Cerebral Blood Flow Regulation in Pregnancy, Hypertension, and Hypertensive Disorders of Pregnancy
Abstract
:1. Introduction to Cerebral Blood Flow (CBF) Regulation
1.1. CBF Regulation in the Normotensive and Hypertensive Non-Pregnant State
1.2. CBF Regulation in Pregnancy and Preeclampsia
1.3. Consequences of Impaired CBF Regulation
1.4. Limitations of Current Knowledge of CBF Regulation in Hypertensive Disorders of Pregnancy
2. Conclusion and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lassen, N.A. Cerebral blood flow and oxygen consumption in man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; Kornbluth, J. Cerebral blood flow and autoregulation: Current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef]
- Armstead, W.M. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesthesiol. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Ter Laan, M.; van Dijk, J.M.; Elting, J.W.; Staal, M.J.; Absalom, A.R. Sympathetic regulation of cerebral blood flow in humans: A review. Br. J. Anaesth. 2013, 111, 361–367. [Google Scholar] [CrossRef]
- Cipolla, M.J. The Cerebral Circulation. In Integrated Systems Physiology: From Molecule to Function; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2009. [Google Scholar]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [Green Version]
- Chalak, L.F.; Zhang, R. New Wavelet Neurovascular Bundle for Bedside Evaluation of Cerebral Autoregulation and Neurovascular Coupling in Newborns with Hypoxic-Ischemic Encephalopathy. Dev. Neurosci. 2017, 39, 89–96. [Google Scholar] [CrossRef]
- Claassen, J.A.; Meel-van den Abeelen, A.S.; Simpson, D.M.; Panerai, R.B. International Cerebral Autoregulation Research Network. Transfer function analysis of dynamic cerebral autoregulation: A white paper from the International Cerebral Autoregulation Research Network. J. Cereb. Blood Flow Metab. 2016, 36, 665–680. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Czosnyka, M.; Smielewski, P.; Varsos, G.V.; Kasprowicz, M.; Brady, K.M.; Pickard, J.D.; Kirkpatrick, P.J. Cerebral autoregulation after subarachnoid hemorrhage: Comparison of three methods. J. Cereb. Blood Flow Metab. 2013, 33, 449–456. [Google Scholar] [CrossRef]
- Zarrinkoob, L.; Ambarki, K.; Wahlin, A.; Birgander, R.; Eklund, A.; Malm, J. Blood flow distribution in cerebral arteries. J. Cereb. Blood Flow Metab. 2015, 35, 648–654. [Google Scholar] [CrossRef]
- Pires, P.W.; Dams Ramos, C.M.; Matin, N.; Dorrance, A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1598–H1614. [Google Scholar] [CrossRef]
- Barry, D.I. Cerebral blood flow in hypertension. J. Cardiovasc. Pharmacol. 1985, 7 (Suppl. 2), S94–S98. [Google Scholar] [CrossRef]
