Magnitude of Reduction and Speed of Remission of Suicidality for Low Amplitude Seizure Therapy (LAP-ST) Compared to Standard Right Unilateral Electroconvulsive Therapy: A Pilot Double-Blinded Randomized Clinical Trial
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Eligibility
2.3. Assessments
2.4. Procedure
2.5. Low Amplitude Seizure Therapy (LAP-ST) or Standard RUL ECT
2.6. Anesthesia
2.7. Electroencephalography (EEG) Acquisition and Ictal Monitoring
2.8. Data Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Treatment Characteristics, Parameters and Seizure Duration
3.3. Suicidality Remission
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS). Available online: https://www.cdc.gov/injury/wisqars/index.html (accessed on 1 March 2019).
- Kochanek, K.D.; Murphy, S.; Xu, J.; Arias, E. Mortality in the United States. 2016. Available online: https://stacks.cdc.gov/view/cdc/60902 (accessed on 1 March 2019).
- Ivey-Stephenson, A.Z.; Crosby, A.E.; Jack, S.P.D.; Haileyesus, T.; Kresnow-Sedacca, M.J. Suicide Trends Among and Within Urbanization Levels by Sex, Race/Ethnicity, Age Group, and Mechanism of Death - United States, 2001–2015. MMWR Surveill Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Curtin, S.C.; Warner, M.; Hedegaard, H. Increase in Suicide in the United States, 1999–2014. Available online: https://stacks.cdc.gov/view/cdc/39008 (accessed on 1 March 2019).
- Isometsä, E. Suicidal Behaviour in Mood Disorders—Who, When, and Why? Can. J. Psychiatry 2014, 59, 120–130. [Google Scholar] [CrossRef]
- Marčinko, D.; Marčinko, V.; Karlovic, D.; Marčinko, A.; Martinac, M.; Begic, D.; Jakovljevič, M. Serum lipid levels and suicidality among male patients with schizoaffective disorder. Prog. Neuro-Psychoph. 2008, 32, 193–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrazek, D.A.; Hornberger, J.C.; Altar, C.A.; Degtiar, I. A Review of the Clinical, Economic, and Societal Burden of Treatment-Resistant Depression: 1996–2013. Psychiatr. Serv. 2014, 65, 977–987. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Bergfeld, I.O.; Mantione, M.; Figee, M.; Schuurman, P.R.; Lok, A.; Denys, D. Treatment-resistant depression and suicidality. J. Affect. Disord. 2018, 235, 362–367. [Google Scholar] [CrossRef] [PubMed]
