Potential Antioxidant Properties of Enzymatic Hydrolysates from Stichopus japonicus against Hydrogen Peroxide-Induced Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the Enzymatic Hydrolysates from S. japonicus
2.3. Measurement of Yield and Proximate Composition
2.4. Ultrafiltration and Molecular Distribution of SJH
2.5. Amino Acid Profile
2.6. Free Radical Scavenging Activity
2.7. Cell Line and Cell Culture
2.8. Determination of Cell Viability and Intracellular ROS Generation in H2O2 Exposed Vero Cells
2.9. Detection of Apoptosis Using Propidium Iodide/Hoechst 33342 Double Fluorescent Staining
2.10. Cell Cycle Analysis by Flow Cytometry
2.11. Origin and Maintenance of Parental Zebrafish
2.12. Treatment of Zebrafish Embryos with α-chy-III
2.13. Measurement of Heart Rate and Survival Rate
2.14. Measurement of Cell Death, Intracellular ROS, and Lipid Peroxidation in H2O2 Exposed Zebrafish Embryos
2.15. Statistical Analysis
3. Results
3.1. The Yield, Chemical Composition, and Free Radical Scavenging Activities of Enzymatic Hydrolysates of S. japonicus
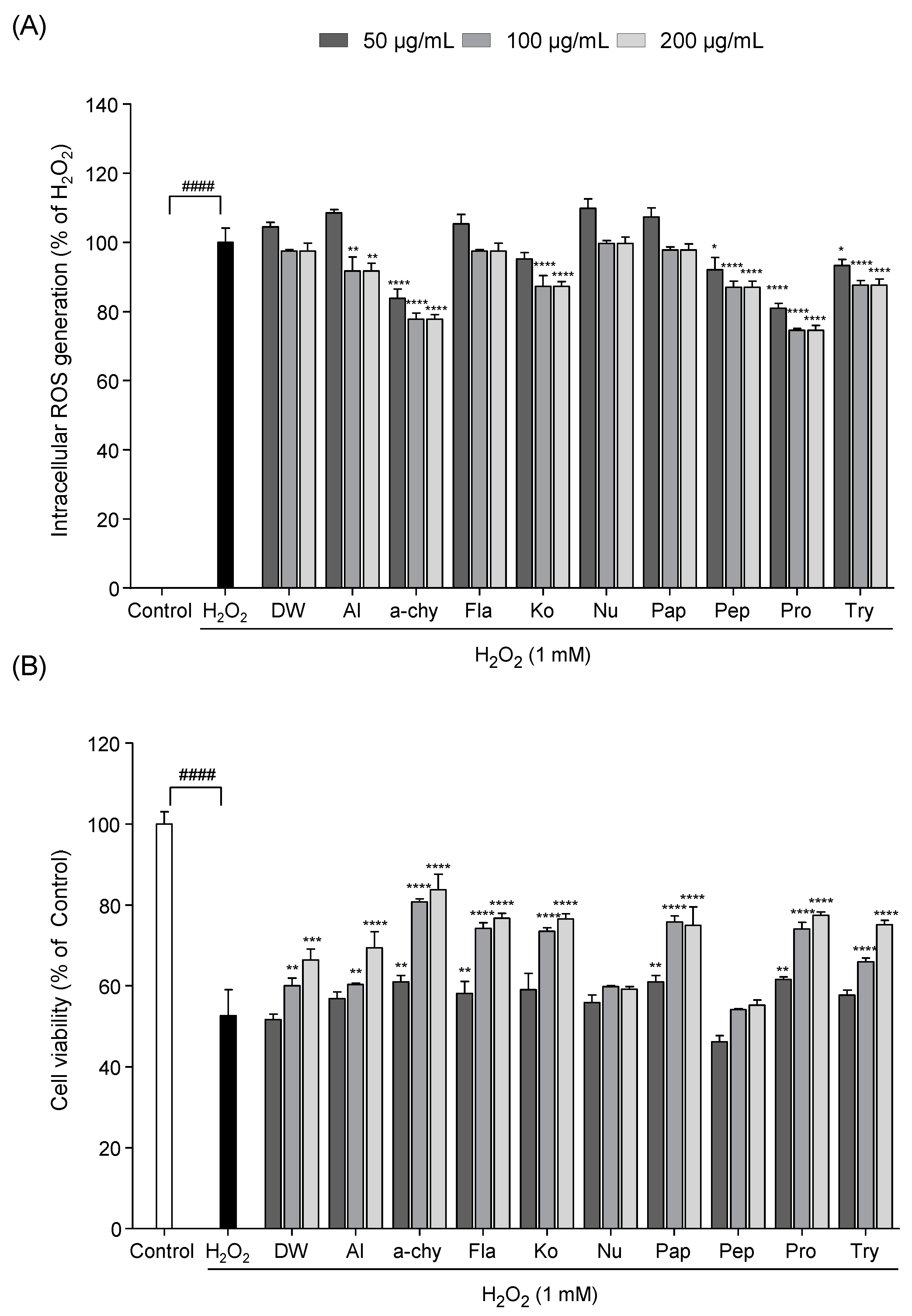
3.2. Screening of the Potential Antioxidant Effect of SJH
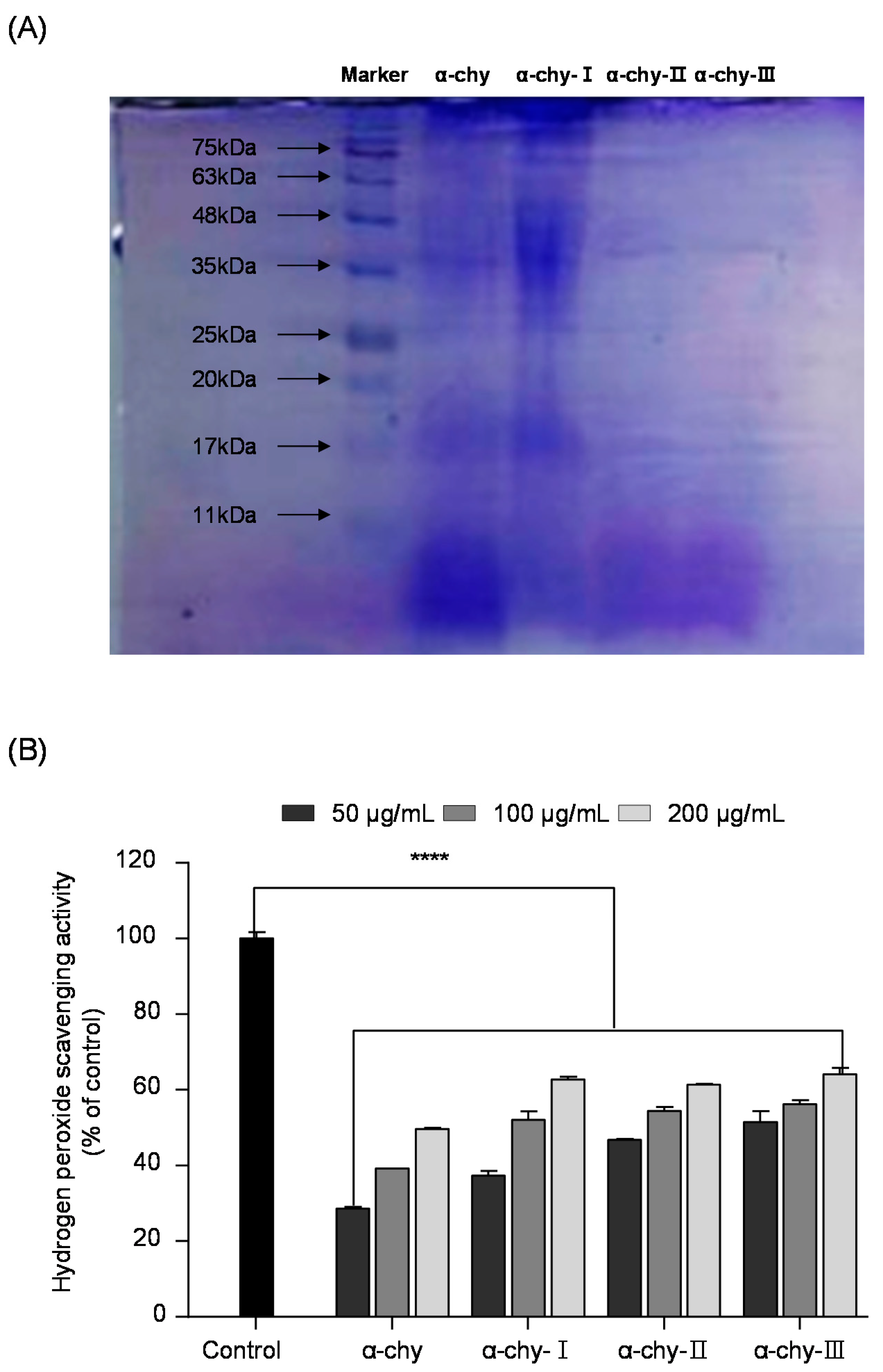
3.3. Separation and Molecular Weight Distribution of α-chy and Its UF Fractions
3.4. Amino Acid Profiles of α-chy and Its UF Fractions
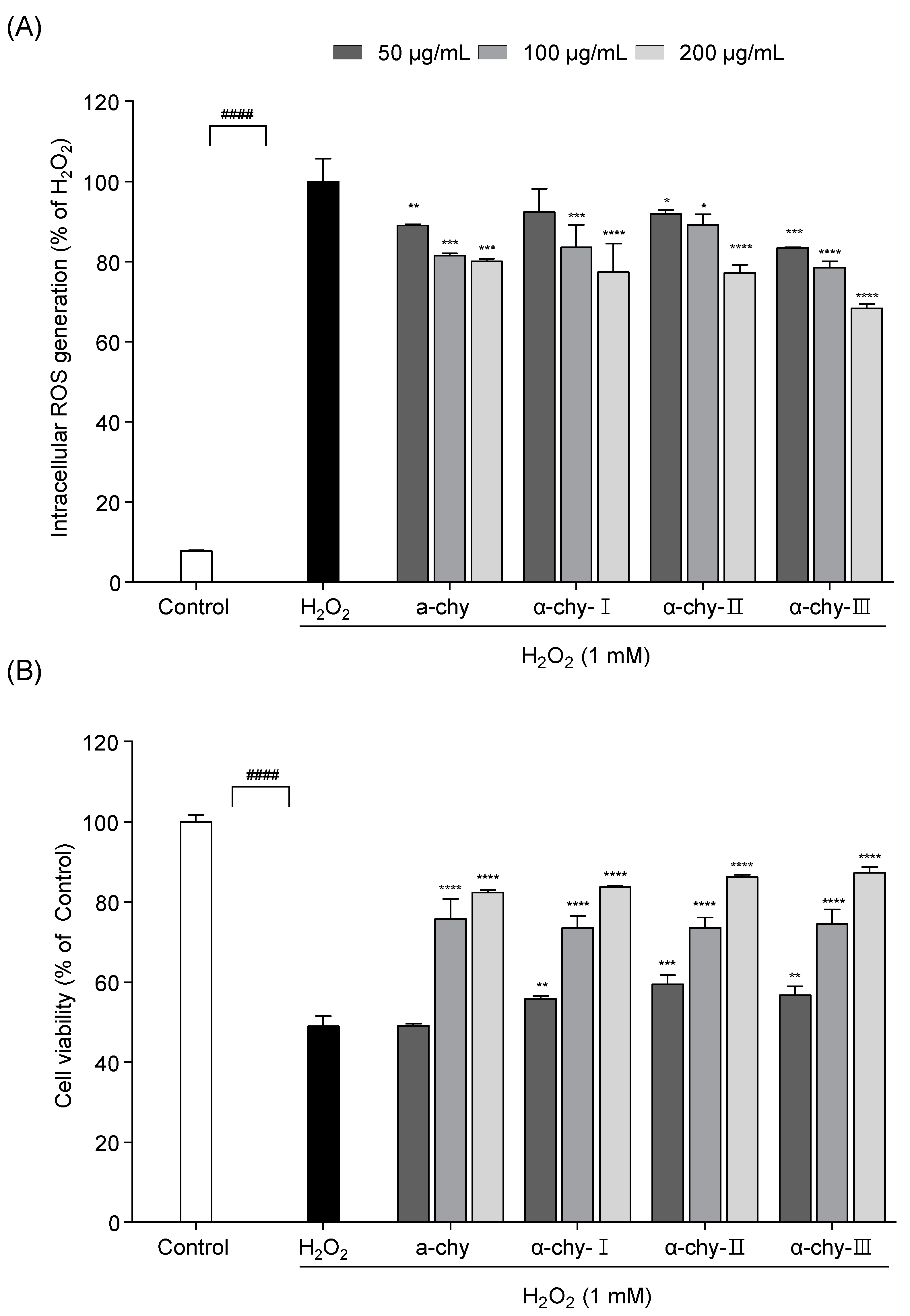
3.5. Effect of α-chy and Its UF Fractions against H2O2 Induced Oxidative Stress in Vero Cells
3.6. Effect of α-chy-III against H2O2 Induced Apoptosis in Vero Cells
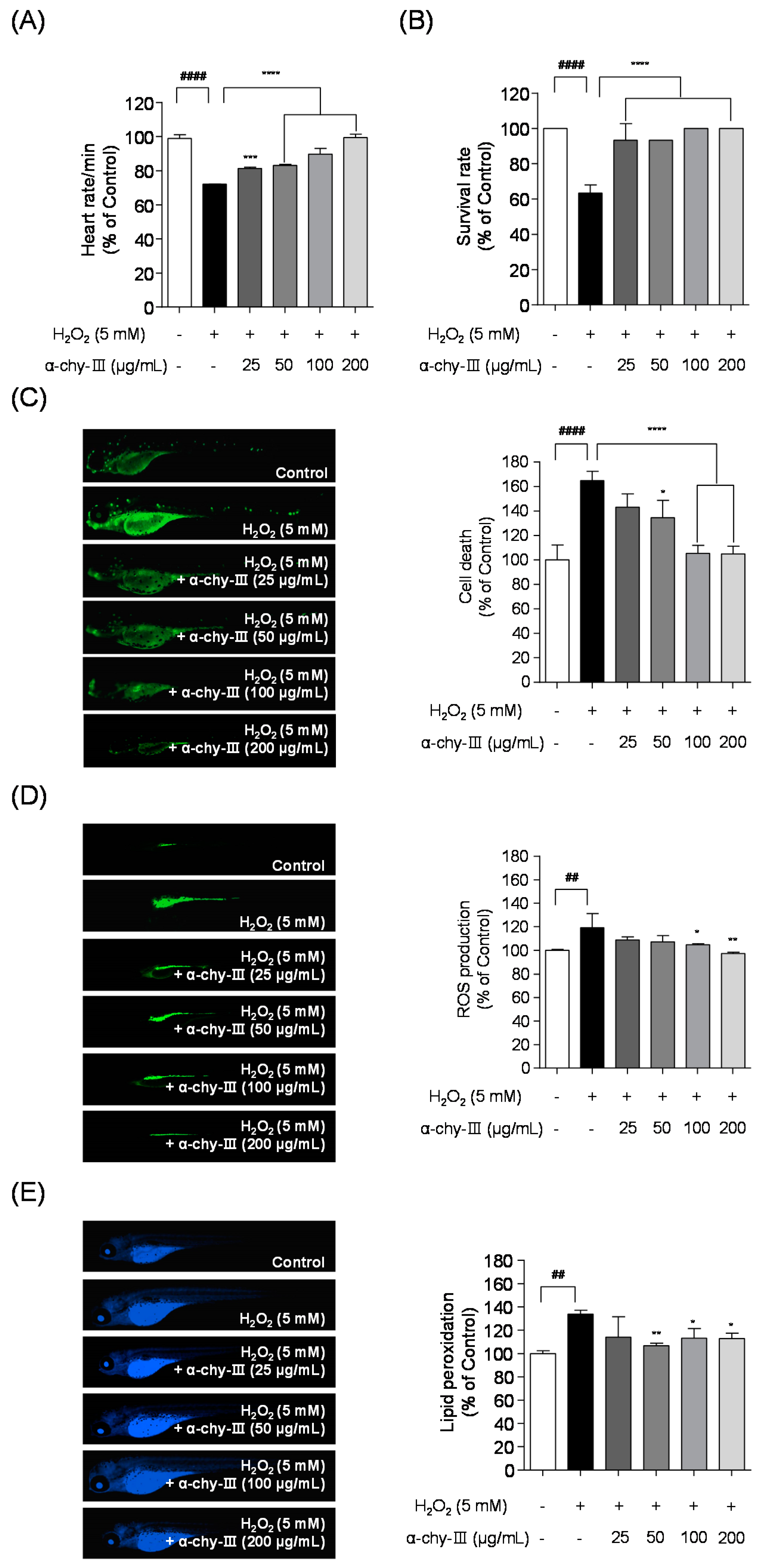
3.7. Effect of α-chy-III against H2O2 Induced Cell Death, ROS Generation, and Lipid Peroxidation in Zebrafish Enbryos
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar]
- Madkour, L.H. Function of reactive oxygen species (ROS) inside the living organisms and sources of oxidants. Pharm. Sci. Anal. Res. J. 2019, 2, 180023. [Google Scholar]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm. Unters. Forsch. 1993, 196, 329. [Google Scholar] [PubMed]