- Nobili, F.; Rodriguez, G.; Marenco, S.; De Carli, F.; Gambaro, M.; Castello, C.; Pontremoli, R.; Rosadini, G. Regional cerebral blood flow in chronic hypertension. A correlative study. Stroke 1993, 24, 1148–1153. [Google Scholar] [CrossRef]
- Dai, W.; Lopez, O.L.; Carmichael, O.T.; Becker, J.T.; Kuller, L.H.; Gach, H.M. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke 2008, 39, 349–354. [Google Scholar] [CrossRef]
- Kim, T.; Richard Jennings, J.; Kim, S.G. Regional cerebral blood flow and arterial blood volume and their reactivity to hypercapnia in hypertensive and normotensive rats. J. Cereb. Blood Flow Metab. 2014, 34, 408–414. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Q.; Huang, S.; Li, W.; Muir, E.R.; Long, J.A.; Duong, T.Q. Cerebral angiography, blood flow and vascular reactivity in progressive hypertension. Neuroimage 2015, 111, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, T.; Shigematsu, J.; Tobimatsu, S.; Takahashi, S.; Kato, M. Persistent hypertension does not alter the cerebral blood flow and glucose utilization in young-adult Dahl salt-sensitive rats. J. Neurol. Sci. 2002, 197, 19–26. [Google Scholar] [CrossRef]
- Smeda, J.S.; Payne, G.W. Alterations in autoregulatory and myogenic function in the cerebrovasculature of Dahl salt-sensitive rats. Stroke 2003, 34, 1484–1490. [Google Scholar] [CrossRef]
- Kazama, K.; Wang, G.; Frys, K.; Anrather, J.; Iadecola, C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1890–H1899. [Google Scholar] [CrossRef] [Green Version]
- Kazama, K.; Anrather, J.; Zhou, P.; Girouard, H.; Frys, K.; Milner, T.A.; Iadecola, C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ. Res. 2004, 95, 1019–1026. [Google Scholar] [CrossRef]
- Toth, P.; Tucsek, Z.; Sosnowska, D.; Gautam, T.; Mitschelen, M.; Tarantini, S.; Deak, F.; Koller, A.; Sonntag, W.E.; Csiszar, A.; et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J. Cereb. Blood Flow Metab. 2013, 33, 1732–1742. [Google Scholar] [CrossRef]
- Capone, C.; Faraco, G.; Park, L.; Cao, X.; Davisson, R.L.; Iadecola, C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H397–H407. [Google Scholar] [CrossRef]
- Poston, L.; McCarthy, A.L.; Ritter, J.M. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacol. Ther. 1995, 65, 215–239. [Google Scholar] [CrossRef]
- Amburgey, O.A.; Reeves, S.A.; Bernstein, I.M.; Cipolla, M.J. Resistance artery adaptation to pregnancy counteracts the vasoconstricting influence of plasma from normal pregnant women. Reprod. Sci. 2010, 17, 29–39. [Google Scholar] [CrossRef]
