Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins
Abstract
:1. Introduction
1.1. The Human Eye
1.2. Reactive Oxygen Species within the Eye
2. Antioxidant Defense Systems in the Eye
2.1. Enzymatic Antioxidants of the Ocular Surface
2.1.1. Primary Antioxidant Enzymes
2.1.2. Secondary Antioxidant Enzymes
2.2. Enzymatic Antioxidants of the Lens
2.3. Enzymatic Antioxidants of the Retina
3. Metallothionein Antioxidant System
3.1. General Properties
3.1.1. Classification
3.1.2. Functions
3.1.3. The Zn-MT Redox Cycle as an Antioxidant Defense Mechanism
3.1.4. MTs Regulation
- Article I.
- Metal response elements (MRE): The MTF-1 transcription factor is a central regulator of the metal inducible expression levels of MT1 and MT2. The binding of zinc to MTF-1 enables its union to MREs in the promoter region, which initiates gene transcription [146,147]. Heavy metal ions like Zn, Cu, Cd or Hg, as well as hypoxia, oxidative stress, glucocorticoids, nitric oxide and high temperature induce the transcriptional activity of MTF-1 [152,153,154,155].
- Article II.
- Article III.
- Article IV.
- STAT-binding sites: Elements activated by signal transducers and activators of transcription (STAT) proteins mediate transcriptional responses to cytokines [162]. Pro-inflammatory cytokines secreted by the activated macrophages during acute inflammation induce the expression of MTs [163]. STAT proteins convert the cytokine signal into gene expression programs regulating the inflammatory response in different cell types [164,165] and, in a cooperative mechanism of negative feedback, MTs inhibit the release of pro-inflammatory cytokines [166,167].
- Article V.
3.2. Oxidative Stress, Age-Related Diseases and MTs in the Human Eye
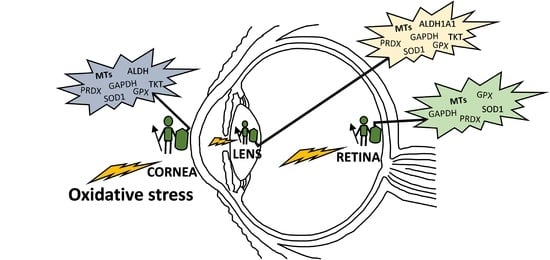
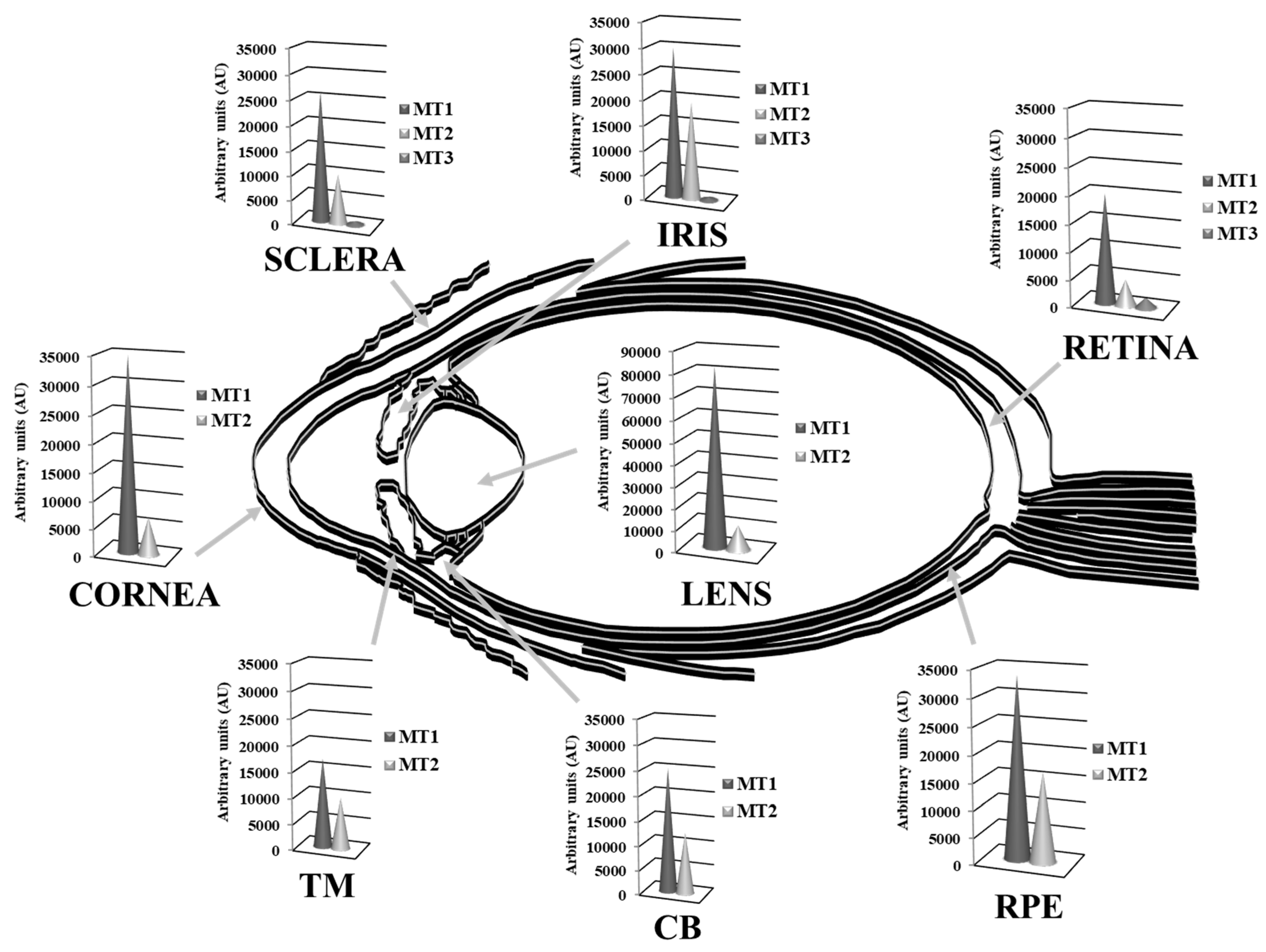
3.2.1. Antioxidant Activity of MTs in the Ocular Surface
3.2.2. Antioxidant Activity of MTs in the Lens
3.2.3. Antioxidant Activity of MTs in the Retina-RPE
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5HT1a | 5-hydroxy-tryptophan 1a |
| 8-OH-DPAT | 8-hydroxy-2-(di-n-propylamino)-tetralin |
| A2E | N-retinylidene-N-retinylethanolamine |
| AAPH | 2,2′-azobis(2-methylpropionamidine) dihydrochloride |
| AHR | Aryl hydrocarbon receptor |
| ALDH | Aldehyde dehydrogenase |
| AMD | Age-related macular degeneration |
| AP-1 | Activator proteins 1 |
| AP-2 | Activator proteins 2 |
| ARE | Antioxidant response element |
| AU | Arbitrary units |
| C/EBPα | CCAAT/enhancer-binding protein alpha |
| CAT | Catalase |
| CB | Ciliary body |
| CNS | Central nervous system |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GADPH | Glyceraldehyde-3-phosphate dehydrogenase |
| GPX | Glutathione peroxidase |
| GREs | Glucocorticoid response elements |
| GSH | Glutathione |
| GSR | Glutathione reductase |
| GSS | Glutathione synthetase |
| GSSG | Glutathione disulfide |
| HNE | 4-hydroxynonenal |
| HO1 | Heme oxygenase 1 |
| HRPEsv | Human RPE cells |
| HSP70 | Heat shock protein 70 |
| IL1α | Interleukin-1α |
| INL | Inner nuclear layer |
| LRP-2 | Membrane receptor protein megalin |
| MPP+ | 1-methyl-4-phenylpyridinium ion |
| MRE | Metal response elements |
| MTF-1 | Metal regulatory transcription factor 1 |
| MTs | Metallothioneins |
| NF-1 | Nuclear factor 1 |
| NFL | Nerve fiber layer |
| NMDA | N-methyl-D-aspartate |
| NqO1 | NAD(P)H dehydrogenase quinone 1 |
| O2· | Superoxide anion |
| ONL | Outer nuclear layer |
| PPP | Pentose phosphate pathway |
| PRDXs | Peroxiredoxins |
| PZ120 | 120-kilodalton zinc finger protein |
| RGC | Retinal ganglion cell |
| RNS | Reactive nitrogen species |
| ROM1 | Retinal outer segment membrane protein 1 |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SEL | Selenoproteins |
| SOD | Superoxide dismutase |
| SRXN | Sulfiredoxin |
| STAT | Signal transducers and activators of transcription |
| TBP | TATA-binding protein |
| TFIID | Transcription factor II D |
| TKT | Transketolase |
| TM | Trabecular meshwork |
| TXN | Thioredoxin |
| UV | Ultraviolet |
| USF1 | Upstream stimulatory factor 1 |
| WT | Wild-type |
References
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F. The Eye, Basic Sciences in Practice, 3rd ed.; Saunders Elsevier: Edinburgh, UK, 2008; Volume 540, ISBN 978-0702028410. [Google Scholar]
- Levin, L.; Nilsson, S.; Ver Hoeve, J.; Wu, S.; Kaufman, P.; Alm, A. Adler’s Physiology of the Eye, 11th ed.; Saunders Elsevier: St. Louis/Mosby, MO, USA, 2011; ISBN 9780323057141. [Google Scholar]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J.L.; Liew, G.; White, A.J.R. Oxidative stress and reactive oxygen species: A review of their role in ocular disease. Clin. Sci. 2017, 131, 2865–2883. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.P.; Chihuailaf, R.H. Antioxidants and the integrity of ocular tissues. Vet. Med. Int. 2011, 905153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojcik, K.A.; Kaminska, A.; Blasiak, J.; Szaflik, J.; Szaflik, J.P. Oxidative stress in the pathogenesis of keratoconus and Fuchs endothelial corneal dystrophy. Int. J. Mol. Sci. 2013, 14, 19294–19308. [Google Scholar] [CrossRef] [Green Version]
- Vinson, J.A. Oxidative stress in cataracts. Pathophysiology 2006, 13, 151–162. [Google Scholar] [CrossRef]
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.B. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010, 44, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Varma, S.D.; Chand, D.; Sharma, Y.R.; Kuck, J.F., Jr.; Richards, R.D. Oxidative stress on lens and cataract formation: Role of light and oxygen. Curr. Eye Res. 1984, 3, 35–57. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 16, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Sci. Rep. 2016, 6, 25792. [Google Scholar] [CrossRef] [Green Version]
- Beatty, S.; Koh, H.H.; Phil, M.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Ankamah, E.; Sebag, J.; Ng, E.; Nolan, J.M. Vitreous Antioxidants, Degeneration, and Vitreo-Retinopathy: Exploring the Links. Antioxidants 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, L.; Gonzalez-Iglesias, H.; Garcia, M.; Ghosh, S.; Sanz-Medel, A.; Coca-Prados, M. The stoichiometric transition from Zn6Cu1-metallothionein to Zn7-metallothionein underlies the up-regulation of metallothionein (MT) expression: Quantitative analysis of MT-metal load in eye cells. J. Biol. Chem. 2012, 287, 28456–28469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchitti, S.A.; Chen, Y.; Thompson, D.C.; Vasiliou, V. Ultraviolet radiation: Cellular antioxidant response and the role of ocular aldehyde dehydrogenase enzymes. Eye Contact Lens 2011, 37, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Brennan, L.A.; McGreal, R.S.; Kantorow, M. Oxidative stress defense and repair systems of the ocular lens. Front. Biosci. 2012, 4, 141–155. [Google Scholar] [CrossRef]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Saccà, S.C.; Roszkowska, A.M.; Izzotti, A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat. Res. 2013, 752, 153–171. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Laher, I. Systems Biology of Free Radicals and Antioxidants; Springer: Heidelberg/Berlin, Germany, 2014; Chapter LXI; p. 4178. ISBN 978-3-642-30017-2. [Google Scholar]
- Hammond, B.R.; Johnson, B.A.; George, E.R. Oxidative photodegradation of ocular tissues: Beneficial effects of filtering and exogenous antioxidants. Exp. Eye Res. 2014, 129, 135–150. [Google Scholar] [CrossRef] [Green Version]
- Lassen, N.; Black, W.J.; Estey, T.; Vasiliou, V. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Semin. Cell. Dev. Biol. 2008, 19, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.G.; Zhu, J.H.; Cheng, W.H.; Bao, Y.; Ho, Y.S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2016, 96, 307–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, A.W.; Maldonado, M.; Sanchez-Hidalgo, M.; Tan, D.X.; Reiter, R.J. Protective effects of melatonin in experimental free radical-related ocular diseases. J. Pineal Res. 2006, 40, 101–109. [Google Scholar] [CrossRef]
- Brennan, L.A.; Kantorow, M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other repair systems in cataract and macular degenerations. Exp. Eye. Res. 2009, 88, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Babizhayev, M.A.; Yegorov, Y.E. Reactive Oxygen Species and the Aging Eye: Specific Role of Metabolically Active Mitochondria in Maintaining Lens Function and in the Initiation of the Oxidation-Induced Maturity Onset Cataract—A Novel Platform of Mitochondria-Targeted Antioxidants With Broad Therapeutic Potential for Redox Regulation and Detoxification of Oxidants in Eye Diseases. Am. J. Ther. 2016, 23, e98–e117. [Google Scholar] [CrossRef] [PubMed]
