Efficacy of Verbally Describing One’s Own Body Movement in Motor Skill Acquisition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
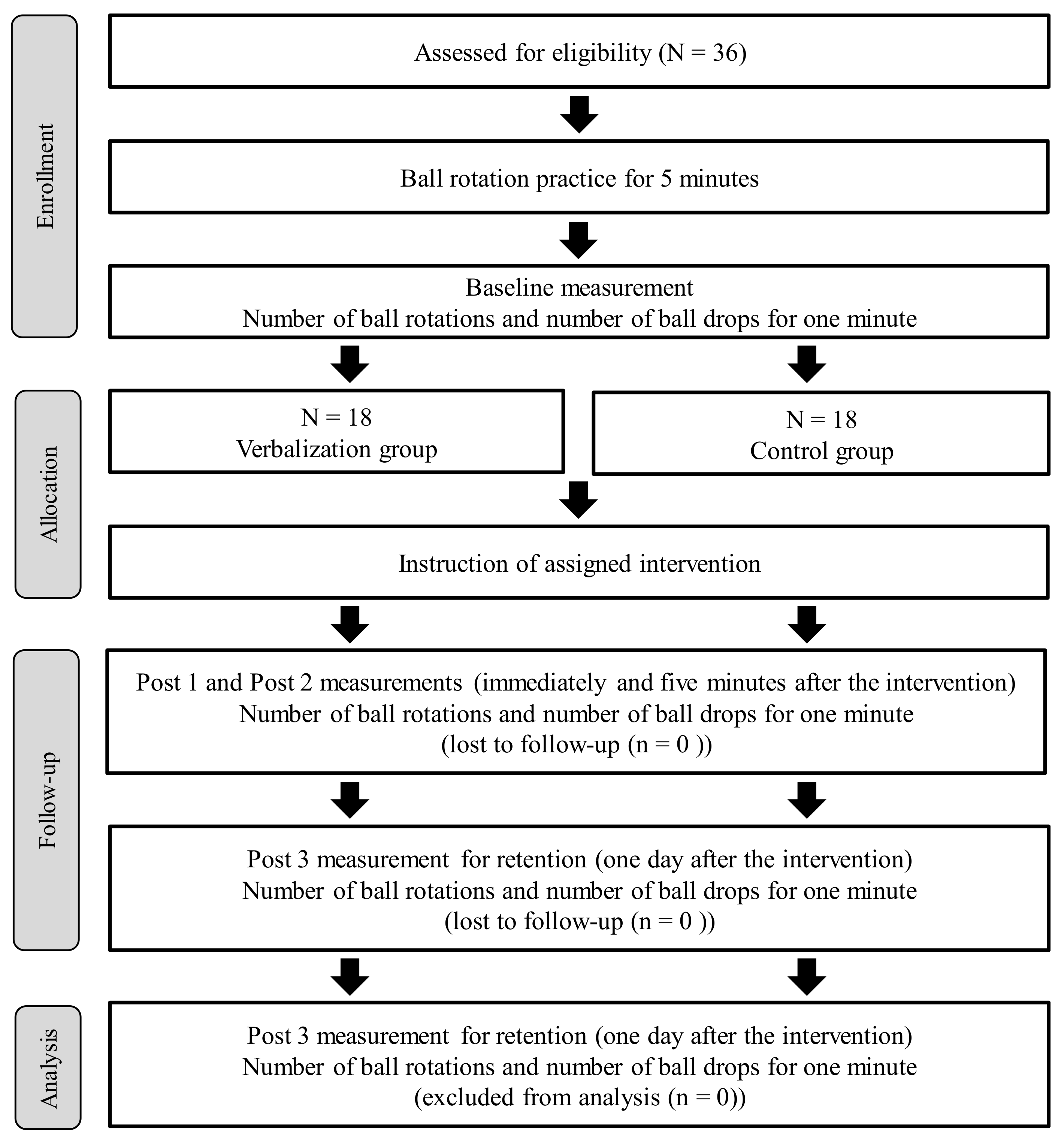
2.2. Procedure
2.3. Dependent Variables and Data Analyses
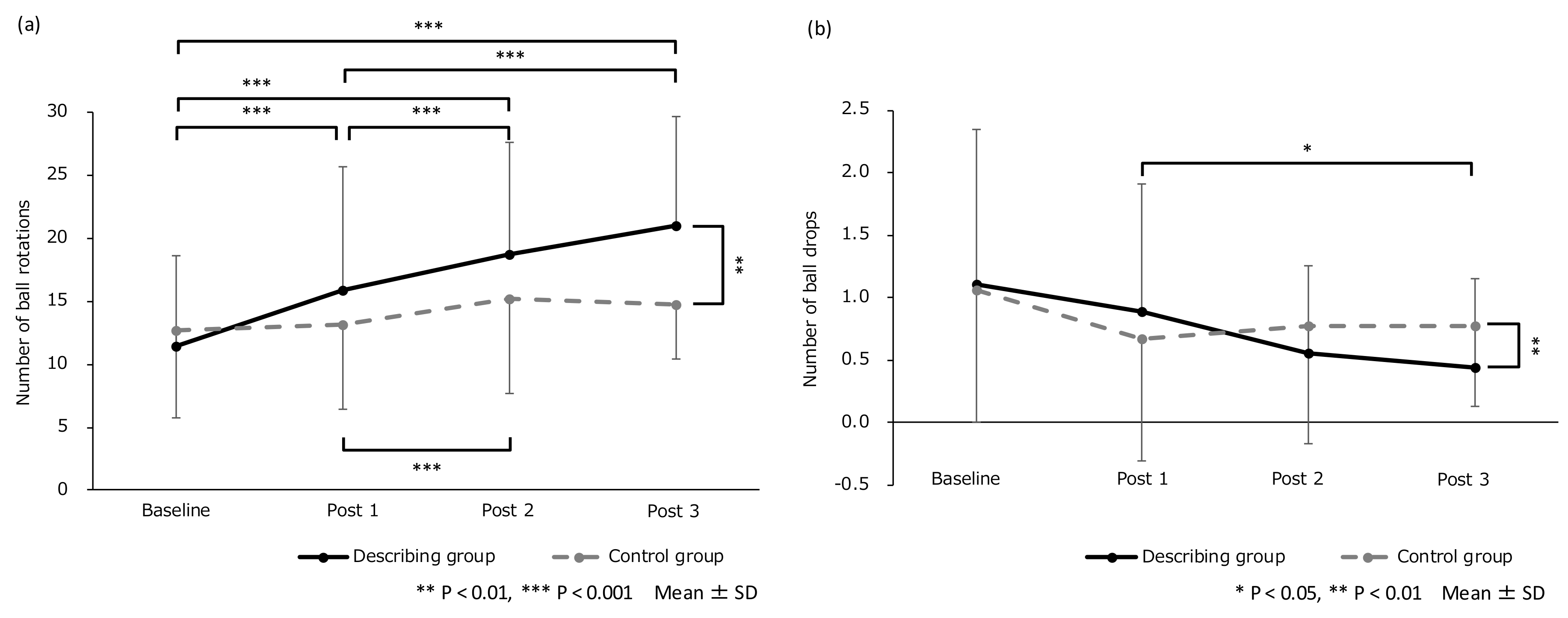
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mulder, T. Motor imagery and action observation: Cognitive tools for rehabilitation. J. Neural Transm. 2007, 114, 1265–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avanzino, L.; Gueugneau, N.; Bisio, A.; Ruggeri, P.; Papaxanthis, C.; Bove, M. Motor cortical plasticity induced by motor learning through mental practice. Front. Behav. Neurosci. 2015, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, S.J.; Levine, P.; Leonard, A.C. Effects of mental practice on affected limb use and function in chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Kim, J.-S.; Lee, G.-C. Effects of motor imagery training on balance and gait abilities in post-stroke patients: A randomized controlled trial. Clin. Rehabil. 2012, 27, 675–680. [Google Scholar] [CrossRef]
- Moseley, G.L. Graded motor imagery for pathologic pain: A randomized controlled trial. Neurology 2006, 67, 2129–2134. [Google Scholar] [CrossRef]
- Moseley, G.L. Is successful rehabilitation of complex regional pain syndrome due to sustained attention to the affected limb? A randomised clinical trial. Pain 2005, 114, 54–61. [Google Scholar] [CrossRef]
- Moseley, G.L. Graded motor imagery is effective for long-standing complex regional pain syndrome: A randomised controlled trial. Pain 2004, 108, 192–198. [Google Scholar] [CrossRef]
- Roland, P.E.; Larsen, B.; Lassen, N.A.; Skinhoj, E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J. Neurophysiol. 1980, 43, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Stephan, K.M.; Fink, G.R.; Passingham, R.E.; Silbersweig, D.; Ceballos-Baumann, A.O.; Frith, C.D.; Frackowiak, R.S. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J. Neurophysiol. 1995, 73, 373–386. [Google Scholar] [CrossRef] [Green Version]
- Luft, A.R.; Skalej, M.; Stefanou, A.; Klose, U.; Voigt, K. Comparing motion- and imagery-related activation in the human cerebellum: A functional MRI study. Hum. Brain Mapp. 1998, 6, 105–113. [Google Scholar] [CrossRef]