- van Veen, T.R.; Panerai, R.B.; Haeri, S.; van den Berg, P.P.; Zeeman, G.G.; Belfort, M.A. Changes in cerebral autoregulation in the second half of pregnancy and compared to non-pregnant controls. Pregnancy Hypertens. 2016, 6, 380–383. [Google Scholar] [CrossRef]
- Nevo, O.; Soustiel, J.F.; Thaler, I. Maternal cerebral blood flow during normal pregnancy: A cross-sectional study. Am. J. Obstet. Gynecol. 2010, 203, 475.e1–475.e6. [Google Scholar] [CrossRef]
- Batur Caglayan, H.Z.; Nazliel, B.; Cinar, M.; Ataoglu, E.; Moraloglu, O.; Irkec, C. Assessment of maternal cerebral blood flow velocity by transcranial Doppler ultrasound before delivery and in the early postpartum period. J. Matern.-Fetal Neonatal Med. 2019, 32, 584–589. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Hirashima, C.; Ohkuchi, A.; Matsubara, S.; Furukawa, M.; Watanabe, T.; Suzuki, M. Hydrocephalus after intraventricular hemorrhage in eclamptic woman with HELLP syndrome. Hypertens. Pregnancy 2006, 25, 255–257. [Google Scholar] [CrossRef]
- Zunker, P.; Ley-Pozo, J.; Louwen, F.; Schuierer, G.; Holzgreve, W.; Ringelstein, E.B. Cerebral hemodynamics in pre-eclampsia/eclampsia syndrome. Ultrasound Obstet. Gynecol. 1995, 6, 411–415. [Google Scholar] [CrossRef]
- Matsuda, H.; Sakaguchi, K.; Shibasaki, T.; Takahashi, H.; Kawakami, Y.; Furuya, K.; Kikuchi, Y. Cerebral edema on MRI in severe preeclamptic women developing eclampsia. J. Perinat. Med. 2005, 33, 199–205. [Google Scholar] [CrossRef]
- Topuz, S.; Kalelioğlu, I.; Iyibozkurt, A.C.; Akhan, S.; Has, R.; Tunaci, M.; Ibrahimoğlu, L. Cranial imaging spectrum in hypertensive disease of pregnancy. Clin. Exp. Obstet. Gynecol. 2008, 35, 194–197. [Google Scholar]
- Mitas, L.; Rogulski, L. Acute cortical blindness in preeclampsia—A case of reversible posterior encephalopathy syndrome. Ginekol. Pol. 2012, 83, 469–472. [Google Scholar]
- Aygün, B.K.; Baykuş, Y.; Berilgen, S.; Kavak, B.; Celik, H.; Gürateş, B. Posterior Reversible Encephalopathy Syndrome in severe preeclampsia: Case report and literature review. J. Turk. Ger. Gynecol. Assoc. 2010, 11, 216–219. [Google Scholar] [CrossRef]
- Van Veen, T.R.; Panerai, R.B.; Haeri, S.; Griffioen, A.C.; Zeeman, G.G.; Belfort, M.A. Cerebral autoregulation in normal pregnancy and preeclampsia. Obstet. Gynecol. 2013, 122, 1064–1069. [Google Scholar] [CrossRef]
- van Veen, T.R.; Panerai, R.B.; Haeri, S.; Singh, J.; Adusumalli, J.A.; Zeeman, G.G.; Belfort, M.A. Cerebral autoregulation in different hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2015, 212, 513.e1–513.e7. [Google Scholar] [CrossRef] [Green Version]
- Belfort, M.A.; Tooke-Miller, C.; Allen, J.C., Jr.; Varner, M.A.; Grunewald, C.; Nisell, H.; Herd, J.A. Pregnant women with chronic hypertension and superimposed pre-eclampsia have high cerebral perfusion pressure. BJOG 2001, 108, 1141–1147. [Google Scholar]