- Ohia, S.E.; Opere, C.A.; Leday, A.M. Pharmacological consequences of oxidative stress in ocular tissues. Mutat. Res. 2005, 579, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Glutathione peroxidases and redox-regulated transcription factors. Biol. Chem. 2006, 387, 1329–1335. [Google Scholar] [CrossRef]
- Ma, W.; Kleiman, N.J.; Sun, F.; Li, D.; Spector, A. Peroxide toxicity in conditioned lens epithelial cells—Evaluation of multi-defense systems. Exp. Eye Res. 2003, 77, 711–720. [Google Scholar] [CrossRef]
- Detienne, G.; De Haes, W.; Mergan, L.; Edwards, S.L.; Temmerman, L.; Van Bael, S. beyond ROS clearance: Peroxiredoxins in stress signaling and aging. Ageing Res. Rev. 2018, 44, 33–48. [Google Scholar] [CrossRef]
- Piatigorsky, J. Gene Sharing and Evolution the Diversity of Protein Functions; Harvard University Press: Cambridge, UK, 2007; Chapter XV; p. 320. ISBN 978-0674023413. [Google Scholar]
- Cooper, D.L.; Isola, N.R.; Stevenson, K.; Baptist, E.W. Members of the ALDH gene family are lens and corneal crystallins. Adv. Exp. Med. Biol. 1993, 328, 169–179. [Google Scholar] [CrossRef]
- Sax, C.M.; Kays, W.T.; Salamon, C.; Chervenak, M.M.; Xu, Y.S.; Piatigorsky, J. Transketolase gene expression in the cornea is influenced by environmental factors and developmentally controlled events. Cornea 2000, 19, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Lampi, K.J.; Wilmarth, P.A.; Murray, M.R.; David, L.L. Lens β-crystallins: The role of deamidation and related modifications in aging and cataract. Prog. Biophys. Mol. Biol. 2014, 115, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anbarasu, K.; Sivakumar, J. Multidimensional significance of crystallin protein-protein interactions and their implications in various human diseases. Biochim. Biophys. Acta 2016, 1860, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Thompson, D.C.; Koppaka, V.; Jester, J.V.; Vasiliou, V. Ocular aldehyde dehydrogenases: Protection against ultraviolet damage and maintenance of transparency for vision. Prog. Retin. Eye Res. 2013, 33, 28–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estey, T.; Piatigorsky, J.; Lassen, N.; Vasiliou, V. ALDH3A1: A corneal crystallin with diverse functions. Exp. Eye Res. 2007, 84, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Voulgaridou, G.P.; Tsochantaridis, I.; Tolkas, C.; Franco, R.; Giatromanolaki, A.; Panayiotidis, M.I.; Pappa, A. Aldehyde dehydrogenase 3A1 confers oxidative stress resistance accompanied by altered DNA damage response in human corneal epithelial cells. Free Radic. Biol. Med. 2020, 150, 66–74. [Google Scholar] [CrossRef]
- Xu, I.M.; Lai, R.K.; Lin, S.H.; Tse, A.P.; Chiu, D.K.; Koh, H.Y.; Law, C.T.; Wong, C.M.; Cai, Z.; Wong, C.C.; et al. Transketolase counteracts oxidative stress to drive cancer development. Proc. Natl. Acad. Sci. USA 2016, 113, E725–E734. [Google Scholar] [CrossRef] [Green Version]
- Romi, F.; Helgeland, G.; Gilhus, N.E. Heat-shock proteins in clinical neurology. Eur. Neurol. 2011, 66, 65–69. [Google Scholar] [CrossRef]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet radiation oxidative stress affects eye health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef] [Green Version]
- Stagos, D.; Chen, Y.; Cantore, M.; Jester, J.V.; Vasiliou, V. Corneal aldehyde dehydrogenases: Multiple functions and novel nuclear localization. Brain Res. Bull. 2010, 81, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Piatigorsky, J. Lens and cornea: The “refracton hypothesis”. Semin. Cell. Dev. Biol. 2008, 19, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, V.M.; Beyer, E.C. Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox Signal. 2009, 11, 339–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crouch, R.K.; Patrick, J.; Goosey, J.; Coles, W.H. The effect of age on corneal and lens superoxide dismutase. Curr. Eye Res. 1984, 3, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.; Vasavada, A.R.; Praveen, M.R.; Ananthan, R.; Reddy, G.B.; Tripathi, H.; Ganatra, D.A.; Arora, A.I.; Patel, A.R. Exploration of molecular factors impairing superoxide dismutase isoforms activity in human senile cataractous lenses. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6224–6233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, N.C.; Harris, D.L.; Zhu, C.C. Age-related gene response of human corneal endothelium to oxidative stress and DNA damage. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1641–1649. [Google Scholar] [CrossRef] [Green Version]
- Reddan, J.R.; Steiger, C.A.; Dziedzic, D.C.; Gordon, S.R. Regional differences in the distribution of catalase in the epithelium of the ocular lens. Cell. Mol. Biol. 1996, 42, 209–219. [Google Scholar]
- Sepasi Tehrani, H.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Wahlig, S.; Lovatt, M.; Mehta, J.S. Functional role of peroxiredoxin 6 in the eye. Free Radic. Biol. Med. 2018, 126, 210–220. [Google Scholar] [CrossRef]
- Shibata, S.; Shibata, N.; Shibata, T.; Sasaki, H.; Singh, D.P.; Kubo, E. The role of Prdx6 in the protection of cells of the crystalline lens from oxidative stress induced by UV exposure. Jpn. J. Ophthalmol. 2016, 60, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Ganea, E.; Harding, J.J. Glutathione-related enzymes and the eye. Curr. Eye Res. 2006, 31, 1–11. [Google Scholar] [CrossRef]
- Reddy, V.N.; Giblin, F.J.; Lin, L.R.; Dang, L.; Unakar, N.J.; Musch, D.C.; Boyle, D.L.; Takemoto, L.J.; Ho, Y.S.; Knoernschild, T.; et al. Glutathione peroxidase-1 deficiency leads to increased nuclear light scattering, membrane damage, and cataract formation in gene-knockout mice. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3247–3255. [Google Scholar]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Roberts, B.R. Glutathione peroxidase 4: A new player in neurodegeneration? Mol. Psychiatry 2017, 22, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Xiao, T.; Vergara, L.A.; Srivastava, S.; Nees, D.; Piatigorsky, J.; Ansari, N.H. Role of aldehyde dehydrogenase isozymes in the defense of rat lens and human lens epithelial cells against oxidative stress. Investig. Ophthalmol. Vis. Sci. 2005, 46, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Sax, C.M.; Salamon, C.; Kays, W.T.; Guo, J.; Yu, F.X.; Cuthbertson, R.A.; Piatigorsky, J. Transketolase is a major protein in the mouse cornea. J. Biol. Chem. 1996, 271, 33568–33574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, P.C. Models, Molecules and Mechanisms in Biogerontology: Physiological Abnormalities, Diseases and Interventions; Springer: Singapore, 2019; Chapter XL; p. 436. [Google Scholar] [CrossRef]
- Cejková, J.; Vejrazka, M.; Pláteník, J.; Stípek, S. Age-related changes in superoxide dismutase, glutathione peroxidase, catalase and xanthine oxidoreductase/xanthine oxidase activities in the rabbit cornea. Exp. Gerontol. 2004, 39, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alonso, J.M.; Ghosh, S.; Coca-Prados, M. Cloning of the bovine plasma selenium-dependent glutathione peroxidase (GP) cDNA from the ocular ciliary epithelium: Expression of the plasma and cellular forms within the mammalian eye. J. Biochem. 1993, 114, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Z.; Ucgun, N.I.; Yildirim, F. The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration. Clinics 2011, 66, 743–746. [Google Scholar] [CrossRef]
- Tan, S.M.; Stefanovic, N.; Tan, G.; Wilkinson-Berka, J.L.; de Haan, J.B. Lack of the antioxidant glutathione peroxidase-1 (GPx1) exacerbates retinopathy of prematurity in mice. Investig. Ophthalmol. Vis. Sci. 2013, 54, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef] [Green Version]
- Donato, L.; Scimone, C.; Alibrandi, S.; Nicocia, G.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. Discovery of GLO1 New Related Genes and Pathways by RNA-Seq on A2E-Stressed Retinal Epithelial Cells Could Improve Knowledge on Retinitis Pigmentosa. Antioxidants 2020, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, G.; Wood, J.P.; Knoops, B.; Casson, R.J. Expression and distribution of peroxiredoxins in the retina and optic nerve. Brain. Struct. Funct. 2016, 221, 3903–3925. [Google Scholar] [CrossRef] [Green Version]
- Andley, U.P. Crystallins in the eye: Function and pathology. Prog. Retin. Eye Res. 2007, 26, 78–98. [Google Scholar] [CrossRef]
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration. Biochim. Biophys. Acta. 2016, 1860, 258–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasák, M.; Hasler, D.W. Metallothioneins: New functional and structural insights. Curr. Opin. Chem. Biol. 2000, 4, 177–183. [Google Scholar] [CrossRef]
- Bell, S.G.; Vallee, B.L. The metallothionein/thionein System: An Oxidoreductive Metabolic Zinc Link. ChemBioChem 2009, 10, 55–62. [Google Scholar] [CrossRef]
