A Reductive Metabolic Switch Protects Infants with Transposition of Great Arteries Undergoing Atrial Septostomy against Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Analytical Procedures
2.2.1. Lipid Peroxidation Biomarkers
2.2.2. Untargeted Ultra-Performance Liquid Chromatography Coupled to Time-of-Flight Mass Spectrometry (UPLC-TOFMS) Metabolomic Analysis
2.2.3. Data Processing and Statistical Analysis
3. Results
3.1. Clinical Results
3.2. Lipid Peroxidation Biomarkers
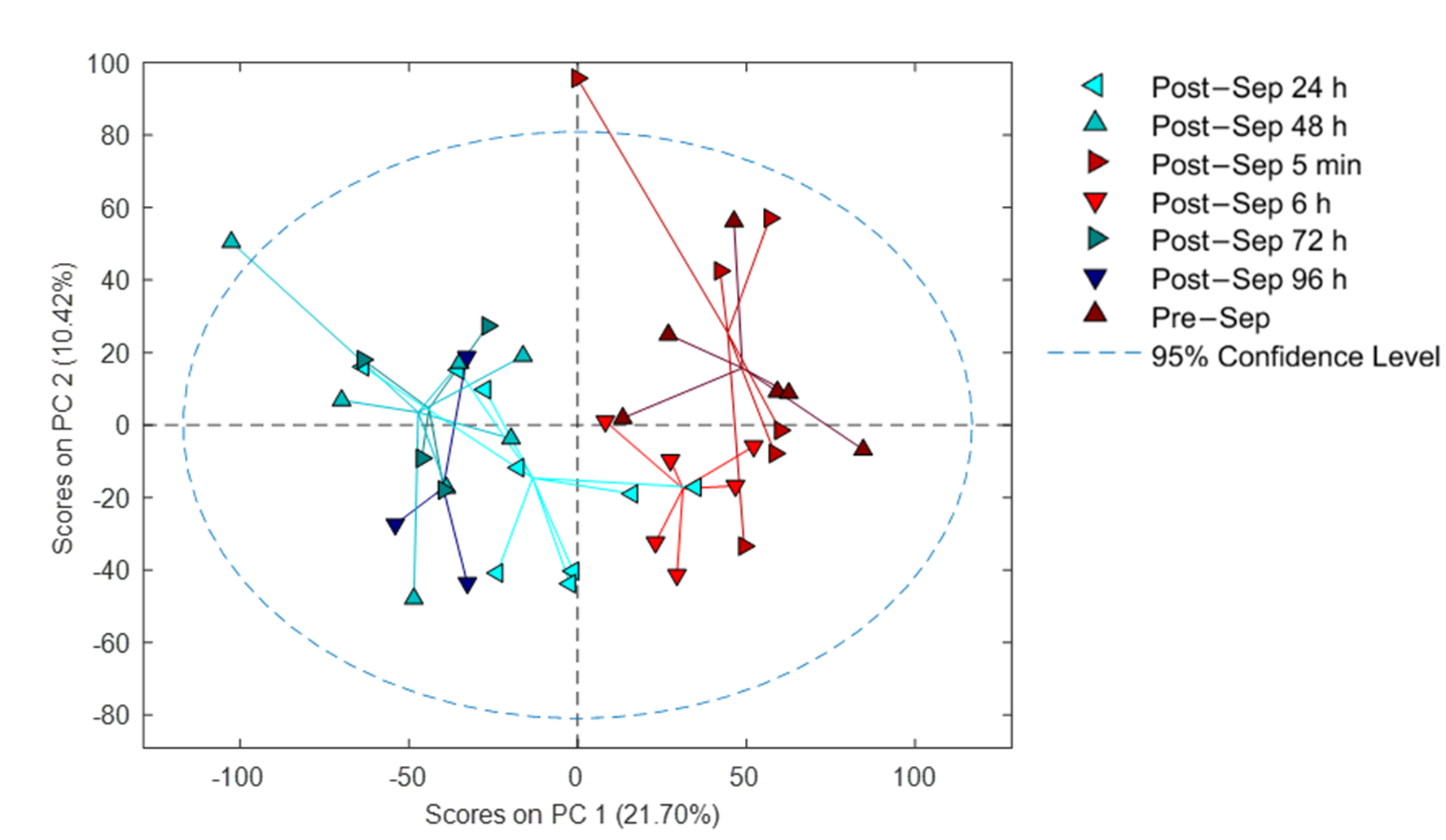
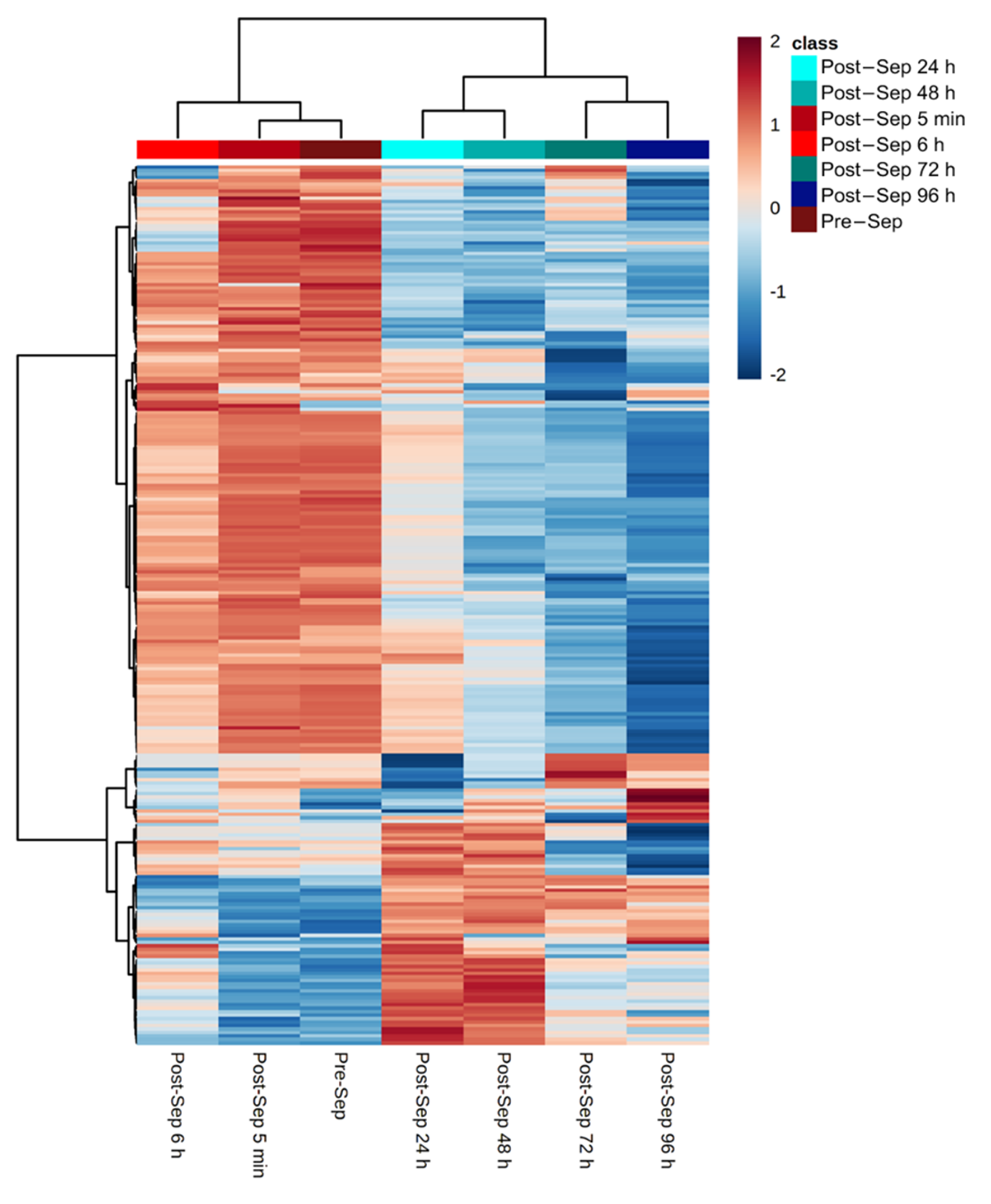
3.3. Effect of Time on the Plasma Metabolome
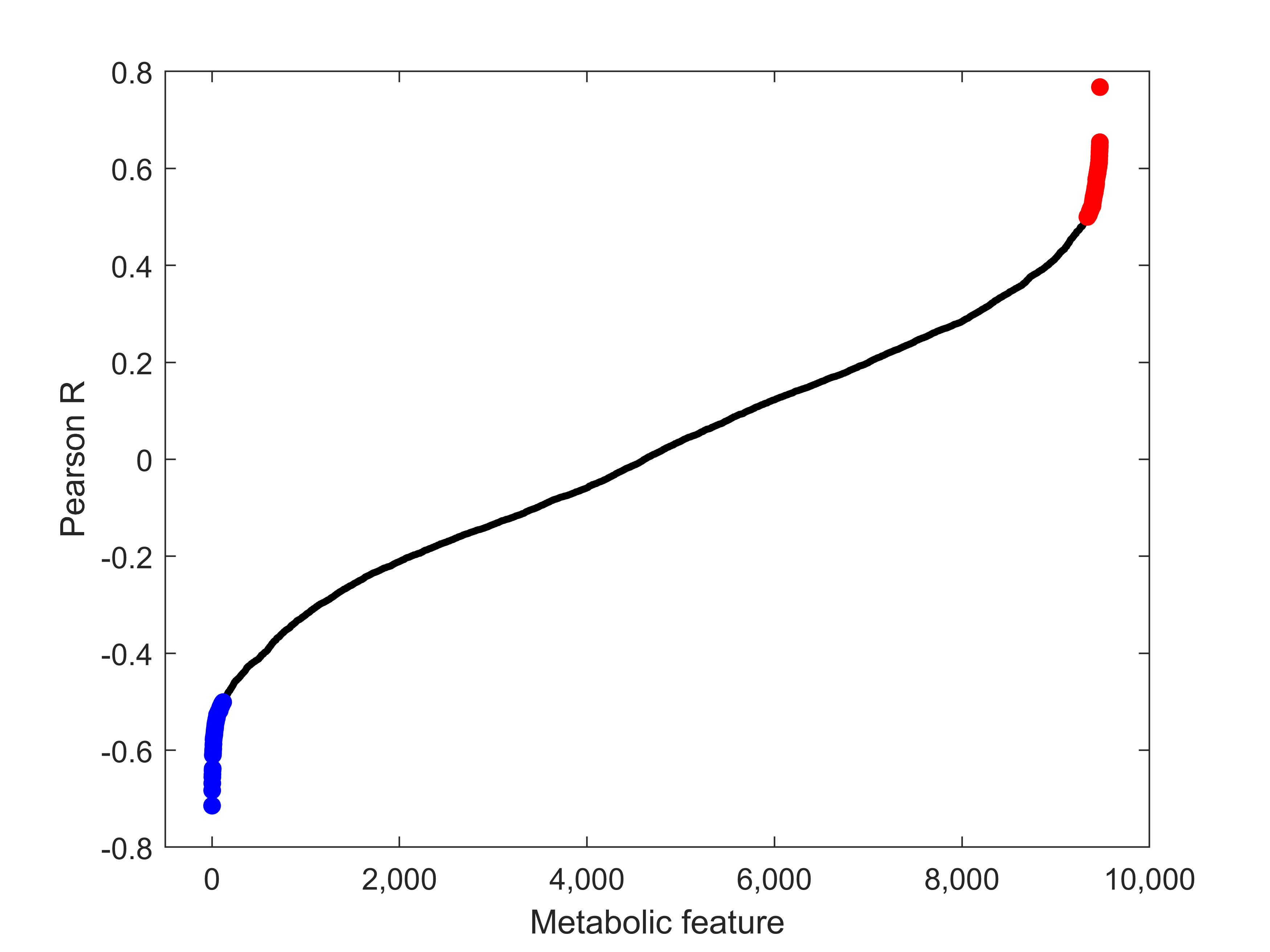
3.4. Correlation of Metabolic Features with FTOE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Linde, D.; Konings, E.E.M.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.M.; Roos-Hesselink, J.W. Birth Prevalence of Congenital Heart Disease