- Asanka Sanjeewa, K.K.; Lee, W.W.; Kim, J.-I.; Jeon, Y.-J. Exploiting biological activities of brown seaweed Ishige okamurae Yendo for potential industrial applications: A review. J. Appl. Phycol. 2017, 29, 3109–3119. [Google Scholar]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar]
- Yang, X.R.; Qiu, Y.T.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Purification and Characterization of Antioxidant Peptides Derived from Protein Hydrolysate of the Marine Bivalve Mollusk Tergillarca granosa. Mar. Drugs 2019, 17, 251. [Google Scholar]
- Azizah, N.; Ochiai, Y.; Nurilmala, M. Collagen Peptides from Pangasius Fish Skin as Antioxidants. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012055. [Google Scholar]
- Giannetto, A.; Esposito, E.; Lanza, M.; Oliva, S.; Riolo, K.; Di Pietro, S.; Abbate, J.M.; Briguglio, G.; Cassata, G.; Cicero, L. Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste: In Vitro and In Vivo Biological Activities. Mar. Drugs 2020, 18, 86. [Google Scholar]
- Ktari, N.; Salem, R.B.S.-B.; Bkhairia, I.; Slima, S.B.; Nasri, R.; Salah, R.B.; Nasri, M. Functional properties and biological activities of peptides from zebra blenny protein hydrolysates fractionated using ultrafiltration. Food Biosci. 2020, 34, 100539. [Google Scholar]
- Guo, Y.; Zou, X.; Chen, Y.; Wang, D.; Wang, S. Sustainability of wildlife use in traditional Chinese medicine. Conserv. China’s Biodivers. 1997, 1, 3. [Google Scholar]
- Oh, G.-W.; Ko, S.-C.; Lee, D.H.; Heo, S.-J.; Jung, W.-K. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): A review. Fish. Aquat. Sci. 2017, 20, 28. [Google Scholar] [CrossRef]
- Mills, D.J.; Duy, N.D.; Juinio-Meñez, M.A.; Raison, C.M.; Zarate, J.M. Overview of sea cucumber aquaculture and sea-ranching research in the South-East Asian region. Asia Pac. Trop. Sea Cucumber Aquac. ACIAR Proc. 2012, 136, 22–31. [Google Scholar]