- Kägi, J.H. Overview of Metallothionein. Methods Enzymol. 1991, 205, 613–626. [Google Scholar] [CrossRef]
- Shaw, C.F.; Savas, M.M.; Petering, D.H. Ligand substitution and sulfhydryl reactivity of metallothionein. Methods Enzymol. 1991, 205, 401–414. [Google Scholar] [CrossRef]
- Sutherland, D.E.K.; Summers, K.L.; Stillman, M.J. Noncooperative Metalation of Metallothionein 1a and Its Isolated Domains with Zinc. Biochemistry 2012, 51, 6690–6700. [Google Scholar] [CrossRef]
- Ngu, T.T.; Stillman, M.J. Metal-binding mechanisms in metallothioneins. Dalton Trans. 2009, 5425–5433. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. Int. J. Mol. Sci. 2017, 18, 1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Maret, W. Human metallothionein metallomics. J. Anal. At. Spectrom. 2008, 23, 1055–1062. [Google Scholar] [CrossRef]
- Moleirinho, A.; Carneiro, J.; Matthiesen, R.; Silva, R.M.; Amorim, A.; Azevedo, L. Gains, losses and changes of function after gene duplication: Study of the metallothionein family. PLoS ONE 2011, 6, e18487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasákm, M. Advances in metallothionein structure and functions. J. Trace Elem. Med. Biol. 2005, 19, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Quaife, C.J.; Findley, S.D.; Erickson, J.C.; Froelick, G.J.; Kelly, E.J.; Zambrowicz, B.P.; Palmiter, R.D. Induction of a new metallothionein isoform (Mt-Iv) occurs during differentiation of stratified squamous epithelia. Biochemistry 1994, 33, 7250–7259. [Google Scholar] [CrossRef]
- Moffatt, P.; Seguin, C. Expression of the gene encoding metallothionein-3 in organs of the reproductive system. DNA Cell. Biol. 1998, 17, 501–510. [Google Scholar] [CrossRef]
- Uchida, Y.; Takio, K.; Titani, K.; Ihara, Y.; Tomonaga, M. The growth inhibitory factor that is deficient in the Alzheimers-disease brain is a 68-amino acid metallothionein-like protein. Neuron 1991, 7, 337–347. [Google Scholar] [CrossRef]
- Bremner, I. Nutritional and physiological significance of metallothionein. Experientia Suppl. 1987, 52, 81–107. [Google Scholar] [CrossRef]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009, 109, 4682–4707. [Google Scholar] [CrossRef]
- Zeng, J.; Vallee, B.L.; Kagi, J.H. Zn transfer from transcription factor IIIA fingers to thionein clusters. Proc. Natl. Acad. Sci. USA 1991, 88, 9984–9988. [Google Scholar] [CrossRef] [Green Version]
- Maret, W. Metallothionein/disulfide interactions, oxidative stress, and the mobilization of cellular zinc. Neurochem. Int. 1995, 27, 111–117. [Google Scholar] [CrossRef]
- Kang, Y.J. Metallothionein redox cycle and function. Exp. Biol. Med. 2006, 231, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Formigari, A.; Irato, P.; Santon, A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: Biochemical and cytochemical aspects. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M.; Cherian, M.G. Toxicological significance of metallothionein. Methods Enzymol. 1991, 205, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordberg, M.; Nordberg, G.F. Toxicological aspects of metallothionein. Cell. Mol. Biol. 2000, 46, 451–463. [Google Scholar] [PubMed]
- Ngu, T.T.; Stillman, M.J. Arsenic binding to human metallothionein. J. Am. Chem. Soc. 2006, 128, 12473–12483. [Google Scholar] [CrossRef]
- Gumulec, J.; Masarik, M.; Krizkova, S.; Adam, V.; Hubalek, J.; Hrabeta, J.; Eckschlager, T.; Stiborova, M.; Kizek, R. Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma. Curr. Med. Chem. 2011, 18, 5041–5051. [Google Scholar] [CrossRef] [Green Version]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef]
- Maret, W.; Vallee, B.L. Thiolate ligands in metallothionein confer redox activity on zinc clusters. Proc. Natl. Acad. Sci. USA 1998, 95, 3478–3482. [Google Scholar] [CrossRef] [Green Version]
- Maret, W. The function of zinc metallothionein: A link between cellular zinc and redox state. J. Nutr. 2000, 130 (Suppl. 5), 1455S–1458S. [Google Scholar] [CrossRef]
- Chiaverini, N.; De Ley, M. Protective effect of metallothionein on oxidative stress-induced DNA damage. Free Radic. Res. 2010, 44, 605–613. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Stiborova, M.; Adam, V.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044–6066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Koropatnick, J.; Cherian, M.G. Metallothionein protects DNA from copper-induced but not iron-induced cleavage in vitro. Chem. Biol. Interact. 1995, 96, 143–155. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.S.; Pitt, B.R. Metallothioneins and cell death by anticancer drugs. Ann. Rev. Pharmacol. Toxicol. 1995, 35, 635–653. [Google Scholar] [CrossRef]
- Hidalgo, J.; Aschner, M.; Zatta, P.; Vasak, M. Roles of the metallothionein family of proteins in the central nervous system. Brain Res. Bull. 2001, 55, 133–145. [Google Scholar] [CrossRef]
- Thirumoorthy, N.; Sunder, A.S.; Kumar, K.M.; Kumar, M.S.; Ganesh, G.; Chatterjee, M. A review of metallothionein isoforms and their role in pathophysiology. World J. Surg. Oncol. 2011, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, T.; Fatima, S. Metallothionein: Classification, biochemical features and clinical applications. J. Prot. Proteom. 2014, 5, 25–33. [Google Scholar]
- Jakovac, H.; Kezele, T.G.; Radošević-Stašić, B. Expression Profiles of Metallothionein I/II and Megalin in Cuprizone Model of De- and Remyelination. Neuroscience 2018, 388, 69–86. [Google Scholar] [CrossRef]
- Ebadi, M.; Iversen, P.L.; Hao, R.; Cerutis, D.R.; Rojas, P.; Happe, H.K.; Murrin, L.C.; Pfeiffer, R.F. Expression and regulation of brain metallothionein. Neurochem. Int. 1995, 27, 1–22. [Google Scholar] [CrossRef]
- Yamada, M.; Hayashi, S.; Hozumi, I.; Inuzuka, T.; Tsuji, S.; Takahashi, H. Subcellular localization of growth inhibitory factor in rat brain: Light and electron microscopic immunohistochemical studies. Brain Res. 1996, 735, 257–264. [Google Scholar] [CrossRef]
- Aschner, M.; Cherian, M.G.; Klaassen, C.D.; Palmiter, R.D.; Erickson, J.C.; Bush, A.I. Metallothioneins in brain—The role in physiology and pathology. Toxicol. Appl. Pharmacol. 1997, 142, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Penkowa, M.; Giralta, M.; Camats, J.; Molinero, A.; Campbell, I.L.; Palmiter, R.D.; Hidalgo, J. Role of metallothionein-III following central nervous system damage. Neurobiol. Dis. 2003, 13, 22–36. [Google Scholar] [CrossRef]
- West, A.K.; Hidalgo, J.; Eddins, D.; Levin, E.D.; Aschner, M. Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology 2008, 29, 488–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The multipurpose protein. Cell. Mol. Life. Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Redox biochemistry of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Giacconi, R.; Cipriano, C.; Costarelli, L.; Muti, E.; Tesei, S.; Giuli, C.; Papa, R.; Marcellini, F.; Mariani, E.; et al. Zinc, metallothioneins, and longevity–effect of zinc supplementation: Zincage study. Ann. N. Y. Acad. Sci. 2007, 1119, 129–146. [Google Scholar] [CrossRef]
- Levenson, C.W.; Morris, D. Zinc and neurogenesis: Making new neurons from development to adulthood. Adv. Nutr. 2011, 2, 96–100. [Google Scholar] [CrossRef]
- Chung, R.S.; Penkowa, M.; Dittmann, J.; King, C.E.; Bartlett, C.; Asmussen, J.W.; Hidalgo, J.; Carrasco, J.; Leung, Y.K.; Walker, A.K.; et al. Redefining the role of metallothionein within the injured brain: Extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. J. Biol. Chem. 2008, 283, 15349–15358. [Google Scholar] [CrossRef] [Green Version]
- Chung, R.S.; Hidalgo, J.; West, A.K. New insight into the molecular pathways of metallothionein-mediated neuroprotection and regeneration. J. Neurochem. 2008, 104, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ambjørn, M.; Asmussen, J.W.; Lindstam, M.; Gotfryd, K.; Jacobsen, C.; Kiselyov, V.V.; Moestrup, S.K.; Penkowa, M.; Bock, E.; Berezin, V. Metallothionein and a peptide modeled after metallothionein, EmtinB, induce neuronal differentiation and survival through binding to receptors of the low-density lipoprotein receptor family. J. Neurochem. 2008, 104, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, J.W.; Sperling, M.L.V.; Penkowa, M. Intraneuronal signaling pathways of metallothionein. J. Neurosci. Res. 2009, 87, 2926–2936. [Google Scholar] [CrossRef] [PubMed]
- West, A.K.; Leung, J.Y.K.; Chung, R.S. Neuroprotection and regeneration by extracellular metallothionein via lipoprotein-receptor-related proteins. J. Biol. Inorg. Chem. 2011, 16, 1115. [Google Scholar] [CrossRef] [PubMed]