Worldwide: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellinger, D.C.; Wypij, D.; Rivkin, M.J.; DeMaso, D.R.; Robertson, R.L.; Dunbar-Masterson, C.; Rappaport, L.A.; Wernovsky, G.; Jonas, R.A.; Newburger, J.W. Adolescents with D-Transposition of the Great Arteries Corrected with the Arterial Switch Procedure: Neuropsychological Assessment and Structural Brain Imaging. Circulation 2011, 124, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Kingdom, T.; Saini, B.; Chau, V.; Post, M.; Blaser, S.; Macgowan, C.; Miller, S.P.; Seed, M. Cerebral Oxygen Delivery Is Reduced in Newborns with Congenital Heart Disease. J. Thorac. Cardiovasc. Surg. 2016, 152, 1095–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Macgowan, C.K.; Sled, J.G.; Yoo, S.-J.; Manlhiot, C.; Porayette, P.; Grosse-Wortmann, L.; Jaeggi, E.; McCrindle, B.W.; Kingdom, J.; et al. Reduced Fetal Cerebral Oxygen Consumption Is Associated with Smaller Brain Size in Fetuses With Congenital Heart Disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef] [Green Version]
- Park, I.S.; Yoon, S.Y.; Min, J.Y.; Kim, Y.H.; Ko, J.K.; Kim, K.S.; Seo, D.M.; Lee, J.H. Metabolic Alterations and Neurodevelopmental Outcome of Infants with Transposition of the Great Arteries. Pediatr. Cardiol. 2006, 27, 569–576. [Google Scholar] [CrossRef]
- Van der Laan, M.E.; Verhagen, E.A.; Bos, A.F.; Berger, R.M.F.; Kooi, E.M.W. Effect of Balloon Atrial Septostomy on Cerebral Oxygenation in Neonates with Transposition of the Great Arteries. Pediatr. Res. 2013, 73, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, D.; Lindsay, M.; Zhang, Y.; Lardaro, T.; Osen, H.; Chang, D.C.; Brenner, J.I.; Abdullah, F. Analysis of 8681 Neonates with Transposition of the Great Arteries: Outcomes with and without Rashkind Balloon Atrial Septostomy. Cardiol. Young 2010, 20, 373–380. [Google Scholar] [CrossRef]
- Hiremath, G.; Natarajan, G.; Math, D.; Aggarwal, S. Impact of Balloon Atrial Septostomy in Neonates with Transposition of Great Arteries. J. Perinatol. 2011, 31, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Hypoxic-Ischemic Encephalopathy: A Review for the Clinician|Cerebrovascular Disease|JAMA Pediatrics|JAMA Network. Available online: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2118582 (accessed on 13 July 2020).
- Terraneo, L.; Samaja, M. Comparative Response of Brain to Chronic Hypoxia and Hyperoxia. Int. J. Mol. Sci 2017, 18, 1914. [Google Scholar] [CrossRef] [Green Version]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sánchez-Illana, A.; Nuñez-Ramiro, A.; Kuligowski, J.; Cháfer-Pericás, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and Oxidative Stress in the Perinatal Period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef]
- Redox Signaling during Hypoxia in Mammalian Cells|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S2213231717302355?tken=9F2B800D98BB8EAC354ADDF5283F82835DF2689AA00C41C7A45BC70C1B1C24E5567F1645B0354F153F5ECD84CE7FAF8E (accessed on 13 July 2020).