- Abedin, M.Z.; Karim, A.A.; Latiff, A.A.; Gan, C.-Y.; Ghazali, F.C.; Barzideh, Z.; Ferdosh, S.; Akanda, M.J.H.; Zzaman, W.; Karim, M.R. Biochemical and radical-scavenging properties of sea cucumber (Stichopus vastus) collagen hydrolysates. Nat. Prod. Res. 2014, 28, 1302–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.-T.; Zhao, C.-C.; Han, J.-R.; Yan, J.-N.; Du, Y.-N.; Shang, W.-H.; Wu, H.-T. Antioxidant activity of sea cucumber (Stichopus japonicus) gut hydrolysates-ribose Maillard reaction products derived from organic reagent extraction. J. Food Meas. Charact. 2019, 13, 2790–2797. [Google Scholar] [CrossRef]
- Popov, A.; Artyukov, A.; Glazunov, V.; Mandron, E.; Krivoshapko, O.; Kozlovskaya, E. Antitumor and anticoagulant activities of collagen protein from the holothurian Apostichopus japonicas modified by proteolytic enzymes. Russ. J. Mar. Biol. 2011, 37, 217–222. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tang, Q.; Wang, Y.; Chang, Y.; Zhao, Q.; Xue, C. Antioxidation activities of low-molecular-weight gelatin hydrolysate isolated from the sea cucumber Stichopus japonicus. J. Ocean Univ. China 2010, 9, 94–98. [Google Scholar] [CrossRef]
- Byun, H.-G.; Kim, S.-K. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process. Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Chandler, S.F.; Dodds, J.H. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of solanum laciniatum. Plant Cell Rep. 1983, 2, 205–208. [Google Scholar] [CrossRef]
- Saito, H.; Yamagata, T.; Suzuki, S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J. Biol. Chem. 1968, 243, 1536–1542. [Google Scholar] [CrossRef]