- Auderset, L.; Landowski, L.M.; Foa, L.; Young, K.M. Low density lipoprotein receptor related proteins as regulators of neural stem and progenitor cell function. Stem Cells Int. 2016, 2108495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoda, R.; Achanzar, W.E.; Qu, W.; Nagamine, T.; Takagi, H.; Mori, M.; Waalkes, M.P. Metallothionein is a potential negative regulator of apoptosis. Toxicol. Sci. 2003, 73, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Santon, A.; Albergoni, V.; Sturniolo, G.C.; Irato, P. Evaluation of MT expression and detection of apoptotic cells in LEC rat kidneys. Biochim. Biophys. Acta 2004, 1688, 223–231. [Google Scholar] [CrossRef]
- Telford, W.G.; Fraker, P.J. Preferential induction of apoptosis in mouse CD4(+)CD8(+)alphabeta- tcr(lo)CD3-epsilon(lo) thymocytes by zinc. J. Cell. Physiol. 1995, 164, 259–270. [Google Scholar] [CrossRef]
- Perry, D.K.; Smyth, M.J.; Stennicke, H.R.; Salvesen, G.S.; Duriez, P.; Poirier, G.G.; Hannun, Y.A. Zinc is a potent inhibitor of the apoptotic protease, caspase-3—A novel target for zinc in the inhibition of apoptosis. J. Biol. Chem. 1997, 272, 18530–18533. [Google Scholar] [CrossRef] [Green Version]
- Bozym, R.A.; Chimienti, F.; Giblin, L.J.; Gross, G.W.; Korichneva, I.; Li, Y.; Libert, S.; Maret, W.; Parviz, M.; Frederickson, C.J.; et al. Free zinc ions outside a narrow concentration range are toxic to a variety of cells in vitro. Exp. Biol. Med. (Maywood) 2010, 235, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Cano-Gauci, D.F.; Sarkar, B. Reversible zinc exchange between metallothionein and the estrogen receptor zinc finger. FEBS Lett. 1996, 386, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Moffatt, P.; Denizeau, F. Metallothionein in physiological and physiopathological processes. Drug Metab. Rev. 1997, 29, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Jacob, C.; Vallee, B.L.; Fischer, E.H. Inhibitory sites in enzymes: Zinc removal and reactivation by thionein. Proc. Natl. Acad. Sci. USA 1999, 96, 1936–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meplan, C.; Verhaegh, G.; Richard, M.J.; Hainaut, P. Metal ions as regulators of the conformation and function of the tumour suppressor protein p53: Implications for carcinogenesis. Proc. Nutr. Soc. 1999, 58, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Meplan, C.; Richard, M.J.; Hainaut, P. Metalloregulation of the tumor suppressor protein p53: Zinc mediates the renaturation of p53 after exposure to metal chelators in vitro and in intact cells. Oncogene 2000, 19, 5227–5236. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Mageed, A.B.; Agrawal, K.C. Activation of nuclear factor kappa B: Potential role in metallothionein-mediated mitogenic response. Cancer Res. 1998, 58, 2335–2338. [Google Scholar]
- Kim, C.H.; Kim, J.H.; Lee, J.; Ahn, Y.S. Zinc-Induced NF-kappa B inhibition can be modulated by changes in the intracellular metallothionein level. Toxicol. Appl. Pharmacol. 2003, 190, 189–196. [Google Scholar] [CrossRef]
- Butcher, H.L.; Kennette, W.A.; Collins, O.; Zalups, R.K.; Koropatnick, J. Metallothionein mediates the level and activity of nuclear factor kappa B in murine fibroblasts. J. Pharmacol. Exp. Ther. 2004, 310, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Tsangaris, G.T.; Vamvoukakis, J.; Politis, I.; Kattamis, A.C.; Tzortzatou-Stathopoulou, F. Metallothionein expression prevents apoptosis. II: Evaluation of the role of metallothionein expression on the chemotherapy-induced apoptosis during the treatment of acute leukemia. Anticancer Res. 2000, 20, 4407–4411. [Google Scholar]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogénesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. Different redox states of metallothionein/thionein in biological tissue. Biochem. J. 2007, 402, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maret, W. Molecular aspects of human cellular zinc homeostasis: Redox control of zinc potentials and zinc signals. Biometals 2009, 22, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Iglesias, H.; Alvarez, L.; García, M.; Petrash, C.; Sanz-Medel, A.; Coca-Prados, M. Metallothioneins (MTs) in the human eye: A perspective article on the zinc-MT redox cycle. Metallomics 2014, 6, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.; Stillman, M.J. The “magic numbers” of metallothionein. Metallomics 2011, 3, 444–463. [Google Scholar] [CrossRef]
- Krezel, A.; Hao, Q.; Maret, W. The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef]
- Chen, Y.; Maret, W. Catalytic selenols couple the redox cycles of metallothionein and glutathione. Eur. J. Biochem. 2001, 268, 3346–3353. [Google Scholar] [CrossRef]
- Maret, W.; Krezel, A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol. Med. 2007, 13, 371–375. [Google Scholar] [CrossRef]
- Laukens, D.; Waeytens, A.; De Bleser, P.; Cuvelier, C.; De Vos, M. Human metallothionein expression under normal and pathological conditions: Mechanisms of gene regulation based on in silico promoter analysis. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 301–317. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Hamer, D.H. Cell specificity and an effect of ras on human metallothionein gene expression. Proc. Natl. Acad. Sci. USA 1986, 83, 3346–3350. [Google Scholar] [CrossRef] [Green Version]
- Varshney, U.; Jahroudi, N.; Foster, R.; Gedamu, L. Structure, organization, and regulation of human metallothionein IF gene: Differential and cell-type-specific expression in response to heavy metals and glucocorticoids. Mol. Cell. Biol. 1986, 6, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, K.; Jacob, S.T. Regulation of metallothionein gene expression. Prog. Nucleic. Acid. Res. Mol. Biol. 2001, 66, 357–384. [Google Scholar] [CrossRef] [PubMed]
- Haq, F.; Mahoney, M.; Koropatnick, J. Signaling events for metallothionein induction. Mutat. Res. 2003, 533, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, L.A.; Lynes, M.A. Stress proteins as agents of immunological change: Some lessons from metallothionein. Cell Stress Chaperones 1996, 1, 99–108. [Google Scholar] [CrossRef]
- Takahashi, S. Molecular functions of metallothionein and its role in hematological malignancies. J. Hematol. Oncol. 2012, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakulsak, N. Metallothionein: An overview on its metal homeostatic regulation in mammals. Int. J. Morphol. 2012, 30, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Kambe, T. The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336–357. [Google Scholar] [CrossRef] [Green Version]
- Westin, G.; Schaffner, W. A zinc responsive factor interacts with a metal regulated enhancer element (MRE) of the mouse metallothionein I gene. EMBO J. 1988, 7, 3763–3770. [Google Scholar] [CrossRef]
- Dalton, T.; Palmiter, R.D.; Andrews, G.K. Transcriptional induction of the mouse metallothionein I gene in hydrogen peroxide treated Hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res. 1994, 22, 5016–5023. [Google Scholar] [CrossRef] [Green Version]
- Murphy, B.J.; Andrews, G.K.; Bittel, D.; Discher, D.J.; McCue, J.; Green, C.J.; Yanovsky, M.; Giaccia, A.; Sutherland, R.M.; Laderoute, K.R.; et al. Activation of metallothionein gene expression by hypoxia involves metal response elements and metal transcription factor 1. Cancer Res. 1999, 59, 1315–1322. [Google Scholar]
- Günther, V.; Lindert, U.; Schaffner, W. The taste of heavy metals: Gene regulation by MTF1. Biochim. Biophys. Acta 2012, 1823, 1416–1425. [Google Scholar] [CrossRef] [Green Version]
- Andrews, G.K.; Geiser, J. Expression of the mouse metallothionein-I and-II genes provides a reproductive advantage during maternal dietary Zn deficiency. J. Nutr. 1999, 129, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Klassen, R.B.; Crenshaw, K.; Kozyraki, R.; Verroust, P.J.; Tio, L.; Atrian, S.; Allen, P.L.; Hammond, T.G. Megalin mediates renal uptake of heavy metalmetallothionein complexes. Am. J. Physiol. Renal. Physiol. 2004, 287, F393–F403. [Google Scholar] [CrossRef]
- Samson, S.L.A.; Gedamu, L. Molecular analyses of metallothionein gene regulation. Prog. Nucleic Acid Res. Mol. Biol. 1998, 59, 257–288. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000, 59, 95–104. [Google Scholar] [CrossRef]
- Kelly, E.; Sandgren, E.; Brinster, R.; Palmiter, R. A pair of adjacent glucocorticoid response elements regulate expression of two mouse metallothionein genes. Proc. Natl. Acad. Sci. USA 1997, 94, 10045–10050. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.; Cousins, R. Metallothionein expression in animals: A physiological perspective on function. J. Nutr. 2000, 130, 1085–1088. [Google Scholar] [CrossRef] [Green Version]
- Ivashkiv, L.B.; Hu, X. Signaling by STATs. Arthritis Res Ther. 2004, 6, 159–168. [Google Scholar] [CrossRef] [Green Version]
- De, S.K.; McMaster, M.T.; Andrews, G.K. Endotoxin induction of murine metallothionein gene expression. J. Biol. Chem. 1990, 265, 15267–15274. [Google Scholar] [CrossRef]