- Riskin-Mashiah, S.; Belfort, M.A. Preeclampsia is associated with global cerebral hemodynamic changes. J. Soc. Gynecol. Investig. 2005, 12, 253–256. [Google Scholar] [CrossRef]
- Demirtaş, O.; Gelal, F.; Vidinli, B.D.; Demirtaş, L.O.; Uluç, E.; Baloğlu, A. Cranial MR imaging with clinical correlation in preeclampsia and eclampsia. Diagn. Interv. Radiol. 2005, 11, 189–194. [Google Scholar]
- Manfredi, M.; Beltramello, A.; Bongiovanni, L.G.; Polo, A.; Pistoia, L.; Rizzuto, N. Eclamptic encephalopathy: Imaging and pathogenetic considerations. Acta Neurol. Scand. 1997, 96, 277–282. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuzaki, K.; Harada, M.; Nishitani, H.; Matsuda, T. Cerebral hyperperfusion in a patient with eclampsia with perfusion-weighted magnetic resonance imaging. Radiat. Med. 2005, 23, 376–379. [Google Scholar]
- Oehm, E.; Reinhard, M.; Keck, C.; Els, T.; Spreer, J.; Hetzel, A. Impaired dynamic cerebral autoregulation in eclampsia. Ultrasound Obstet. Gynecol. 2003, 22, 395–398. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Fukuuchi, Y.; Nogawa, S.; Dembo, T.; Tomita, Y.; Tanaka, K. Recovery of decreased local cerebral blood flow detected by the xenon/CT CBF method in a patient with eclampsia. Keio J. Med. 2000, 49, A71–A74. [Google Scholar]
- Williams, K.P.; Wilson, S. Maternal cerebral blood flow changes associated with eclampsia. Am. J. Perinatol. 1995, 12, 189–191. [Google Scholar] [CrossRef]
- Zunker, P.; Georgiadis, A.L.; Czech, N.; Golombeck, K.; Brossmann, J.; Deuschl, G. Impaired cerebral glucose metabolism in eclampsia: A new finding in two cases. Fetal Diagn. Ther. 2003, 18, 41–46. [Google Scholar] [CrossRef]
- Janzarik, W.G.; Ehlers, E.; Ehmann, R.; Gerds, T.A.; Schork, J.; Mayer, S.; Gabriel, B.; Weiller, C.; Prömpeler, H.; Reinhard, M. Dynamic cerebral autoregulation in pregnancy and the risk of preeclampsia. Hypertension 2014, 63, 161–166. [Google Scholar] [CrossRef]
- Barnes, J.N.; Harvey, R.E.; Miller, K.B.; Jayachandran, M.; Malterer, K.R.; Lahr, B.D.; Bailey, K.R.; Joyner, M.J.; Miller, V.M. Cerebrovascular Reactivity and Vascular Activation in Postmenopausal Women with Histories of Preeclampsia. Hypertension 2018, 71, 110–117. [Google Scholar] [CrossRef]
- Cipolla, M.J. Cerebrovascular function in pregnancy and eclampsia. Hypertension 2007, 50, 14–24. [Google Scholar] [CrossRef]
- Hammer, E.S.; Cipolla, M.J. Cerebrovascular Dysfunction in Preeclamptic Pregnancies. Curr. Hypertens. Rep. 2015, 17, 64. [Google Scholar] [CrossRef]