- Asaduzzaman, A.; Chun, B.-S. Recovery of functional materials with thermally stable antioxidative properties in squid muscle hydrolyzates by subcritical water. J. Food Sci. Technol. 2015, 52, 793–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Huang, K.; Yang, X.; Xu, H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta 1999, 1472, 643–650. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Kim, S.Y.; Kim, Y.T.; Kim, E.A.; Lee, S.H.; Ko, S.C.; Wijesinghe, W.A.; Samarakoon, K.W.; Kim, Y.S.; Cho, J.H.; et al. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr. Polym. 2014, 99, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57–63. [Google Scholar] [CrossRef]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A microplate assay for the detection of oxidative products using 2′, 7′-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef]
- Agarwal, A.; Kasinathan, A.; Ganesan, R.; Balasubramanian, A.; Bhaskaran, J.; Suresh, S.; Srinivasan, R.; Aravind, K.B.; Sivalingam, N. Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species-independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells. Nutr. Res. 2018, 51, 67–81. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.; Jeon, Y.J.; Ramasamy, P.; Wahid, M.E.; Vairappan, C.S. Anticancer activity and mediation of apoptosis in human HL-60 leukaemia cells by edible sea cucumber (Holothuria edulis) extract. Food Chem. 2013, 139, 326–331. [Google Scholar] [CrossRef]
- Kang, M.-C.; Kim, K.-N.; Kang, S.-M.; Yang, X.; Kim, E.-A.; Song, C.B.; Nah, J.-W.; Jang, M.-K.; Lee, J.-S.; Jung, W.-K. Protective effect of dieckol isolated from Ecklonia cava against ethanol caused damage in vitro and in zebrafish model. Environ. Toxicol. Pharmacol. 2013, 36, 1217–1226. [Google Scholar] [CrossRef]
- Toral-Granda, V.; Lovatelli, A.; Vasconcellos, M. Sea Cucumbers: A Global Review of Fisheries and Trade; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008; Volume 516. [Google Scholar]
- Hamel, J.-F.; Mercier, A. Synchronous gamete maturation and reliable spawning induction method in holothurians. FAO Fish. Tech. Pap. 2005, 3, 359–372. [Google Scholar]