- Pfitzner, E.; Kliem, S.; Baus, D.; Litterst, C.M. The Role of STATs in Inflammation and Inflammatory Diseases. Curr Pharm Des. 2004, 10, 2839–2850. [Google Scholar] [CrossRef]
- Lee, D.K.; Carrasco, J.; Hidalgo, J.; Andrews, G.K. Identification of a signal transducer and activator of transcription (STAT) binding site in the mouse metallothionein-I promoter involved in interleukin-6-induced gene expression. Biochem. J. 1999, 337, 59–65. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Itoh, N.; Kawasaki, A.; Matsuyama, A.; Matsuda, K.; Nakanishi, T.; Tanaka, K. Metallothionein modulates lipopolysaccharide-stimulated tumour necrosis factor expression in mouse peritoneal macrophages. Biochem. J. 2002, 361, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.J.; Kimura, T.; Sato, B.G.; Shi, Y.; Andrews, G.K. Metallothionein induction by hypoxia involves cooperative interactions between metal-responsive transcription factor-1 and hypoxia-inducible transcription factor- 1alpha. Mol. Cancer Res. 2008, 6, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, G.K.; Lee, D.K.; Ravindra, R.; Lichtlen, P.; Sirito, M.; Sawadogo, M.; Schaffner, W. The transcription factors MTF 1 and USF1 cooperate to regulate mouse metallothionein I expression in response to the essential metal zinc in visceral endoderm cells during early development. EMBO J. 2001, 20, 1114–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaRochelle, O.; Labbe, S.; Harrisson, J.F.; Simard, C.; Tremblay, V.; St-Gelais, G.; Govindan, M.V.; Séguin, C. Nuclear factor 1 and metal transcription factor 1 synergistically activate the mouse metallothionein 1 gene in response to metal ions. J. Biol. Chem. 2008, 283, 8190–8201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imoto, A.; Okada, M.; Okazaki, T.; Kitasato, H.; Harigae, H.; Takahashi, S. Metallothionein 1 isoforms and vimentin are direct PU.1 downstream target genes in leukemia cells. J. Biol. Chem. 2010, 285, 10300–10309. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Nakano, H.; Takahashi, S. Epigenetic regulation of the metallothionein 1A promoter by PU.1 during differentiation of THP 1 cells. Biochem. Biophys. Res. Commun. 2013, 433, 349–353. [Google Scholar] [CrossRef]
- Tang, C.M.; Westling, J.; Seto, E. trans repression of the human metallothionein IIA gene promoter by PZ120, a novel 120 kilo¬dalton zinc finger protein. Mol. Cell. Biol. 1999, 19, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Smith, M.; Glass, J. Stable expression of C/EBPalpha in prostate cancer cells down regulates metallothionein and increases zinc induced toxicity. Prostate 2005, 62, 209–216. [Google Scholar] [CrossRef]
- Ghoshal, K.; Li, Z.; Jacob, S.T. Overexpression of the large subunit of the protein Ku suppresses metallothionein I induction by heavy metals. Proc. Natl. Acad. Sci. USA 1998, 95, 10390–10395. [Google Scholar] [CrossRef] [Green Version]
- Majumder, S.; Kutay, H.; Datta, J.; Summers, D.; Jacob, S.T.; Kalpana-Ghoshal, K. Epigenetic regulation of metallothionein-i gene expression: Differential regulation of methylated and unmethylated promoters by DNA methyltransferases and methyl CpG binding proteins. J. Cell. Biochem. 2006, 97, 1300–1316. [Google Scholar] [CrossRef]
- Ghoshal, K.; Datta, J.; Majumder, S.; Bai, S.; Dong, X.; Parthun, M.; Jacob, S.T. Inhibitors of Histone Deacetylase and DNA Methyltransferase synergistically activate the methylated metallothionein I promoter by activating the transcription factor MTF-1 and forming an open chromatin structure. Mol. Cell. Biol. 2002, 22, 8302–8319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKim, J.M.; Choudhuri, S.; Klaassen, C.D. In vitro degradation of apo-, zinc-, and cadmium metallothionein by cathepsins B, C, and D. Toxicol. Appl. Pharmacol. 1992, 116, 117–124. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Choudhuri, S.; McKim, J.M.; Lehman-McKeeman, L.D.; Kershaw, W.C. In vitro and in vivo studies on the degradation of metallothionein. Environ. Health Perspect. 1994, 102, 141–146. [Google Scholar] [CrossRef]
- Shcherbik, N.; Pestov, D.G. The Impact of Oxidative Stress on Ribosomes: From Injury to Regulation. Cells 2019, 8, 1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilkis, I.; Silman, I.; Weiner, L. Generation of Reactive Oxygen Species by Photosensitizers and their Modes of Action on Proteins. Curr. Med. Chem. 2018, 25, 5528–5539. [Google Scholar] [CrossRef]
- Brem, R.; Guven, M.; Karran, P. Oxidatively-generated damage to DNA and proteins mediated by photosensitized UVA. Free Radic. Biol. Med. 2017, 107, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Hauck, A.K.; Huang, Y.; Hertzel, A.V.; Bernlohr, D.A. Adipose oxidative stress and protein carbonylation. J. Biol. Chem. 2019, 294, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Kamari, F.; Hallaj, S.; Dorosti, F.; Alinezhad, F.; Taleschian-Tabrizi, N.; Farhadi, F.; Aslani, H. Phototoxicity of environmental radiations in human lens: Revisiting the pathogenesis of UV-induced cataract. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2065–2077. [Google Scholar] [CrossRef]
- Truscott, R.J.W.; Friedrich, M.G. Molecular processes implicated in human age-related nuclear cataract. IOVS 2019, 60, 5007–5021. [Google Scholar] [CrossRef] [Green Version]
- Richardson, R.B.; Ainsbury, E.A.; Prescott, C.R.; Lovicu, F.J. Etiology of posterior subcapsular cataracts based on a review of risk factors including, aging, diabetes, and ionizing radiation. Int. J. Radiat. Biol. 2020, 96, 1339–1361. [Google Scholar] [CrossRef] [PubMed]
- van Kuijk, F.J. Effects of ultraviolet light on the eye: Role of protective glasses. Environ. Health Perspect. 1991, 96, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E. Ocular phototoxicity. J. Photochem. Photobiol. B 2001, 64, 136–143. [Google Scholar] [CrossRef]
- Hamba, N.; Gerbi, A.; Tesfaye, S. Histopathological effects of ultraviolet radiation exposure on the ocular structures in animal studies—Literature review. Transl. Res. Anat. 2020, 100086. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef]
- Deng, R.; Hua, X.; Li, J.; Chi, W.; Zhang, Z.; Lu, F.; Zhang, L.; Pflugfelder, S.C.; Li, D.Q. Oxidative stress markers induced by hyperosmolarity in primary human corneal epithelial cells. PLoS ONE 2015, 10, e0126561. [Google Scholar] [CrossRef] [Green Version]
- Kojima, T.; Wakamatsu, T.H.; Dogru, M.; Ogawa, Y.; Igarashi, A.; Ibrahim, O.M.; Inaba, T.; Shimizu, T.; Noda, S.; Obata, H.; et al. Age-related dysfunction of the lacrimal gland and oxidative stress: Evidence from the Cu,Zn-superoxide dismutase-1 (Sod1) knockout mice. Am. J. Pathol. 2012, 180, 1879–1896. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Dogru, M.; Matsumoto, Y.; Igarashi, A.; Kojima, T.; Wakamatsu, T.H.; Inaba, T.; Shimizu, T.; Shimazaki, J.; Tsubota, K. Oxidative stress induced age dependent meibomian gland dysfunction in Cu, Zn-superoxide dismutase-1 (Sod1) knockout mice. PLoS ONE 2014, 9, e99328. [Google Scholar] [CrossRef] [Green Version]
- Uchino, Y.; Kawakita, T.; Miyazawa, M.; Ishii, T.; Onouchi, H.; Yasuda, K.; Ogawa, Y.; Shimmura, S.; Ishii, N.; Tsubota, K. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS ONE 2012, 7, e45805. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Shibuya, M.; Nakashima, H.; Hisamura, R.; Masuda, N.; Imagawa, T.; Uehara, M.; Tsubota, K. Involvement of oxidative stress on corneal epithelial alterations in a blink-suppressed dry eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1552–1558. [Google Scholar] [CrossRef]
- Choi, W.; Lian, C.; Ying, L.; Kim, G.E.; You, I.C.; Park, S.H.; Yoon, K.C. Expression of Lipid Peroxidation Markers in the Tear Film and Ocular Surface of Patients with Non- Sjogren Syndrome: Potential Biomarkers for Dry Eye Disease. Curr. Eye Res. 2016, 41, 1143–1149. [Google Scholar] [CrossRef]
- Perez-Garmendia, R.; Lopez de Eguileta Rodriguez, A.; Ramos-Martinez, I.; Martínez Zuñiga, N.; Gonzalez-Salinas, R.; Quiroz-Mercado, H.; Zeneto, E.; Ramírez Hernández, E.; Hernández-Zimbrón, L.F. Interplay between oxidative stress, inflammation, and amyloidosis in the anterior segment of the eye; its pathological implications. Oxid. Med. Cell. Longev. 2020, 2020, 6286105. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Dogru, M.; Kawashima, M.; Nakamura, S.; Tsubota, K. Advances in the diagnosis and treatment of dry eye. Prog. Retin. Eye Res. 2020, 78, 100842. [Google Scholar] [CrossRef] [PubMed]
- Arden, G.B.; Sivaprasad, S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr. Diabetes Rev. 2011, 7, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M. Role of vascular reactive oxygen species in development of vascular abnormalities in diabetes. Diabetes Res. Clin. Pract. 2007, 77 (Suppl. 1), S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.M.; Chari, S. Lipid peroxidation and antioxidant status in patients with diabetic retinopathy. Indian J. Physiol. Pharmacol. 2005, 49, 187–192. [Google Scholar]
