Google Scholar] [PubMed]
- Kashiwabuchi, N.; Ikeda, K.; Araki, K.; Hirano, T.; Shibuki, K.; Takayama, C.; Inoue, Y.; Kutsuwada, T.; Yagi, T.; Kang, Y.; et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell 1995, 81, 245–252. [Google Scholar] [CrossRef] [Green Version]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, T.; Tatsumi, K.; Takimoto, Y.; Nishimura, T.; Imai, T.; Yamanaka, T.; Takeda, N.; Wanaka, A.; Kitahara, T. Vestibular Compensation after Vestibular Dysfunction Induced by Arsanilic Acid in Mice. Brain Sci. 2019, 9, 329. https://doi.org/10.3390/brainsci9110329
Ito T, Tatsumi K, Takimoto Y, Nishimura T, Imai T, Yamanaka T, Takeda N, Wanaka A, Kitahara T. Vestibular Compensation after Vestibular Dysfunction Induced by Arsanilic Acid in Mice. Brain Sciences. 2019; 9(11):329. https://doi.org/10.3390/brainsci9110329
Chicago/Turabian StyleIto, Taeko, Kouko Tatsumi, Yasumitsu Takimoto, Tadashi Nishimura, Takao Imai, Toshiaki Yamanaka, Noriaki Takeda, Akio Wanaka, and Tadashi Kitahara. 2019. "Vestibular Compensation after Vestibular Dysfunction Induced by Arsanilic Acid in Mice" Brain Sciences 9, no. 11: 329. https://doi.org/10.3390/brainsci9110329
APA StyleIto, T., Tatsumi, K., Takimoto, Y., Nishimura, T., Imai, T., Yamanaka, T., Takeda, N., Wanaka, A., & Kitahara, T. (2019). Vestibular Compensation after Vestibular Dysfunction Induced by Arsanilic Acid in Mice. Brain Sciences, 9(11), 329. https://doi.org/10.3390/brainsci9110329