- Zhou, X.; Wang, C.; Jiang, A. Antioxidant peptides isolated from sea cucumber Stichopus japonicus. Eur. Food Res. Technol. 2012, 234, 441–447. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, H.-T.; Zhu, B.-W.; Dong, X.-P.; Zhang, M.-M.; Li, Y.-L. Identification of antioxidative oligopeptides derived from autolysis hydrolysates of sea cucumber (Stichopus japonicus) guts. Eur. Food Res. Technol. 2012, 234, 895–904. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Wang, F.; Xing, C. A zebrafish (Danio rerio) model for high-throughput screening food and drugs with uric acid-lowering activity. Biochem. Biophys. Res. Commun. 2019, 508, 494–498. [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Meshalkina, D.A.; Petrov, D.V.; Okovityi, S.V.; Musienko, P.E.; Kalueff, A.V. Pharmacological screening of a new alpha-2 adrenergic receptor agonist, mafedine, in zebrafish. Neurosci. Lett. 2019, 701, 234–239. [Google Scholar] [CrossRef]
- Falcão, M.A.P.; de Souza, L.S.; Dolabella, S.S.; Guimarães, A.G.; Walker, C.I.B. Zebrafish as an alternative method for determining the embryo toxicity of plant products: A systematic review. Environ. Sci. Pollut. Res. 2018, 25, 35015–35026. [Google Scholar] [CrossRef]
- Eimon, P.M.; Rubinstein, A.L. The use of in vivo zebrafish assays in drug toxicity screening. Expert Opin. Drug Metab. Toxicol. 2009, 5, 393–401. [Google Scholar] [CrossRef]
- Deveau, A.P.; Bentley, V.L.; Berman, J.N. Using zebrafish models of leukemia to streamline drug screening and discovery. Exp. Hematol. 2017, 45, 1–9. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Mosafa, L.; Moghadam, M.; Shahedi, M. Papain enzyme supported on magnetic nanoparticles: Preparation, characterization and application in the fruit juice clarification. Chin. J. Catal. 2013, 34, 1897–1904. [Google Scholar] [CrossRef]