- Robison, W.G., Jr.; Jacot, J.L.; Katz, M.L.; Glover, J.P. Retinal vascular changes induced by the oxidative stress of alpha-tocopherol deficiency contrasted with diabetic microangiopathy. J. Ocul. Pharmacol. Ther. 2000, 16, 109–120. [Google Scholar] [CrossRef]
- Martín-Gallán, P.; Carrascosa, A.; Gussinyé, M.; Domínguez, C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic. Biol. Med. 2003, 34, 1563–1574. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, L.; Xin, G.; Li, S.; Ma, L.; Xu, Y.; Zhuang, M.; Xiong, Q.; Wei, Z.; Xing, Z.; et al. Oxidative stress mediated by lipid metabolism contributes to high glucose-induced senescence in retinal pigment epithelium. Free Radic. Biol. Med. 2019, 130, 48–58. [Google Scholar] [CrossRef]
- Chen, B.H.; Jiang, D.Y.; Tang, L.S. Advanced glycation end-products induce apoptosis involving the signaling pathways of oxidative stress in bovine retinal pericytes. Life Sci. 2006, 79, 1040–1048. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Abbas, S.N. Diabetes-induced mitochondrial dysfunction in the retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5327–5334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.C.W.; Wilkinson-Berka, J.L.; Deliyanti, D.; Hunter, D.; Fung, A.; Liew, G.; White, A. The role of reactive oxygen species in the pathogenesis and treatment of retinal diseases. Exp. Eye Res. 2020, 201, 108255. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Moran, E.P.; Wang, Z.; Chen, J.; Sapieha, P.; Smith, L.E.; Mam, J.X. Neurovascular cross talk in diabetic retinopathy: Pathophysiological roles and therapeutic implications. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H738–K749. [Google Scholar] [CrossRef] [Green Version]
- Zadeh, J.K.; Ruemmler, R.; Hartmann, E.K.; Ziebart, A.; Ludwig, M.; Patzak, A.; Xia, N.; Li, H.; Pfeiffer, N.; Gericke, A. Responses of retinal arterioles and ciliary arteries in pigs with acute respiratory distress syndrome (ARDS). Exp. Eye Res. 2019, 184, 152–161. [Google Scholar] [CrossRef]
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [Green Version]
- Benoist d’Azy, C.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Oxidative and Anti-Oxidative Stress Markers in Chronic Glaucoma: A Systematic Review and Meta- Analysis. PLoS ONE 2016, 11, e0166915. [Google Scholar] [CrossRef]
- Gericke, A.; Mann, C.; Zadeh, J.K.; Musayeva, A.; Wolff, I.; Wang, M.; Pfeiffer, N.; Daiber, A.; Li, H.; Xia, N.; et al. Elevated Intraocular Pressure Causes Abnormal Reactivity of Mouse Retinal Arterioles. Oxid. Med. Cell. Longev. 2019, 29, 9736047. [Google Scholar] [CrossRef] [Green Version]
- Zanon-Moreno, V.; Marco-Ventura, P.; Lleo-Perez, A.; Pons-Vazquez, S.; Garcia-Medina, J.J.; Vinuesa-Silva, I.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Oxidative stress in primary open-angle glaucoma. J. Glaucoma 2008, 17, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Yang, X.; Luo, C.; Peng, Y.; Sun, S.L.; Sun, D. Mechanisms of immune system activation in glaucoma: Oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest. Ophthalmol. Vis. Sci. 2007, 48, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Wax, M.B. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J. Neurosci. 2000, 20, 8693–8700. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef] [Green Version]
- Tezel, G. The immune response in glaucoma: A perspective on the roles of oxidative stress. Exp. Eye Res. 2011, 93, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Izzotti, A.; Bagnis, A.; Saccà, S.C. The role of oxidative stress in glaucoma. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Lopez-Bernal, M.D.; Cobo-Martinez, A.; Zanon-Moreno, V.; Pinazo-Duran, M.D.; del-Rio-Vellosillo, M. Glaucoma and antioxidants: Review and update. Antioxidants 2020, 9, 1031. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Cao, W.; Sun, X. The association of oxidative stress status with open-angle glaucoma and exfoliation glaucoma: A systematic review and meta-analysis. J. Ophthalmol. 2019, 2019, 1803619. [Google Scholar] [CrossRef] [Green Version]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage-induced inflammation initiates age- related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Aspects Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [Green Version]
- Klettner, A. Oxidative stress induced cellular signaling in RPE cells. Front. Biosci. 2012, 4, 392–411. [Google Scholar] [CrossRef]
- Nowak, J.Z. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: Focus on age-related macular degeneration. Pharmacol. Rep. 2013, 65, 288–304. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Zhou, J.; Ben-Shabat, S.; Vollmer, H.; Itagaki, Y.; Nakanishi, K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1222–1227. [Google Scholar]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 1999, 5, 32. [Google Scholar] [PubMed]
- Ham, W.T.; Ruffolo, J.J.; Mueller, H.A.; Clarke, A.M.; Moon, M.E. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Investig. Ophthalmol. Vis. Sci. 1978, 17, 1029–1035. [Google Scholar]
- Tong, Y.; Wang, S. Not all stressors are equal: Mechanism of stressors on RPE cell degeneration. Front. Cell Dev. Biol. 2020, 8, 591067. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal pigment epithelium and age-related macular degeneration: A review of major disease mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef]
- Duncan, K.E.; Stillman, M.J. Metal-dependent protein folding: Metallation of metallothionein. J. Inorg. Biochem. 2006, 100, 2101–2107. [Google Scholar] [CrossRef]
- Romero-Isart, N.; Vasák, M. Advances in the structure and chemistry of metallothioneins. J. Inorg. Biochem. 2002, 88, 388–396. [Google Scholar] [CrossRef]
- Oppermann, B.; Zhang, W.; Magabo, K.; Kantorow, M. Identification and spatial analysis of metallothioneins expressed by the adult human lens. Investig. Ophthalmol. Vis. Sci. 2001, 42, 188–193. [Google Scholar]
- Tate, D.J.; Miceli, M.V.; Newsome, D.A. Expression of metallothionein isoforms in human chorioretinal complex. Curr. Eye Res. 2002, 24, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Shimazawa, M.; Hara, H. Physiological Roles of Metallothioneins in Central Nervous System Diseases. Biol. Pharm. Bull. 2018, 41, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Vasák, M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta 1985, 827, 36–44. [Google Scholar] [CrossRef]
- Ohashi, Y.; Dogru, M.; Tsubota, K. Laboratory findings in tear fluid analysis. Clin. Chim. Acta 2006, 369, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Karnati, R.; Laurie, D.E.; Laurie, G.W. Lacritin and the Tear Proteome as Natural Replacement Therapy for Dry Eye. Exp. Eye Res. 2013, 117, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Lauweryns, B.; van den Oord, J.J.; Missotten, L. The transitional zone between limbus and peripheral cornea. An immunohistochemical study. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1991–1999. [Google Scholar]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef]
- Lu, H.; Hunt, D.M.; Ganti, R.; Davis, A.; Dutt, K.; Alam, J.; Hunt, R.C. Metallothionein protects retinal pigment epithelial cells against apoptosis and oxidative stress. Exp. Eye Res. 2002, 74, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Carnes, M.U.; Allingham, R.R.; Ashley-Koch, A.; Hauser, M.A. Transcriptome analysis of adult and fetal trabecular meshwork, cornea, and ciliary body tissues by RNA sequencing. Exp. Eye Res. 2018, 167, 91–99. [Google Scholar] [CrossRef]
- Gottsch, J.D.; Bowers, A.L.; Margulies, E.H.; Seitzman, G.D.; Kim, S.W.; Saha, S.; Jun, A.S.; Stark, W.J.; Liu, S.H. Serial analysis of gene expression in the corneal endothelium of Fuchs’ dystrophy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 594–599. [Google Scholar] [CrossRef] [Green Version]
- De Roo, A.-K.; Wouters, J.; Govaere, O.; Foets, B.; van den Oord, J.J. Identification of circulating fibrocytes and dendritic derivatives in corneal endothelium of patients with Fuch’s dystrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 670–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, B.; Ziaei, A.; Fuchsluger, T.; Schmedt, T.; Chen, Y.; Jurkunas, U.V. p53-regulated increase in oxidative-stress–induced apoptosis in Fuchs endothelial corneal dystrophy: A native tissue model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9291–9297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaluzhny, Y.; Kinuthia, M.W.; Lapointe, A.M.; Truong, T.; Klausner, M.; Hayden, P. Oxidative stress in corneal injuries of different origin: Utilization of 3D human corneal epithelial tissue model. Exp. Eye Res. 2020, 190, 107867. [Google Scholar] [CrossRef] [PubMed]