- McCall, W.V.; Lisanby, S.H.; Rosenquist, P.B.; Dooley, M.; Husain, M.M.; Knapp, R.G.; Petrides, G.; Rudorfer, M.V.; Young, R.C.; McClintock, S.M.; et al. Effects of continuation electroconvulsive therapy on quality of life in elderly depressed patients: A randomized clinical trial. J. Psychiatr. Res. 2018, 97, 65–69. [Google Scholar] [CrossRef]
- Al-Harbi, K.S.; Harbi, A. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer. Adher. 2012, 6, 369–388. [Google Scholar] [CrossRef] [Green Version]
- Fink, M.; Kellner, C.H.; McCall, W.V. The Role of ECT in Suicide Prevention. J. ECT 2014, 30, 5–9. [Google Scholar] [CrossRef]
- Sienaert, P. Based on a True Story? The Portrayal of ECT in International Movies and Television Programs. Brain Stimul. 2016, 9, 882–891. [Google Scholar] [CrossRef]
- Matthews, A.M.; Rosenquist, P.B.; McCall, W.V. Representations of ECT in English-Language Film and Television in the New Millennium. J. ECT 2016, 32, 1–191. [Google Scholar] [CrossRef]
- Isometsä, E.T.; Henriksson, M.M.; Heikkinen, M.E.; Lönnqvist, J.K. Completed suicide and recent electroconvulsive therapy in Finland. Convuls. Ther. 1996, 12, 152–155. [Google Scholar]
- Aoki, Y.; Yamaguchi, S.; Ando, S.; Sasaki, N.; Bernick, P.J.; Akiyama, T. The experience of electroconvulsive therapy and its impact on associated stigma: A meta-analysis. Int. J. Soc. Psychiatry 2016, 62, 708–718. [Google Scholar] [CrossRef]
- Youssef, N.A.; Sidhom, E. Feasibility, safety, and preliminary efficacy of Low Amplitude Seizure Therapy (LAP-ST): A proof of concept clinical trial in man. J. Affect. Disord. 2017, 222, 1–6. [Google Scholar] [CrossRef]
- Youssef, N.; Sidhom, E. Examination of Cognitive Profile and Variability in the Current Amplitude Domain of Low Current Amplitude ECT. In Proceedings of the Society of Biological Psychiatry (SOBP) 69th Annual Meeting, New York, NY, USA, 8 May 2014; Elsevier Science Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Youssef, N.; Sidhom, E. A Proof of concept clinical trial of feasibility course of low Amplitude Seizure Therapy (LAP-ST) in man: Less cognitive side effects with retention of efficacy. Brain Stimul. 2017, 10, 368. [Google Scholar] [CrossRef]