- Kang, M.-C.; Lee, H.; Choi, H.-D.; Jeon, Y.-J. Antioxidant properties of a sulfated polysaccharide isolated from an enzymatic digest of Sargassum thunbergii. Int. J. Biol. Macromol. 2019, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, S.Y.; Fernando, I.S.; Sanjeewa, K.A.; Wang, L.; Lee, S.H.; Ko, S.C.; Kang, M.C.; Jayawardena, T.U.; Jeon, Y.J. Free radical scavenging activity of the peptide from the Alcalase hydrolysate of the edible aquacultural seahorse (Hippocampus abdominalis). J. Food Biochem. 2019, 43, e12833. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Han, L.; Hong, H.; Pan, J.; Liu, H.; Luo, Y. Purification and identification of novel antioxidant peptides from silver carp muscle hydrolysate after simulated gastrointestinal digestion and transepithelial transport. Food Chem. 2020, 2, 128275. [Google Scholar] [CrossRef]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.; Kang, S.I.; Lee, J.-S.; Jeon, Y.-J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Bai, M.; Wang, L.; Liu, H.; Xu, K.; Deng, J.; Huang, R.; Yin, Y. Imbalanced dietary methionine-to-sulfur amino acid ratio can affect amino acid profiles, antioxidant capacity, and intestinal morphology of piglets. Anim. Nutr. 2020, 6, 447–456. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. Cytoprotective antioxidant function of tyrosine and tryptophan residues in transmembrane proteins. Eur. J. Biochem. 2000, 267, 5687–5692. [Google Scholar] [CrossRef] [Green Version]
- Guru, A.; Lite, C.; Freddy, A.J.; Issac, P.K.; Pasupuleti, M.; Saraswathi, N.; Arasu, M.V.; Al-Dhabi, N.A.; Arshad, A.; Arockiaraj, J. Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Dev. Comp. Immunol. 2020, 114, 103863. [Google Scholar] [CrossRef]
- Jing, H.; Kitts, D.D. Antioxidant activity of sugar–lysine Maillard reaction products in cell free and cell culture systems. Arch. Biochem. Biophys. 2004, 429, 154–163. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Q.; Wang, Y.; Qin, D.; Luo, Q.; Zhang, H. Self-assembly, rheological properties and antioxidant activities of chitosan grafted with tryptophan and phenylalanine. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124763. [Google Scholar] [CrossRef]