- Porro, C.A.; Francescato, M.P.; Cettolo, V.; Diamond, M.E.; Baraldi, P.; Zuiani, C.; Bazzocchi, M.; di Prampero, P.E. Primary motor and sensory cortex activation during motor performance and motor imagery: A functional magnetic resonance imaging study. J. Neurosci. 1996, 16, 7688–7698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, M.; Decety, J.; Raybaudi, M.; Massarelli, R.; Delon-Martin, C.; Segebarth, C.; Morand, S.; Gemignani, A.; Decorps, M.; Jeannerod, M. Possible involvement of primary motor cortex in mentally simulated movement: A functional magnetic resonance imaging study. Neuroreport 1996, 7, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Montoya, P.; Erb, M.; Hülsmann, E.; Flor, H.; Klose, U.; Birbaumer, N.; Grodd, W. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: An fMRI study. J. Cogn. Neurosci. 1999, 11, 491–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinear, C.M.; Byblow, W.D.; Steyvers, M.; Levin, O.; Swinnen, S.P. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp. Brain Res. 2006, 168, 157–164. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Sakamoto, M.; Muraoka, T.; Kanosue, K. Influence of touching an object on corticospinal excitability during motor imagery. Exp. Brain Res. 2009, 196, 529–535. [Google Scholar] [CrossRef]
- Mizuguchi, N.; Sakamoto, M.; Muraoka, T.; Nakagawa, K.; Kanazawa, S.; Nakata, H.; Moriyama, N.; Kanosue, K. The Modulation of Corticospinal Excitability during Motor Imagery of Actions with Objects. PLoS ONE 2011, 6, e26006. [Google Scholar] [CrossRef] [Green Version]
- Bufalari, I.; Sforza, A.; Cesari, P.; Aglioti, S.M.; Fourkas, A.D. Motor imagery beyond the joint limits: A transcranial magnetic stimulation study. Biol. Psychol. 2010, 85, 283–290. [Google Scholar] [CrossRef]
- Decety, J.; Jeannerod, M. Mentally simulated movements in virtual reality: Does Fitts’s law hold in motor imagery? Behav. Brain Res. 1995, 72, 127–134. [Google Scholar] [CrossRef]
- Bakker, M.; De Lange, F.; Stevens, J.; Toni, I.; Bloem, B. Motor imagery of gait: A quantitative approach. Exp. Brain Res. 2007, 179, 497–504. [Google Scholar] [CrossRef]
- Stout, D.; Chaminade, T. Stone tools, language and the brain in human evolution. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 75–87. [Google Scholar] [CrossRef]