- Youssef, N.; Ravilla, D.; McCall, W.; Patel, C.; McCloud, L.; Yassa, M.; Rosenquist, P. Magnitude of Reduction & Speed of Remission of Suicidality for Low AmPlitude Seizure Therapy (LAP-ST) Compared to Standard Right Unilateral ECT. Brain Stimul. 2019, 12, 439. [Google Scholar] [CrossRef]
- Youssef, N.; McCall, W.V.; Patel, C.; Momen, M.; Boswell, E.; Yassa, M.; McCloud, L.; Rosenquist, P. S108. Speed of Remission of Suicidality for Low Amplitude Seizure Therapy (LAP-ST) Versus Right Unilateral Ultra-Brief ECT. Biol. Psychiatry 2018, 83, S389. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Åsberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Roy, B.; Shelton, R.C.; Dwivedi, Y. DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation. J. Psychiatr. 2017, 89, 115–124. [Google Scholar] [CrossRef]
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am. J. Psychiatry 2000, 157, 1–45. [Google Scholar]
- Prudic, J.; Sackeim, H.A. Electroconvulsive therapy and suicide risk. J. Clin. Psychiatry 1999, 60, 104–110. [Google Scholar]
- Fink, M. What was learned: studies by the consortium for research in ECT (CORE) 1997–2011. Acta. Psychiatr. Scand. 2014, 129, 417–426. [Google Scholar] [CrossRef]

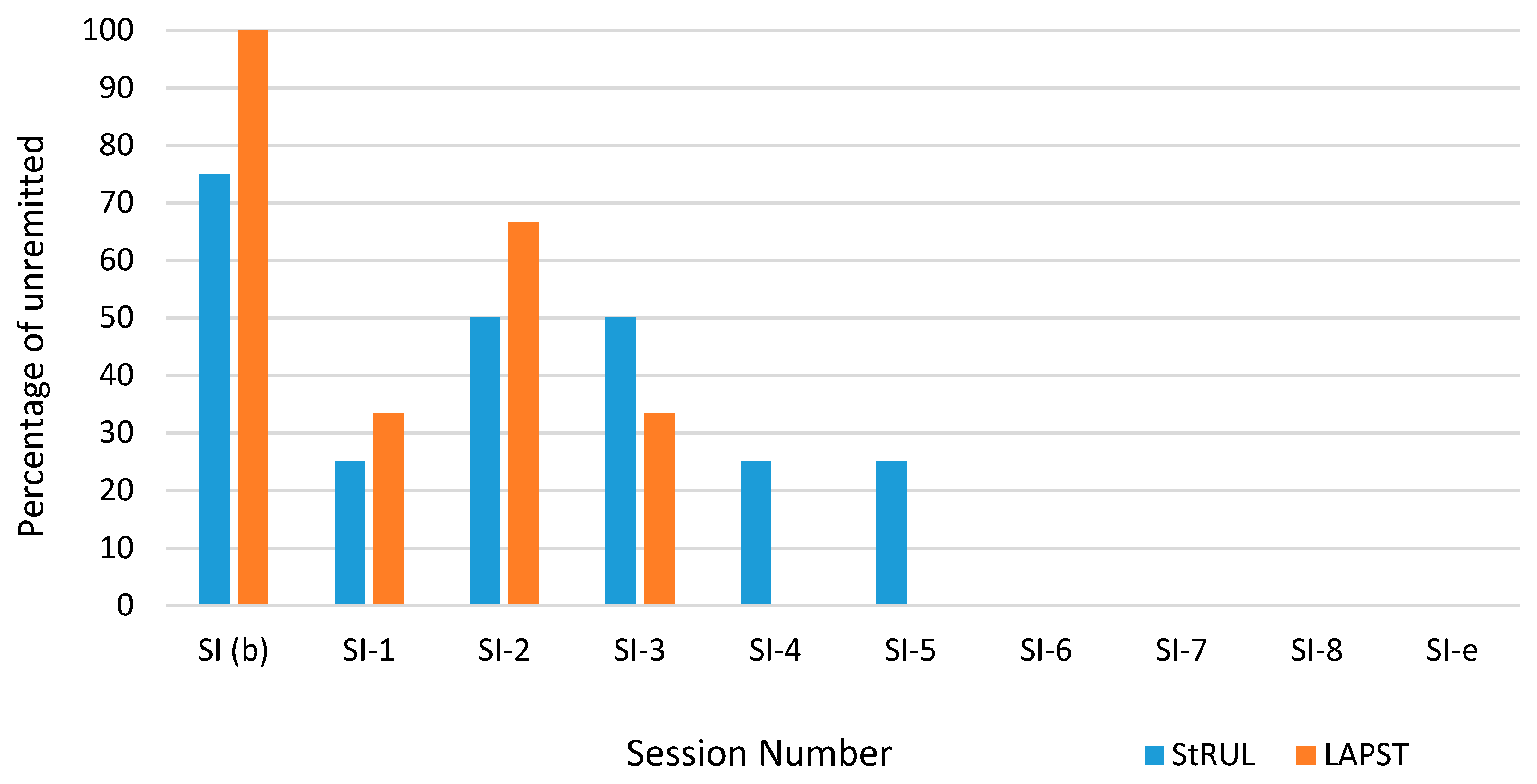
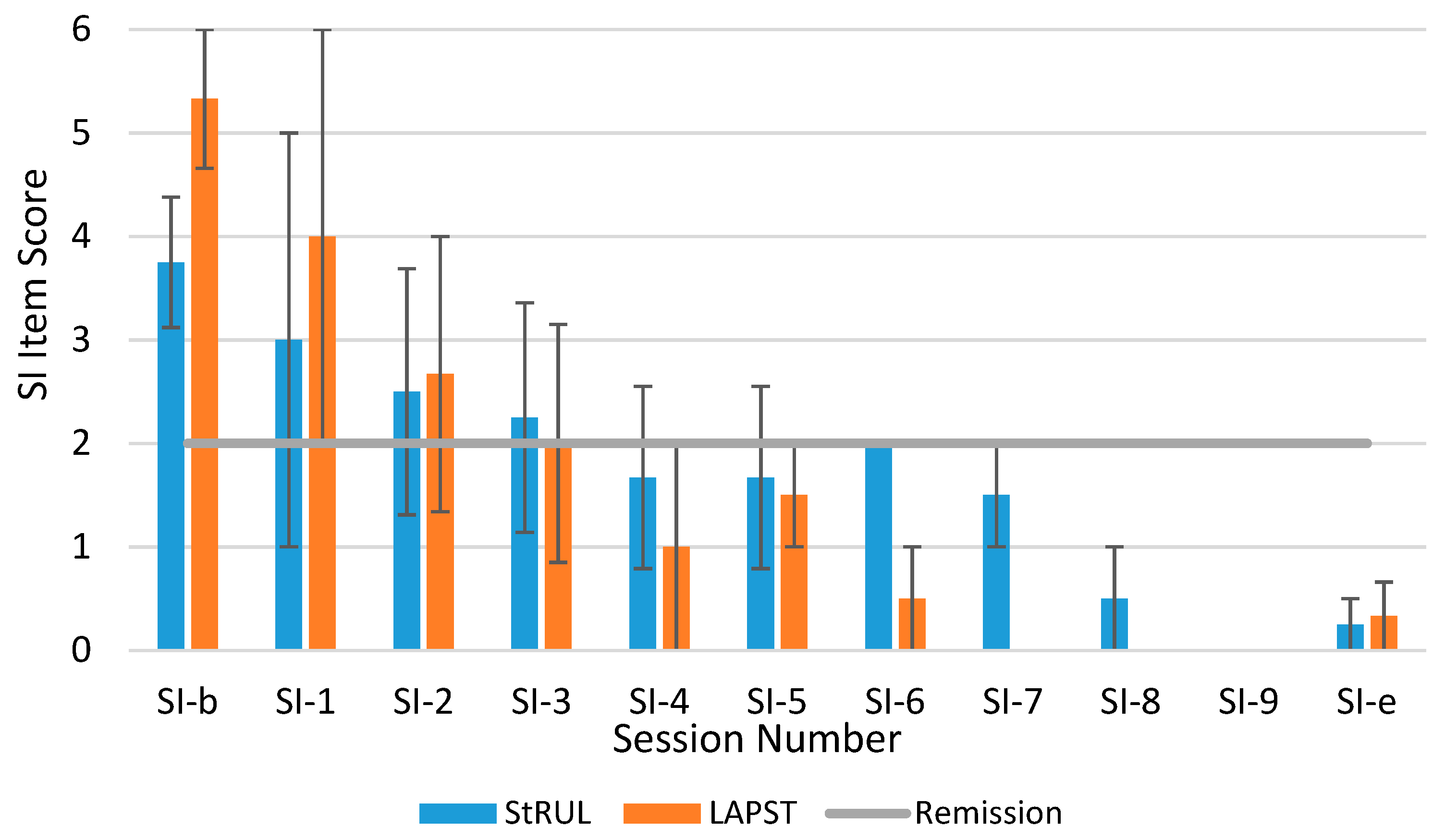

| Variable | Stat | Total Sample (n = 11) | Standard RUL (n = 4) | LAP-ST (n = 3) | Dropouts (n = 4) |
|---|---|---|---|---|---|
| Age | Mean (SD) | 40.64 (13.81) | 40.25 (17.39) | 42.00 (16.09) | 40 (12.57) |
| Median | 44 | 39 | 47 | 37.5 | |
| Range | 24–59 | 24–59 | 24–55 | 29–56 | |
| Female | N (%) | 6 (54.55) | 3 (75.00) | 0 (0.00) | 3 (75.00) |
| Race | |||||
| Black or African American | N (%) | 2 (18.18) | 0 (0.00) | 1 (33.33) | 1 (25.00) |
| White | N (%) | 8 (72.73) | 4 (100.00) | 2 (66.67) | 2 (50.00) |
| Other | N (%) | 1 (9.09) | 0 (0.00) | 0 (0.00) | 1 (25.00) |
| Education | |||||