- Piñeiro-Ramos, J.D.; Núñez-Ramiro, A.; Llorens-Salvador, R.; Parra-Llorca, A.; Sánchez-Illana, Á.; Quintás, G.; Boronat-González, N.; Martínez-Rodilla, J.; Kuligowski, J.; Vento, M.; et al. Metabolic Phenotypes of Hypoxic-Ischemic Encephalopathy with Normal vs. Pathologic Magnetic Resonance Imaging Outcomes. Metabolites 2020, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.-W.; Tamrazi, B.; Hsu, K.-H.; Ho, E.; Reitman, A.J.; Borzage, M.; Blüml, S.; Wisnowski, J.L. Cerebral Lactate Concentration in Neonatal Hypoxic-Ischemic Encephalopathy: In Relation to Time, Characteristic of Injury, and Serum Lactate Concentration. Front. Neurol. 2018, 9, 293. [Google Scholar] [CrossRef]
- Fanos, V.; Pintus, R.; Dessì, A. Clinical Metabolomics in Neonatology: From Metabolites to Diseases. NEO 2018, 113, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Illana, Á.; Thayyil, S.; Montaldo, P.; Jenkins, D.; Quintás, G.; Oger, C.; Galano, J.-M.; Vigor, C.; Durand, T.; Vento, M.; et al. Novel Free-Radical Mediated Lipid Peroxidation Biomarkers in Newborn Plasma. Anal. Chim. Acta 2017, 996, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Illana, Á.; Shah, V.; Piñeiro-Ramos, J.D.; Di Fiore, J.M.; Quintás, G.; Raffay, T.M.; MacFarlane, P.M.; Martin, R.J.; Kuligowski, J. Adrenic Acid Non-Enzymatic Peroxidation Products in Biofluids of Moderate Preterm Infants. Free Radic. Biol. Med. 2019, 142, 107–112. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and Considerations for the Use of System Suitability and Quality Control Samples in Mass Spectrometry Assays Applied in Untargeted Clinical Metabolomic Studies. Metabolomics 2018, 14, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open Source Software for Rapid Proteomics Tools Development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Benton, H.P.; Want, E.J.; Ebbels, T.M.D. Correction of Mass Calibration Gaps in Liquid Chromatography-Mass Spectrometry Metabolomics Data. Bioinformatics 2010, 26, 2488–2489. [Google Scholar] [CrossRef] [Green Version]
- Tautenhahn, R.; Böttcher, C.; Neumann, S. Highly Sensitive Feature Detection for High Resolution LC/MS. BMC Bioinform. 2008, 9, 504. [Google Scholar] [CrossRef] [Green Version]
- Kuhl, C.; Tautenhahn, R.; Böttcher, C.; Larson, T.R.; Neumann, S. CAMERA: An Integrated Strategy for Compound Spectra Extraction and Annotation of Liquid Chromatography/Mass Spectrometry Data Sets. Anal. Chem. 2012, 84, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Illana, Á.; Piñeiro-Ramos, J.D.; Sanjuan-Herráez, J.D.; Vento, M.; Quintás, G.; Kuligowski, J. Evaluation of Batch Effect Elimination Using Quality Control Replicates in LC-MS Metabolite Profiling. Anal. Chim. Acta 2018, 1019, 38–48. [Google Scholar] [CrossRef]
- Quintás, G.; Sánchez-Illana, Á.; Piñeiro-Ramos, J.D.; Kuligowski, J. Chapter Six-Data Quality Assessment in Untargeted LC-MS Metabolomics. In Comprehensive Analytical Chemistry; Jaumot, J., Bedia, C., Tauler, R., Eds.; Data Analysis for Omic Sciences: Methods and Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 82, pp. 137–164. [Google Scholar]