- Suwa, M. Meta-Cognition as a Tool for Storytelling and Questioning What Design Is. Bull. Jpn. Soc. Sci. Des. 2009, 16, 21–26. [Google Scholar]
- Suwa, M. A cognitive model of acquiring embodied expertise through meta-cognitive verbalization. Trans. Jpn. Soc. Artif. Intell. 2008, 23, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Duffau, H.; Capelle, L.; Denvil, D.; Gatignol, P.; Sichez, N.; Lopes, M.; Sichez, J.-P.; Van Effenterre, R. The role of dominant premotor cortex in language: A study using intraoperative functional mapping in awake patients. Neuroimage 2003, 20, 1903–1914. [Google Scholar] [CrossRef]
- Morin, A.; Hamper, B. Self-Reflection and the inner voice: Activation of the left inferior frontal gyrus during perceptual and conceptual self-referential thinking. Open Neuroimaging J. 2012, 6, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Camarda, R.; Fogassi, L.; Gentilucci, M.; Luppino, G.; Matelli, M. Functional organization of inferior area 6 in the macaque monkey. Exp. Brain Res. 1988, 71, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Rodgers, W.; Hall, C.; Buckolz, E. The effect of an imagery training program on imagery ability, imagery use, and figure skating performance. J. Appl. Sport Psychol. 1991, 3, 109–125. [Google Scholar] [CrossRef]
- Kawasaki, T.; Tozawa, R.; Aramaki, H. Effectiveness of using an unskilled model in action observation combined with motor imagery training for early motor learning in elderly people: A preliminary study. Somatosens. Mot. Res. 2018, 35, 204–211. [Google Scholar] [CrossRef]
- Miller, K.J. Executive functions. Pediatr. Ann. 2005, 34, 310–317. [Google Scholar] [CrossRef]
- Toglia, J.P.; Rodger, S.A.; Polatajko, H.J. Anatomy of cognitive strategies: A therapist’s primer for enabling occupational performance. Can. J. Occup. Ther. 2012, 79, 225–236. [Google Scholar] [CrossRef]
- Personnier, P.; Kubicki, A.; Laroche, D.; Papaxanthis, C. Temporal features of imagined locomotion in normal aging. Neurosci. Lett. 2010, 476, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.J.; Cazeaux, J.; Fidler, A.; Jansen, J.; Lefkove, N.; Gregg, M.; Hall, C.; Easley, K.A.; Shenvi, N.; Wolf, S.L. The movement imagery