| High School graduate-diploma or GED | N (%) | 2 (18.18) | 0 (0.00) | 1 (33.33) | 1 (25.00) |
| Some College | N (%) | 4 (36.36) | 2 (50.00) | 1 (33.33) | 1 (25.00) |
| College graduate | N (%) | 4 (36.36) | 1 (25.00) | 1 (33.33) | 2 (50.00) |
| Master’s Degree | N (%) | 1 (9.09) | 1 (25.00) | 0 (0.00) | 0 (0.00) |
| Diagnosis | |||||
| Major Depressive Disorder | N (%) | 4 (36.36) | 2 (50.00) | 1 (33.33) | 1 (25.00) |
| Bipolar Type I | N (%) | 5 (45.45) | 1 (25.00) | 2 (66.67) | 2 (50.00) |
| Bipolar Type II | N (%) | 1 (9.09) | 0 (0.00) | 0 (0.00) | 1 (25.00) |
| Schizoaffective/bipolar type | N (%) | 1 (9.09) | 1 (25.00) | 0 (0.00) | 0 (0.00) |
| Low Amplitude Seizure Therapy (LAP-ST) | Standard Right Unilateral (RUL) ECT | ||||
|---|---|---|---|---|---|
| Titration Session | 6 × ST * (500%) | Titration Session | 6 × ST * (500%) | ||
| Stimulus 1 | PW | 0.3 ms | 0.3 ms | 0.3 ms | 0.3 ms |
| Frequency | 20 Hz | 25 Hz | 20 Hz | 20 Hz | |
| Duration | 2 s | 8 s | 1 s | 6 s | |
| Current | 500 mA | 500 mA | 900 mA | 900 mA | |
| Charge | 12 mC | 60 mC | 10.8 mC | 64.8 mC | |
| Stimulus 2 | PW | 0.3 ms | 0.3 ms | 0.3 ms | 0.3 ms |
| Frequency | 20 Hz | 60 Hz | 20 Hz | 35 Hz | |
| Duration | 4 s | 8 s | 2 s | 7.5 s | |
| Current | 500 mA | 500 mA | 900 mA | 900 mA | |
| Charge | 24 mC | 144 mC | 21.6 mC | 141.75 mC | |
| Stimulus 3 | PW | 0.3 ms | 0.3 ms | 0.3 ms | 0.3 ms |
| Frequency | 20 Hz | 120 Hz | 20 Hz | 65 Hz | |
| Duration | 8 s | 8 s | 4.5 s | 8 s | |
| Current | 500 mA | 500 mA | 900 mA | 900 mA | |
| Charge | 48.0 mC | 288 mC | 48.6 mC | 280.8 mC | |
| Stimulus 4 | PW | 0.5 ms | 0.5 ms | 0.3 ms | 0.34 ms |
| Frequency | 60 Hz | 120 Hz | 25 Hz | 115 Hz | |
| Duration | 8 s | 8 s | 8 s | 8 s | |
| Current | 600 mA | 600 mA | 900 mA | 900 mA | |
| Charge | 108.0 mC | 576 mC | 172.0 mC | 563.0 mC | |
| Parameters | Standard RUL (n = 4) | LAP-ST (n = 3) | ||
|---|---|---|---|---|
| 1st Session | Subsequent Sessions | 1st Session | Subsequent Session | |
| Charge (mC), mean (SD), mean (SD) | 18.9 (5.4) | 122.5 (38.48) | 73.33 (72.04) * | 288.0 (173.1) * |
| Current (mA), mean (SD), mean (SD) | 900 (0) | 900 (0) | 500 (0) | 500 (0) |