- Cipolla, M.J. The adaptation of the cerebral circulation to pregnancy: Mechanisms and consequences. J. Cereb. Blood Flow Metab. 2013, 33, 465–478. [Google Scholar] [CrossRef]
- Ogoh, S. Relationship between cognitive function and regulation of cerebral blood flow. J. Physiol. Sci. 2017, 67, 345–351. [Google Scholar] [CrossRef]
- Theilen, L.H.; Fraser, A.; Hollingshaus, M.S.; Schliep, K.C.; Varner, M.W.; Smith, K.R.; Esplin, M.S. All-Cause and Cause-Specific Mortality After Hypertensive Disease of Pregnancy. Obstet. Gynecol. 2016, 128, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Nelander, M.; Cnattingius, S.; Akerud, H.; Wikstrom, J.; Pedersen, N.L.; Wikstrom, A.K. Pregnancy hypertensive disease and risk of dementia and cardiovascular disease in women aged 65 years or older: A cohort study. BMJ Open 2016, 6, e009880. [Google Scholar] [CrossRef]
- Basit, S.; Wohlfahrt, J.; Boyd, H.A. Pre-eclampsia and risk of dementia later in life: Nationwide cohort study. BMJ 2018, 363, k4109. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Mielke, M.M.; Milic, N.M.; Weissgerber, T.L.; White, W.M.; Kantarci, K.; Mosley, T.H.; Windham, B.G.; Simpson, B.N.; Turner, S.T.; Garovic, V.D. Impaired Cognition and Brain Atrophy Decades After Hypertensive Pregnancy Disorders. Circ. Cardiovasc. Qual. Outcomes 2016, 9 (Suppl. 1), S70–S76. [Google Scholar] [CrossRef]
- Postma, I.R.; Bouma, A.; Ankersmit, I.F.; Zeeman, G.G. Neurocognitive functioning following preeclampsia and eclampsia: A long-term follow-up study. Am. J. Obstet. Gynecol. 2014, 211, 37. [Google Scholar] [CrossRef]
- Siepmann, T.; Boardman, H.; Bilderbeck, A.; Griffanti, L.; Kenworthy, Y.; Zwager, C.; McKean, D.; Francis, J.; Neubauer, S.; Grace, Z.Y.; et al. Long-term cerebral white and gray matter changes after preeclampsia. Neurology 2017, 88, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Sacks, K.N.; Friger, M.; Shoham-Vardi, I.; Sergienko, R.; Spiegel, E.; Landau, D.; Sheiner, E. Long-term neuropsychiatric morbidity in children exposed prenatally to preeclampsia. Early Hum. Dev. 2019, 130, 96–100. [Google Scholar] [CrossRef]
- Rätsep, M.T.; Paolozza, A.; Hickman, A.F.; Maser, B.; Kay, V.R.; Mohammad, S.; Pudwell, J.; Smith, G.N.; Brien, D.; Stroman, P.W.; et al. Brain Structural and Vascular Anatomy Is Altered in Offspring of Pre-Eclamptic Pregnancies: A Pilot Study. AJNR Am. J. Neuroradiol. 2016, 37, 939–945. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Ryan, M.J.; Gilbert, E.L.; Glover, P.H.; George, E.M.; Masterson, C.W.; McLemore, G.R., Jr.; LaMarca, B.; Granger, J.P.; Drummond, H.A. Placental ischemia impairs middle cerebral artery myogenic responses in the pregnant rat. Hypertension 2011, 58, 1126–1131. [Google Scholar] [CrossRef]