questionnaire-revised, (MIQ-RS) is a reliable and valid tool for evaluating motor imagery in stroke populations. Evid. Based Complement. Altern. Med. 2012, 2012, 497289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInnes, K.; Friesen, C.; Boe, S. Specific brain lesions impair explicit motor imagery ability: A systematic review of the evidence. Arch. Phys. Med. Rehabil. 2016, 97, 478–489. [Google Scholar] [CrossRef]
- Cohen, R.G.; Chao, A.; Nutt, J.G.; Horak, F.B. Freezing of gait is associated with a mismatch between motor imagery and motor execution in narrow doorways, not with failure to judge doorway passability. Neuropsychologia 2011, 49, 3981–3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, T.; Mikami, K.; Kamo, T.; Aoki, R.; Ishiguro, R.; Nakamura, H.; Tozawa, R.; Asada, N.; Hiiragi, Y.; Yamada, Y. Motor planning error in Parkinson’s disease and its clinical correlates. PLoS ONE 2018, 13, e0202228. [Google Scholar] [CrossRef] [PubMed]
- Nojima, I.; Oga, T.; Fukuyama, H.; Kawamata, T.; Mima, T. Mirror visual feedback can induce motor learning in patients with callosal disconnection. Exp. Brain Res. 2013, 227, 79–83. [Google Scholar] [CrossRef]
- Nojima, I.; Mima, T.; Koganemaru, S.; Thabit, M.N.; Fukuyama, H.; Kawamata, T. Human motor plasticity induced by mirror visual feedback. J. Neurosci. 2012, 32, 1293–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nojima, I.; Koganemaru, S.; Kawamata, T.; Fukuyama, H.; Mima, T. Action observation with kinesthetic illusion can produce human motor plasticity. Eur. J. Neurosci. 2015, 41, 1614–1623. [Google Scholar] [CrossRef]
- Von Rein, E.; Hoff, M.; Kaminski, E.; Sehm, B.; Steele, C.J.; Villringer, A.; Ragert, P. Improving motor performance without training: The effect of combining mirror visual feedback with transcranial direct current stimulation. J. Neurophysiol. 2015, 113, 2383–2389. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Osumi, M.; Ueta, K.; Kodama, T.; Morioka, S. Changes in electroencephalographic activity during observation, preparation, and execution of a motor learning task. Int. J. Neurosci. 2013, 123, 866–875. [Google Scholar] [CrossRef]