| Pulse Width (Sec), mean (SD) | 0.3 (0) | 0.3 (0) | 0.53 (0.40) | 0.60 (0.36) |
| Frequency (Hz), mean (SD) | 23.75 (7.5) | 31.25 (7.5) | 35 (25.98) | 70 (45.83) |
| Motor Seizure (Sec), mean (SD) | 56.25 (24.30) | 21.25 (8.10) | 27.67 (13.50) | 36.00 (12.53) |
| EEG Seizure (Sec), mean (SD) | 81.5 (49.22) | 42.0 (5.72) | 44.67 (36.14) | 51.33 (21.03) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, N.A.; Ravilla, D.; Patel, C.; Yassa, M.; Sadek, R.; Zhang, L.F.; McCloud, L.; McCall, W.V.; Rosenquist, P.B. Magnitude of Reduction and Speed of Remission of Suicidality for Low Amplitude Seizure Therapy (LAP-ST) Compared to Standard Right Unilateral Electroconvulsive Therapy: A Pilot Double-Blinded Randomized Clinical Trial. Brain Sci. 2019, 9, 99. https://doi.org/10.3390/brainsci9050099
Youssef NA, Ravilla D, Patel C, Yassa M, Sadek R, Zhang LF, McCloud L, McCall WV, Rosenquist PB. Magnitude of Reduction and Speed of Remission of Suicidality for Low Amplitude Seizure Therapy (LAP-ST) Compared to Standard Right Unilateral Electroconvulsive Therapy: A Pilot Double-Blinded Randomized Clinical Trial. Brain Sciences. 2019; 9(5):99. https://doi.org/10.3390/brainsci9050099
Chicago/Turabian StyleYoussef, Nagy A., Dheeraj Ravilla, Cherishma Patel, Mark Yassa, Ramses Sadek, Li Fang Zhang, Laryssa McCloud, William V. McCall, and Peter B. Rosenquist. 2019. "Magnitude of Reduction and Speed of Remission of Suicidality for Low Amplitude Seizure Therapy (LAP-ST) Compared to Standard Right Unilateral Electroconvulsive Therapy: A Pilot Double-Blinded Randomized Clinical Trial" Brain Sciences 9, no. 5: 99. https://doi.org/10.3390/brainsci9050099
APA StyleYoussef, N. A., Ravilla, D., Patel, C., Yassa, M., Sadek, R., Zhang, L. F., McCloud, L., McCall, W. V., & Rosenquist, P. B. (2019). Magnitude of Reduction and Speed of Remission of Suicidality for Low Amplitude Seizure Therapy (LAP-ST) Compared to Standard Right Unilateral Electroconvulsive Therapy: A Pilot Double-Blinded Randomized Clinical Trial. Brain Sciences, 9(5), 99. https://doi.org/10.3390/brainsci9050099