- Clayton, A.M.; Shao, Q.; Paauw, N.D.; Giambrone, A.B.; Granger, J.P.; Warrington, J.P. Postpartum increases in cerebral edema and inflammation in response to placental ischemia during pregnancy. Brain Behav. Immun. 2018, 70, 376–389. [Google Scholar] [CrossRef]
- Warrington, J.P.; Fan, F.; Murphy, S.R.; Roman, R.J.; Drummond, H.A.; Granger, J.P.; Ryan, M.J. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol. Rep. 2014, 2, e12134. [Google Scholar] [CrossRef]
- Johnson, A.C.; Cipolla, M.J. Impaired function of cerebral parenchymal arterioles in experimental preeclampsia. Microvasc. Res. 2018, 119, 64–72. [Google Scholar] [CrossRef]
- Johnson, A.C.; Cipolla, M.J. Altered hippocampal arteriole structure and function in a rat model of preeclampsia: Potential role in impaired seizure-induced hyperemia. J. Cereb. Blood Flow Metab. 2017, 37, 2857–2869. [Google Scholar] [CrossRef]
- Johnson, A.C.; Tremble, S.M.; Chan, S.L.; Moseley, J.; LaMarca, B.; Nagle, K.J.; Cipolla, M.J. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PLoS ONE 2014, 9, e113670. [Google Scholar] [CrossRef]
- Chapman, A.C.; Cipolla, M.J.; Chan, S.L. Effect of pregnancy and nitric oxide on the myogenic vasodilation of posterior cerebral arteries and the lower limit of cerebral blood flow autoregulation. Reprod. Sci. 2013, 20, 1046–1054. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Bishop, N.; Chan, S.L. Effect of pregnancy on autoregulation of cerebral blood flow in anterior versus posterior cerebrum. Hypertension 2012, 60, 705–711. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Smith, J.; Bishop, N.; Bullinger, L.V.; Godfrey, J.A. Pregnancy reverses hypertensive remodeling of cerebral arteries. Hypertension 2008, 51, 1052–1057. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Houston, E.M.; Kraig, R.P.; Bonney, E.A. Differential effects of low-dose endotoxin on the cerebral circulation during pregnancy. Reprod. Sci. 2011, 18, 1211–1221. [Google Scholar] [CrossRef]
- Warrington, J.P. Placental ischemia increases seizure susceptibility and cerebrospinal fluid cytokines. Physiol. Rep. 2015, 3, e12634. [Google Scholar] [CrossRef]
- Johnson, A.C.; Nagle, K.J.; Tremble, S.M.; Cipolla, M.J. The Contribution of Normal Pregnancy to Eclampsia. PLoS ONE 2015, 10, e0133953. [Google Scholar] [CrossRef]
- Alexander, B.T.; Kassab, S.E.; Miller, M.T.; Abram, S.R.; Reckelhoff, J.F.; Bennett, W.A.; Granger, J.P. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 2001, 37, 1191–1195. [Google Scholar] [CrossRef]