- Matsumura, M.; Sadato, N.; Kochiyama, T.; Nakamura, S.; Naito, E.; Matsunami, K.; Kawashima, R.; Fukuda, H.; Yonekura, Y. Role of the cerebellum in implicit motor skill learning: A PET study. Brain Res. Bull. 2004, 63, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A. Learning strategies in physical education: Self-talk, imagery, and goal-setting. J. Phys. Educ. Recreat. Dance 1997, 68, 30–35. [Google Scholar] [CrossRef]
- Anderson, A.; Vogel, P.; Albrecht, R. The effect of instructional self-talk on the overhand throw. Phys. Educ. 1999, 56, 215. [Google Scholar]
- Landin, D. The role of verbal cues in skill learning. Quest 1994, 46, 299–313. [Google Scholar] [CrossRef]

| Describing Group (N = 18) | Control Group (N = 18) | p-Value | |
|---|---|---|---|
| Age (years) | 21.1 ± 0.83 | 21.4 ± 0.51 | 0.16 |
| Gender distribution (% female) | 33 | 33 | 1.00 |
| Distance between wrist to top of middle finger at non-dominant hand (cm) | 19.6 ± 2.8 | 19.3 ± 1.4 | 0.60 |
| Edinburgh handedness inventory score | 92.6 ± 6.6 | 89.9 ± 6.5 | 0.23 |
| Absolute error of mental chronometry (s) | 2.22 ± 1.23 | 1.37 ± 0.97 | 0.69 |
| Baseline | Post 1 | Post 2 | Post 3 | |
|---|---|---|---|---|
| (a) | ||||
| Describing group | 11.46 ± 7.19 | 15.93 ± 9.77 | 18.71 ± 8.98 | 21.01 ± 8.72 |
| Control group | 12.67 ± 6.92 | 13.15 ± 6.73 | 15.24 ± 7.54 | 14.71 ± 4.27 |
| (b) | ||||
| Describing group | 1.11 ± 1.23 | 0.89 ± 1.02 | 0.56 ± 0.70 | 0.44 ± 0.70 |
| Control group | 1.06 ± 1.06 | 0.67 ± 0.97 | 0.78 ± 0.94 | 0.78 ± 0.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki, T.; Kono, M.; Tozawa, R. Efficacy of Verbally Describing One’s Own Body Movement in Motor Skill Acquisition. Brain Sci. 2019, 9, 356. https://doi.org/10.3390/brainsci9120356
Kawasaki T, Kono M, Tozawa R. Efficacy of Verbally Describing One’s Own Body Movement in Motor Skill Acquisition. Brain Sciences. 2019; 9(12):356. https://doi.org/10.3390/brainsci9120356
Chicago/Turabian StyleKawasaki, Tsubasa, Masashi Kono, and Ryosuke Tozawa. 2019. "Efficacy of Verbally Describing One’s Own Body Movement in Motor Skill Acquisition" Brain Sciences 9, no. 12: 356. https://doi.org/10.3390/brainsci9120356
APA StyleKawasaki, T., Kono, M., & Tozawa, R. (2019). Efficacy of Verbally Describing One’s Own Body Movement in Motor Skill Acquisition. Brain Sciences, 9(12), 356. https://doi.org/10.3390/brainsci9120356