- Han, E.S.; Muller, F.L.; Perez, V.I.; Qi, W.; Liang, H.; Xi, L.; Fu, C.; Doyle, E.; Hickey, M.; Cornell, J.; et al. The in vivo gene expression signature of oxidative stress. Physiol. Genom. 2008, 34, 112–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hejtmancik, J.F.; Riazuddin, S.A.; McGreal, R.; Liu, W.; Cvekl, A.; Shiels, A. Lens Biology and Biochemistry. Prog. Mol. Biol. Transl. Sci. 2015, 134, 169–201. [Google Scholar] [CrossRef]
- Cejková, J.; Stípek, S.; Crkovská, J.; Ardan, T. Changes of superoxide dismutase, catalase and glutathione peroxidase in the corneal epithelium after UVB rays. Histochemical and biochemical study. Histol. Histopathol. 2000, 15, 1043–1050. [Google Scholar] [CrossRef]
- Fecondo, J.V.; Augusteyn, R.C. Superoxide dismutase, catalase and glutathione peroxidase in the human cataractous lens. Exp. Eye Res. 1983, 36, 15–23. [Google Scholar] [CrossRef]
- Bova, L.M.; Sweeney, M.H.; Jamie, J.F.; Truscott, R.J. Major changes in human ocular UV protection with age. Investig. Ophthalmol. Vis. Sci. 2001, 42, 200–205. [Google Scholar]
- Kantorow, M.; Kays, T.; Horwitz, J.; Huang, Q.; Sun, J.; Piatigorsky, J.; Carper, D. Differential display detects altered gene expression between cataractous and normal human lenses. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2344–2354. [Google Scholar]
- Whitson, J.A.; Zhang, X.; Medvedovic, M.; Chen, J.; Wei, Z.; Monnier, V.M.; Fan, X. Transcriptome of the GSH-depleted lens reveals changes in detoxification and EMT signaling genes, transport systems, and lipid homeostasis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2666–2684. [Google Scholar] [CrossRef]
- Spector, A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995, 9, 1173–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawse, J.R.; Hejtmancik, J.F.; Huang, Q.; Sheets, N.L.; Hosack, D.A.; Lempicki, R.A.; Horwitz, J.; Kantorow, M. Identification and functional clustering of global gene expression differences between human age-related cataract and clear lenses. Mol. Vis. 2003, 9, 515–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawse, J.R.; Padgaonkar, V.A.; Leverenz, V.R.; Pelliccia, S.E.; Kantorow, M.; Giblin, F.J. The role of metallothionein IIa in defending lens epithelial cells against cadmium and TBHP induced oxidative stress. Mol. Vis. 2006, 12, 342–349. [Google Scholar] [PubMed]
- Saito, T.; Tezuka, T.; Konno, R.; Fujii, N. Protective effects of metallothionein I and II against metal- and ultraviolet radiation-induced damage in cultured lens epithelial cells. Jpn. J. Ophthalmol. 2010, 54, 486–493. [Google Scholar] [CrossRef]
- González-Iglesias, H.; Petrash, C.; Rodríguez-Menéndez, S.; García, M.; Álvarez, L.; Fernández-Vega-Cueto, L.; Fernández, B.; Pereiro, R.; Sanz-Medel, A.; Coca-Prados, M. Quantitative distribution of Zn, Fe and Cu in the human lens and study of the Zn–metallothionein redox system in cultured lens epithelial cells by elemental MS. J. Anal. At. Spectrom. 2017, 32, 1746–1756. [Google Scholar] [CrossRef]
- Bazan, N.G. The metabolism of omega-3 polyunsaturated fatty acids in the eye: The possible role of docosahexaenoic acid and docosanoids in retinal physiology and ocular pathology. Prog. Clin. Biol. Res. 1989, 312, 95–112. [Google Scholar]
- Dargel, R. Lipid peroxidation—A common pathogenetic mechanism? Exp. Toxicol. Pathol. 1992, 44, 169–181. [Google Scholar] [CrossRef]
- Glickman, R.D. Phototoxicity to the retina: Mechanisms of damage. Int. J. Toxicol. 2002, 21, 473–490. [Google Scholar] [CrossRef]
- van Reyk, D.M.; Gillies, M.C.; Davies, M.J. The retina: Oxidative stress and diabetes. Redox Rep. 2003, 8, 187–192. [Google Scholar] [CrossRef]
- Tate, D.J.; Newsome, D.A.; Oliver, P.D. Metallothionein shows an age-related decrease in human macular retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2348–2351. [Google Scholar]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic. Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liles, M.R.; Newsome, D.A.; Oliver, P.D. Antioxidant enzymes in the aging human retinal pigment epithelium. Arch. Ophthalmol. 1991, 109, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Newsome, D.A.; Miceli, M.V.; Tate, D.J.; Alcock, N.W.; Oliver, P.D. Zinc content of human retinal pigment epithelium decreases with age and macular degeneration, but superoxide dismutase activity increases. J. Trace Elem. Exp. Med. 1995, 8, 193–199. [Google Scholar] [CrossRef]
- Decanini, A.; Nordgaard, C.L.; Feng, X.; Ferrington, D.A.; Olsen, T.W. Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am. J. Ophthalmol. 2008, 143, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, W.; Lu, Y.; Zhong, S.; Zhang, M.; Sun, L.; Dong, H.; Wang, M.; Wei, M.; Xie, H.; Qu, H.; et al. A single-cell transcriptome atlas of the aging human and macaque retina. Natl. Sci. Rev. 2020. [Google Scholar] [CrossRef]
- Voigt, A.P.; Mullin, N.K.; Stone, E.M.; Tucker, B.A.; Scheetz, T.E.; Mullins, R.F. Single-cell RNA sequencing in vision research: Insights into human retinal health and disease. Prog. Retin. Eye Res. 2020, 100934. [Google Scholar] [CrossRef]
- Nicolas, M.G.; Fujiki, K.; Murayama, K.; Suzuki, M.T.; Shindo, N.; Hotta, Y.; Iwata, F.; Fujimura, T.; Yoshikawa, Y.; Cho, F.; et al. Studies on the mechanism of early onset macular degeneration in cynomolgus monkeys. II. Suppression of metallothionein synthesis in the retina in oxidative stress. Exp. Eye Res. 1996, 62, 399–408. [Google Scholar] [CrossRef]
- Miceli, M.V.; Tate, D.J.; Alcock, N.W.; Newsome, D.A. Zinc deficiency and oxidative stress in the retina of pigmented rats. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1238–1244. [Google Scholar]
- Noell, W.K.; Organisciak, D.T.; Ando, H.; Braniecki, M.A.; Durlin, C. Ascorbate and dietary protective mechanisms in retinal light damage of rats: Electrophysiological, histological and DNA measurements. Prog. Clin. Biol. Res. 1987, 247, 469–483. [Google Scholar]
- Truscott, R.J. Age-related nuclear cataract-oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Siu, T.L.; Morley, J.W.; Coroneo, M.T. Toxicology of the retina: Advances in understanding the defence mechanisms and pathogenesis of drug- and light-induced retinopathy. Clin. Exp. Ophthalmol. 2008, 36, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tower, J. Effects of light on aging and longevity. Ageing Res. Rev. 2019, 53, 100913. [Google Scholar] [CrossRef] [PubMed]
- Glickman, R.D. The origin of photo-oxidative stress in the aging eye. Prog. Brain Res. 2001, 131, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Baksheeva, V.E.; Tiulina, V.V.; Tikhomirova, N.K.; Gancharova, O.S.; Komarov, S.V.; Philippov, P.P.; Zamyatnin, A.A., Jr.; Senin, I.I.; Zernii, E.Y. Supression of light-induced oxidative stress in the retina by mitochondria-targeted antioxidant. Antioxidants 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wu, W.; Dentchev, T.; Zeng, Y.; Wang, J.; Tsui, I.; Tobias, J.W.; Bennett, J.; Baldwin, D.; Dunaief, J.L. Light damage induced changes in mouse retinal gene expression. Exp. Eye Res. 2004, 79, 239–247. [Google Scholar] [CrossRef]
- Chen, L.; Wu, W.; Dentchev, T.; Wong, R.; Dunaief, J.L. Increased metallothionein in light damaged mouse retinas. Exp. Eye Res. 2004, 79, 287–293. [Google Scholar] [CrossRef]
- Natoli, R.; Zhu, Y.; Valter, K.; Bisti, S.; Eells, J.; Stone, J. Gene and noncoding RNA regulation underlying photoreceptor protection: Microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol. Vis. 2010, 16, 1801–1822. [Google Scholar]
- Tsuruma, K.; Shimazaki, H.; Ohno, Y.; Inoue, Y.; Honda, A.; Imai, S.; Lee, J.; Shimazawa, M.; Satoh, M.; Hara, H. Metallothionein-III deficiency exacerbates light-induced retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7896–7903. [Google Scholar] [CrossRef]
- Michalska, A.E.; Choo, K.H. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc. Natl. Acad. Sci. USA 1993, 90, 8088–8092. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Bremner, I. Oxygen free radicals and metallothionein. Free Radic. Biol. Med. 1993, 14, 325–337. [Google Scholar] [CrossRef]
- Masters, B.A.; Kelly, E.J.; Quaife, C.J.; Brinster, R.L.; Palmiter, R.D. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc. Natl. Acad. Sci. USA 1994, 91, 584–588. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.Y.; Lee, S.J. Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol. Brain 2020, 13, 116. [Google Scholar] [CrossRef]
- Suemori, S.; Shimazawa, M.; Kawase, K.; Satoh, M.; Nagase, H.; Yamamoto, T.; Hara, H. Metallothionein, an endogenous antioxidant, protects against retinal neuron damage in mice. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3975–3982. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.W. Excitotoxic cell death. J. Neurobiol. 1992, 23, 1261–1276. [Google Scholar] [CrossRef]