- Osol, G.; Brekke, J.F.; McElroy-Yaggy, K.; Gokina, N.I. Myogenic tone, reactivity, and forced dilatation: A three-phase model of in vitro arterial myogenic behavior. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2260–H2267. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Osol, G. Vascular smooth muscle actin cytoskeleton in cerebral artery forced dilatation. Stroke 1998, 29, 1223–1228. [Google Scholar] [CrossRef]
- Amburgey, O.A.; Chapman, A.C.; May, V.; Bernstein, I.M.; Cipolla, M.J. Plasma from preeclamptic women increases blood-brain barrier permeability: Role of vascular endothelial growth factor signaling. Hypertension 2010, 56, 1003–1008. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.; Hu, B.; Di, X.; Brennecke, S.P.; Liu, H. Decreased seizure threshold in an eclampsia-like model induced in pregnant rats with lipopolysaccharide and pentylenetetrazol treatments. PLoS ONE 2014, 9, e89333. [Google Scholar] [CrossRef]
- Gillis, E.E.; Williams, J.M.; Garrett, M.R.; Mooney, J.N.; Sasser, J.M. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R62–R70. [Google Scholar] [CrossRef]
- Marshall, S.A.; Hannan, N.J.; Jelinic, M.; Nguyen, T.P.H.; Girling, J.E.; Parry, L.J. Animal models of preeclampsia: Translational failings and why. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R499–R508. [Google Scholar] [CrossRef]
- Atochin, D.N.; Demchenko, I.T.; Astern, J.; Boso, A.E.; Piantadosi, C.A.; Huang, P.L. Contributions of endothelial and neuronal nitric oxide synthases to cerebrovascular responses to hyperoxia. J. Cereb. Blood Flow Metab. 2003, 23, 1219–1226. [Google Scholar] [CrossRef]
- Williams, D. Pregnancy: A stress test for life. Curr. Opin. Obstet. Gynecol. 2003, 15, 465–471. [Google Scholar] [CrossRef]
- Chasan-Taber, L. It Is Time to View Pregnancy as a Stress Test. J. Womens Health (Larchmt) 2016, 25, 2–3. [Google Scholar] [CrossRef] [Green Version]
- MacKay, A.P.; Berg, C.J.; Atrash, H.K. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet. Gynecol. 2001, 97, 533–538. [Google Scholar] [CrossRef]


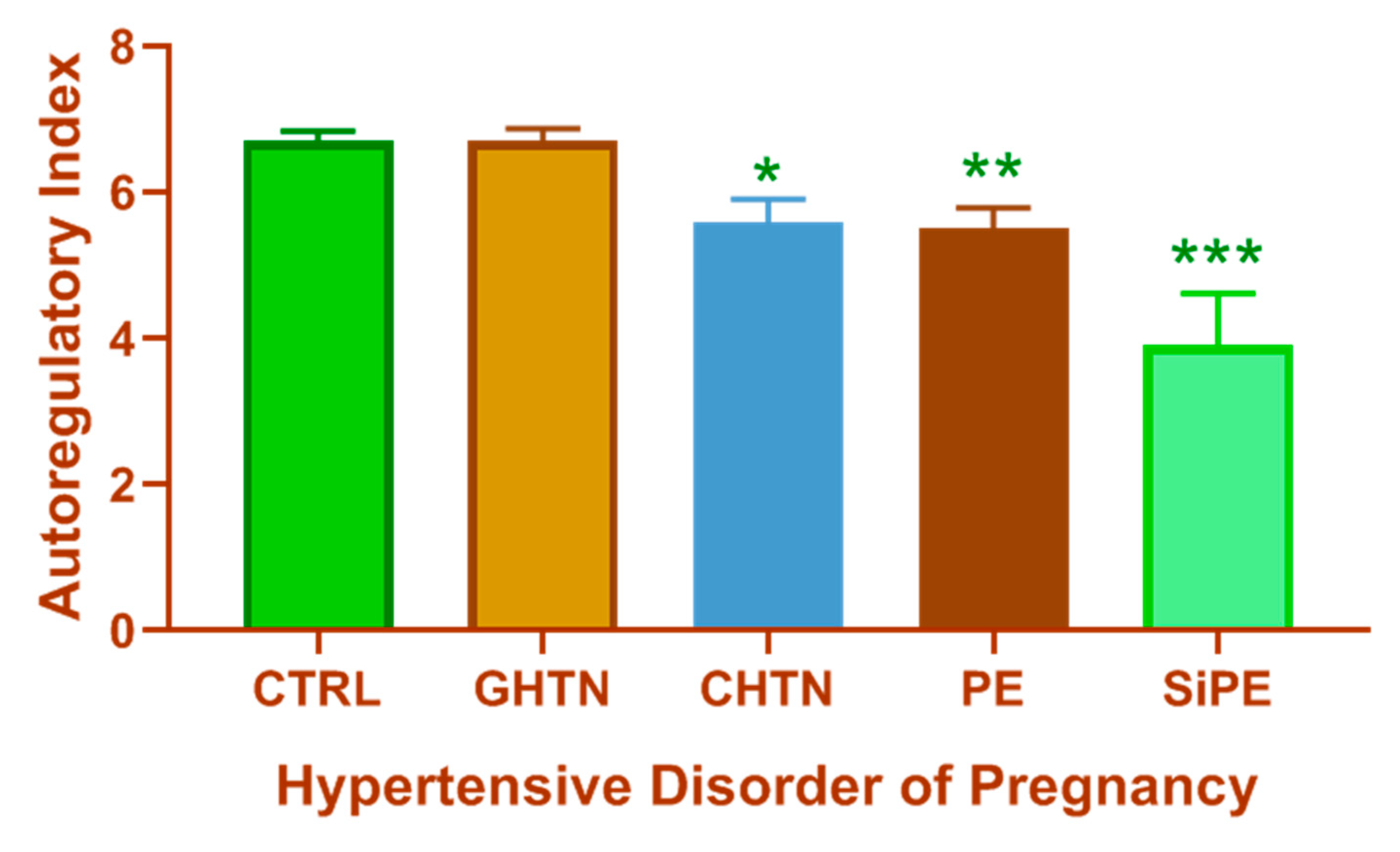
| Static CBF Autoregulation | Dynamic CBF Autoregulation | |
|---|---|---|
| What does it measure? | Steady-state CBF/MAP relationship | Transient changes in CBF in response to rapid MAP changes (CBF recovery time) |
| Autoregulatory Index range | 0–1 0 = absent autoregulation 1 = perfect autoregulation | 0–9 0 = absent autoregulation 9 = perfect autoregulation |
| Methods used to manipulate MAP | Pharmacological agents | Valsalva maneuver Lower body negative pressure Sudden posture changes Thigh-cuff deflation Rapid changes in head positioning Cold pressor stimulus Spontaneous fluctuations in MAP at rest |
| Type of studies predominantly used | Animal Studies | Clinical Studies |