| Sample | Yield (%) | Proximate Composition (%) | ||
|---|---|---|---|---|
| Polysaccharide | Protein | Sulfate | ||
| DW | 43.00 ± 0.01 | 3.18 ± 0.23 | 15.60 ± 0.69 | 7.41 ± 0.03 |
| Al | 42.50 ± 0.01 | 9.35 ± 2.98 *** | 26.19 ± 1.04 *** | 6.06 ± 0.04 |
| α-chy | 96.50 ± 0.06 *** | 11.95 ± 2.07 *** | 34.05 ± 0.97 *** | 5.64 ± 0.08 |
| Fla | 68.50 ± 0.04 *** | 9.35 ± 0.69 *** | 36.15 ± 1.59 *** | 6.58 ± 0.00 |
| Koj | 55.00 ± 0.02 *** | 13.08 ± 5.51 *** | 27.51 ± 1.66 *** | 5.99 ± 0.17 |
| Neu | 55.50 ± 0.01 *** | 13.08 ± 0.46 *** | 31.41 ± 1.38 *** | 5.55 ± 0.13 |
| Pap | 77.50 ± 0.05 *** | 11.46 ± 0.46 *** | 32.05 ± 1.45 *** | 5.64 ± 0.08 |
| Pep | 62.50 ± 0.07 *** | 8.21 ± 1.38 | 35.27 ± 2.97 *** | 4.69 ± 0.08 |
| Pro | 78.50 ± 0.02 *** | 11.46 ± 0.92 *** | 28.58 ± 1.24 *** | 5.58 ± 0.17 |
| Try | 82.00 ± 0.01 *** | 10.64 ± 3.44 *** | 36.24 ± 2.55 *** | 6.14 ± 0.04 |
| Sample | Free Radical Scavenging Activity IC 50 Value, (mg/mL) | ||
|---|---|---|---|
| DPPH | Alkyl | Hydroxyl | |
| DW | 2.94 ± 0.06 | 0.39 ± 0.06 | 1.59 ± 0.02 |
| Al | 3.69 ± 0.04 | 0.38 ± 0.04 | 1.63 ± 0.18 |
| α-chy | 3.37 ± 0.19 | 0.39 ± 0.01 | 1.03 ± 0.26 * |
| Fla | 4.40 ± 0.43 | 0.34 ± 0.02 | 1.40 ± 0.04 |
| Koji | 2.97 ± 0.51 | 0.44 ± 0.02 | 1.27 ± 0.09 |
| Neu | 5.31 ± 0.30 | 0.40 ± 0.02 | 2.96 ± 0.07 |
| Pap | 4.99 ± 0.00 | 0.40 ± 0.01 | 1.47 ± 0.08 |
| Pep | 3.89 ± 0.75 | 0.48 ± 0.03 | 3.46 ± 0.34 |
| Pro | 3.21 ± 0.42 | 0.42 ± 0.04 | 1.12 ± 0.07 * |
| Try | 3.83 ± 0.14 | 0.38 ± 0.06 | 1.59 ± 0.01 |
| Amino Acid | α-chy | α-chy-I | α-chy-II | α-chy-III |
|---|---|---|---|---|
| Aspartic acid | 11.32 | 11.61 | 9.85 | 8.84 |
| Threonine | 5.40 | 5.29 | 4.92 | 4.55 |
| Serine | 5.35 | 4.96 | 4.93 | 4.81 |
| Glutamic acid | 16.91 | 15.91 | 15.96 | 14.54 |