- Salt, T.E. Handbook of Neurotoxicity; Kostrzewa, R.M., Ed.; Springer: New York, NY, USA, 2014; pp. 1273–1285. [Google Scholar]
- Wallin, C.; Weber, S.G.; Sandberg, M. Glutathione efflux induced by NMDA and kainate: Implications in neurotoxicity? J. Neurochem. 1999, 73, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Wallin, C.; Abbas, A.-K.; Tranberg, M.; Weber, S.G.; Wigström, H.; Sandberg, M. Searching for mechanisms of N-Methyl-D-Aspartate-induced glutathione efflux in organotypic hippocampal cultures. Neurochem. Res. 2003, 28, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karasawa, M.; Hosoi, J.; Hashiba, H.; Nose, K.; Tohyama, C.; Abe, E.; Suda, T.; Kuroki, T. Regulation of metallothionein gene expression by 1 alpha,25-dihydroxyvitamin D3 in cultured cells and in mice. Proc. Natl. Acad. Sci. USA 1987, 84, 8810–8813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tate, D.J.; Miceli, M.V.; Newsome, D.A. Zinc protects against oxidative damage in cultured human retinal pigment epithelial cells. Free Radic. Biol. Med. 1999, 26, 704–713. [Google Scholar] [CrossRef]
- Newsome, D.A.; Miceli, M.V.; Liles, M.R.; Tate, D.J.; Oliver, P.D. Antioxidants in the retinal pigment epithelium. Prog. Retinal Eye Res. 1994, 13, 101–123. [Google Scholar] [CrossRef]
- Chen, R.W.; Vasey, E.J.; Whanger, P.D. Accumulation and depletion of zinc in rat liver and kidney metallothionens. J. Nutr. 1977, 107, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Oliver, P.D.; Tate, D.J.; Newsome, D.A. Metallothionein in human retinal pigment epithelial cells: Expression, induction and zinc uptake. Curr. Eye Res. 1992, 11, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.J.; Newsome, D.A. A novel zinc compound (zinc monocysteine) enhances the antioxidant capacity of human retinal pigment epithelial cells. Curr Eye Res. 2006, 31, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Pabis, K.; Gundacker, C.; Giacconi, R.; Basso, A.; Costarelli, L.; Piacenza, F.; Strizzi, S.; Provinciali, M.; Malavolta, M. Zinc supplementation can reduce accumulation of cadmium in aged metallothionein transgenic mice. Chemosphere 2018, 211, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Trovato Battagliola, E.; D’Angelo, R.; Sidoti, A.; Donato, L. Expression of Pro-Angiogenic Markers Is Enhanced by Blue Light in Human RPE Cells. Antioxidants 2020, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; D’Angelo, R.; Alibrandi, S.; Rinaldi, C.; Sidoti, A.; Scimone, C. Effects of A2E-Induced Oxidative Stress on Retinal Epithelial Cells: New Insights on Differential Gene Response and Retinal Dystrophies. Antioxidants 2020, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Bailey, T.A.; Kanuga, N.; Romero, I.A.; Greenwood, J.; Luthert, P.J.; Cheetham, M.E. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, M.; Kurz, T.; Brunk, U.T.; Nilsson, S.E.; Frennesson, C.I. What does the commonly used DCF test for oxidative stress really show? Biochem. J. 2010, 428, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Zareba, M.; Raciti, M.W.; Henry, M.M.; Sarna, T.; Burke, J.M. Oxidative stress in ARPE-19 cultures: Do melanosomes confer cytoprotection? Free Radic. Biol. Med. 2006, 40, 87–100. [Google Scholar] [CrossRef]
- Kurz, T.; Karlsson, M.; Brunk, U.T.; Nilsson, S.E.; Frennesson, C. ARPE-19 retinal pigment epithelial cells are highly resistant to oxidative stress and exercise strict control over their lysosomal redox-active iron. Autophagy 2009, 5, 494–501. [Google Scholar] [CrossRef]
- Karlsson, M.; Frennesson, C.; Gustafsson, T.; Brunk, U.T.; Nilsson, S.E.; Kurz, T. Autophagy of iron-binding proteins may contribute to the oxidative stress resistance of ARPE-19 cells. Exp. Eye Res. 2013, 116, 359–365. [Google Scholar] [CrossRef]
- Karlsson, M.; Kurz, T. Attenuation of iron-binding proteins in ARPE-19 cells reduces their resistance to oxidative stress. Acta Ophthalmol. 2016, 94, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Creagh, E.M.; Carmody, R.J.; Cotter, T.G. Heat shock protein 70 inhibits caspase- dependent and -independent apoptosis in Jurkat T cells. Exp. Cell. Res. 2000, 257, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Baird, S.K.; Kurz, T.; Brunk, U.T. Metallothionein protects against oxidative stress-induced lysosomal destabilization. Biochem J. 2006, 394, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doulias, P.T.; Kotoglou, P.; Tenopoulou, M.; Keramisanou, D.; Tzavaras, T.; Brunk, U.; Galaris, D.; Angelidis, C. Involvement of heat shock protein-70 in the mechanism of hydrogen peroxide-induced DNA damage: The role of lysosomes and iron. Free Radic. Biol. Med. 2007, 42, 567–577. [Google Scholar] [CrossRef]
- Kurz, T.; Gustafsson, B.; Brunk, U.T. Cell sensitivity to oxidative stress is influenced by ferritin autophagy. Free Radic. Biol. Med. 2011, 50, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Xiao, T.; Srivastava, S.; Zhang, W.; Chan, L.L.; Vergara, L.A.; Van Kuijk, F.J.; Ansari, N.H. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol. Appl. Pharmacol. 2005, 204, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.Y.; Yoshida, A.; Tsunoo, H.; Nakajima, H. Mouse liver metallothioneins. Complete amino acid sequence of metallothionein-I. J. Biol. Chem. 1977, 252, 8217–8221. [Google Scholar] [CrossRef]
- Yoshida, A.; Kaplan, B.E.; Kimura, M. Metal-binding and detoxification effect of synthetic oligopeptides containing three cysteinyl residues. Proc. Natl. Acad. Sci. USA 1979, 76, 486–490. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, L.; Gu, S.; Yu, Y.; Huang, H.; Mo, K.; Xu, H.; Zeng, F.; Xiao, Y.; Peng, L.; et al. D609 protects retinal pigmented epithelium as a potential therapy for age-related macular degeneration. Signal Transduct. Target Ther. 2020, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, C.M.; Biswal, M.R.; Li, H.; Han, P.; Ildefonso, C.J.; Lewin, A.S. Repurposing an orally available drug for the treatment of geographic atrophy. Mol. Vis. 2016, 22, 294–310. [Google Scholar] [PubMed]
- Castello, P.R.; Drechsel, D.A.; Patel, M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J. Biol. Chem. 2007, 282, 14186–14193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswal, M.R.; Ahmed, C.M.; Ildefonso, C.J.; Han, P.; Li, H.; Jivanji, H.; Mao, H.; Lewin, A.S. Systemic treatment with a 5HT1a agonist induces anti-oxidant protection and preserves the retina from mitochondrial oxidative stress. Exp. Eye Res. 2015, 140, 94–105. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.J.; Rubio, M.D.; Defagot, C.; Hischberg, L.; Villar, M.J.; Brusco, A. The 5HT1A receptor agonist, 8-OH-DPAT, protects neurons and reduces astroglial reaction after ischemic damage caused by cortical devascularization. Brain Res. 2004, 1030, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Asanuma, M.; Murakami, S.; Takeshima, M.; Torigoe, N.; Kitamura, Y.; Miyoshi, K. Targeting 5-HT(1A) receptors in astrocytes to protect dopaminergic neurons in Parkinsonian models. Neurobiol. Dis. 2013, 59, 244–256. [Google Scholar] [CrossRef]
- Rodríguez-Menéndez, S.; Fernández, B.; García, M.; Álvarez, L.; Fernández, M.L.; Sanz-Medel, A.; Coca-Prados, M.; Pereiro, R.; González-Iglesias, H. Quantitative study of zinc and metallothioneins in the human retina and RPE cells by mass spectrometry-based methodologies. Talanta 2018, 178, 222–230. [Google Scholar] [CrossRef]
- Rodríguez-Menéndez, S.; García, M.; Fernández, B.; Álvarez, L.; Fernández-Vega-Cueto, A.; Coca-Prados, M.; Pereiro, R.; González-Iglesias, H. The Zinc-Metallothionein Redox System Reduces Oxidative Stress in Retinal Pigment Epithelial Cells. Nutrients 2018, 10, 1874. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Barrios, A.; Álvarez, L.; García, M.; Artime, E.; Pereiro, R.; González-Iglesias, H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants 2021, 10, 89. https://doi.org/10.3390/antiox10010089
Álvarez-Barrios A, Álvarez L, García M, Artime E, Pereiro R, González-Iglesias H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants. 2021; 10(1):89. https://doi.org/10.3390/antiox10010089
Chicago/Turabian StyleÁlvarez-Barrios, Ana, Lydia Álvarez, Montserrat García, Enol Artime, Rosario Pereiro, and Héctor González-Iglesias. 2021. "Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins" Antioxidants 10, no. 1: 89. https://doi.org/10.3390/antiox10010089
APA StyleÁlvarez-Barrios, A., Álvarez, L., García, M., Artime, E., Pereiro, R., & González-Iglesias, H. (2021). Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants, 10(1), 89. https://doi.org/10.3390/antiox10010089