| Condition Mimicked | Manipulation | Vascular Bed | CBF Response Type | CBF-Related Response | Ref. |
|---|---|---|---|---|---|
| PE | RUPP | MCA segments | Myogenic Tone | Impaired Myogenic tone in isolated vessels | [62,63] |
| PE | RUPP | MCA territory | Static CBF | Impaired CBF autoregulation | [64] |
| PE | High Cholesterol | Cortical Parenchymal Arterioles | Myogenic Tone | No change in myogenic tone. ↓ Vasodilation in PE group. | [65] |
| PE & Eclampsia | High Cholesterol + PTZ | Hippocampal Arterioles | CBF | ↑ CBF in non-pregnant and pregnant group but no change in PE group during seizure ↓ Basal tone and ↓ vasodilation in PE group. | [66] |
| PE & Eclampsia | RUPP + High Cholesterol + PTZ | PCA Territory | Relative CBF | No difference in relative CBF | [67] |
| Pregnancy | Late Pregnancy | PCA Territory | Static CBF | ↑ Vasodilation in pregnant group. CBF auto regulatory curve shifted to lower pressures. | [68] |
| PE | Acute Phenylephrine Infusion | Anterior + Posterior Cerebrum | Static CBF | Pregnancy ↑ CBF autoregulation threshold in both regions. Acute hypertension ↑ edema in both regions | [69] |
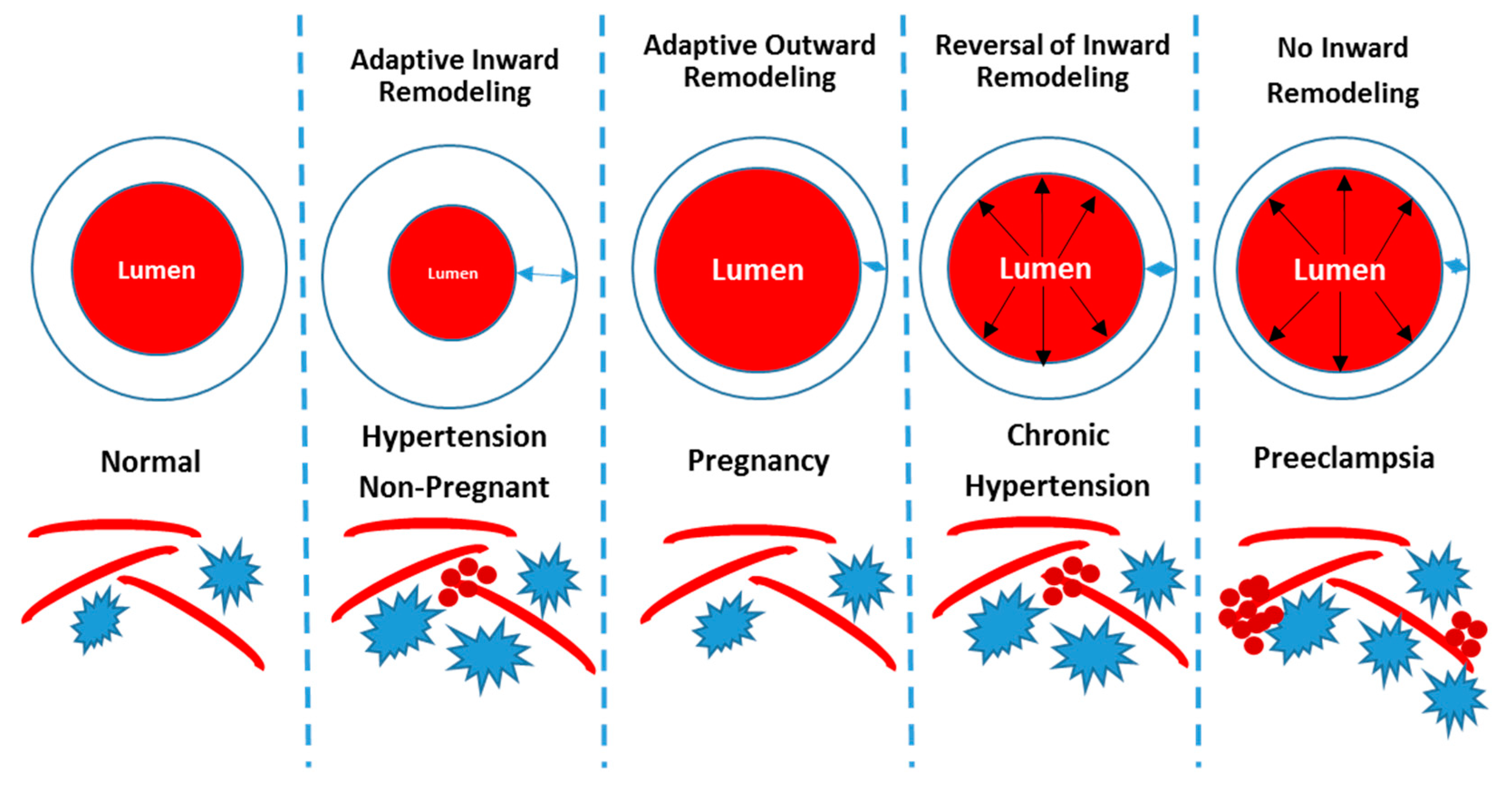
| Chronic Hypertension | L-NAME (2wks before + during gestation) | PCA segments | Vascular Remodeling | Hypertension = inward remodeling Hypertension + Pregnancy = no inward remodeling | [70] |
| PE | LPS (1.5 µg/kg) | PCA | Myogenic Tone | ↓ Myogenic tone in late pregnant LPS rats | [71] |
| Eclampsia | RUPP + PTZ | Global | Brain Water Content | ↑ Water content with seizures in normal pregnant and RUPP rats | [72] |
| Eclampsia | Prenancy + PTZ | Posterior Cerebrum | Brain Water Content | ↑ Water content in pregnant rats following seizures | [73] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones-Muhammad, M.; Warrington, J.P. Cerebral Blood Flow Regulation in Pregnancy, Hypertension, and Hypertensive Disorders of Pregnancy. Brain Sci. 2019, 9, 224. https://doi.org/10.3390/brainsci9090224
Jones-Muhammad M, Warrington JP. Cerebral Blood Flow Regulation in Pregnancy, Hypertension, and Hypertensive Disorders of Pregnancy. Brain Sciences. 2019; 9(9):224. https://doi.org/10.3390/brainsci9090224
Chicago/Turabian StyleJones-Muhammad, Maria, and Junie P. Warrington. 2019. "Cerebral Blood Flow Regulation in Pregnancy, Hypertension, and Hypertensive Disorders of Pregnancy" Brain Sciences 9, no. 9: 224. https://doi.org/10.3390/brainsci9090224
APA StyleJones-Muhammad, M., & Warrington, J. P. (2019). Cerebral Blood Flow Regulation in Pregnancy, Hypertension, and Hypertensive Disorders of Pregnancy. Brain Sciences, 9(9), 224. https://doi.org/10.3390/brainsci9090224