| Proline | 1.79 | 8.53 | 9.75 | 9.79 |
| Glycine | 17.28 | 16.81 | 18.16 | 18.36 |
| Alanine | 8.01 | 6.93 | 7.46 | 7.03 |
| Valine | 3.97 | 4.04 | 3.04 | 2.87 |
| Methionine | 2.10 | 1.55 | 1.66 | 2.23 |
| Isoleucine | 3.30 | 3.42 | 2.62 | 2.50 |
| Leucine | 3.40 | 3.38 | 2.69 | 2.79 |
| Tyrosine | 3.06 | 2.15 | 2.89 | 3.60 |
| Phenylalanine | 3.39 | 2.46 | 2.83 | 3.60 |
| Histidine | 2.78 | 3.60 | 1.47 | 1.54 |
| Lysine | 3.60 | 2.78 | 3.48 | 4.10 |
| Arginine | 8.34 | 6.59 | 8.28 | 8.86 |
| Total | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-G.; Kim, H.-S.; Oh, J.-Y.; Lee, D.-S.; Yang, H.-W.; Kang, M.-C.; Kim, E.-A.; Kang, N.; Kim, J.; Heo, S.-J.; et al. Potential Antioxidant Properties of Enzymatic Hydrolysates from Stichopus japonicus against Hydrogen Peroxide-Induced Oxidative Stress. Antioxidants 2021, 10, 110. https://doi.org/10.3390/antiox10010110
Lee H-G, Kim H-S, Oh J-Y, Lee D-S, Yang H-W, Kang M-C, Kim E-A, Kang N, Kim J, Heo S-J, et al. Potential Antioxidant Properties of Enzymatic Hydrolysates from Stichopus japonicus against Hydrogen Peroxide-Induced Oxidative Stress. Antioxidants. 2021; 10(1):110. https://doi.org/10.3390/antiox10010110
Chicago/Turabian StyleLee, Hyo-Geun, Hyun-Soo Kim, Jae-Young Oh, Dae-Sung Lee, Hye-Won Yang, Min-Cheol Kang, Eun-A Kim, Nalae Kang, Junseong Kim, Soo-Jin Heo, and et al. 2021. "Potential Antioxidant Properties of Enzymatic Hydrolysates from Stichopus japonicus against Hydrogen Peroxide-Induced Oxidative Stress" Antioxidants 10, no. 1: 110. https://doi.org/10.3390/antiox10010110
APA StyleLee, H.-G., Kim, H.-S., Oh, J.-Y., Lee, D.-S., Yang, H.-W., Kang, M.-C., Kim, E.-A., Kang, N., Kim, J., Heo, S.-J., & Jeon, Y.-J. (2021). Potential Antioxidant Properties of Enzymatic Hydrolysates from Stichopus japonicus against Hydrogen Peroxide-Induced Oxidative Stress. Antioxidants, 10(1), 110. https://doi.org/10.3390/antiox10010110