- Kuligowski, J.; Sánchez-Illana, Á.; Sanjuán-Herráez, D.; Vento, M.; Quintás, G. Intra-Batch Effect Correction in Liquid Chromatography-Mass Spectrometry Using Quality Control Samples and Support Vector Regression (QC-SVRC). Analyst 2015, 140, 7810–7817. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, C.-J. LIBSVM: A Library for Support Vector Machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 1–27. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Johnston, M.V.; Trescher, W.H.; Ishida, A.; Nakajima, W. Neurobiology of Hypoxic-Ischemic Injury in the Developing Brain. Pediatr. Res. 2001, 49, 735–741. [Google Scholar] [CrossRef]
- Fatemi, A.; Wilson, M.A.; Johnston, M.V. Hypoxic-Ischemic Encephalopathy in the Term Infant. Clin. Perinatol. 2009, 36, 835–858. [Google Scholar] [CrossRef] [Green Version]
- Teshima, Y.; Akao, M.; Li, R.A.; Chong, T.H.; Baumgartner, W.A.; Johnston, M.V.; Marbán, E. Mitochondrial ATP-Sensitive Potassium Channel Activation Protects Cerebellar Granule Neurons from Apoptosis Induced by Oxidative Stress. Stroke 2003, 34, 1796–1802. [Google Scholar] [CrossRef] [Green Version]
- Millán, I.; Piñero-Ramos, J.D.; Lara, I.; Parra-Llorca, A.; Torres-Cuevas, I.; Vento, M. Oxidative Stress in the Newborn Period: Useful Biomarkers in the Clinical Setting. Antioxidants 2018, 7, 193. [Google Scholar] [CrossRef] [Green Version]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The Return of Metabolism: Biochemistry and Physiology of the Pentose Phosphate Pathway. Biol. Rev. Camb Philos Soc. 2015, 90, 927–963. [Google Scholar] [CrossRef] [Green Version]
- Brekke, E.M.; Morken, T.S.; Widerøe, M.; Håberg, A.K.; Brubakk, A.-M.; Sonnewald, U. The Pentose Phosphate Pathway and Pyruvate Carboxylation after Neonatal Hypoxic-Ischemic Brain Injury. Biol. Rev. 2014, 34, 724–734. [Google Scholar] [CrossRef] [Green Version]
- Kuehne, A.; Emmert, H.; Soehle, J.; Winnefeld, M.; Fischer, F.; Wenck, H.; Gallinat, S.; Terstegen, L.; Lucius, R.; Hildebrand, J.; et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol. Cell 2015, 59, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Ralser, M.; Wamelink, M.M.; Kowald, A.; Gerisch, B.; Heeren, G.; Struys, E.A.; Klipp, E.; Jakobs, C.; Breitenbach, M.; Lehrach, H.; et al. Dynamic Rerouting of the Carbohydrate Flux Is Key to Counteracting Oxidative Stress. J. Biol. 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-P.; Zhou, L.-S.; Zhao, Y.-Z.; Wang, S.-W.; Chen, L.-L.; Liu, L.-X.; Ling, Z.-Q.; Hu, F.-J.; Sun, Y.-P.; Zhang, J.-Y.; et al. Regulation of G6PD Acetylation by SIRT2 and KAT9 Modulates NADPH Homeostasis and Cell Survival during Oxidative Stress. EMBO J. 2014, 33, 1304–1320. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.; Shah, S.M.A.; Munir, N.; Daniyal, M.; Tahir, I.M.; Mahmood, Z.; Irshad, M.; Akhlaq, M.; Sultana, S.; Zainab, R. Hexose Monophosphate Shunt, the Role of Its Metabolites and Associated Disorders: A Review. J. Cell. Physiol. 2019, 234, 14473–14482. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.M.; Das, A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]

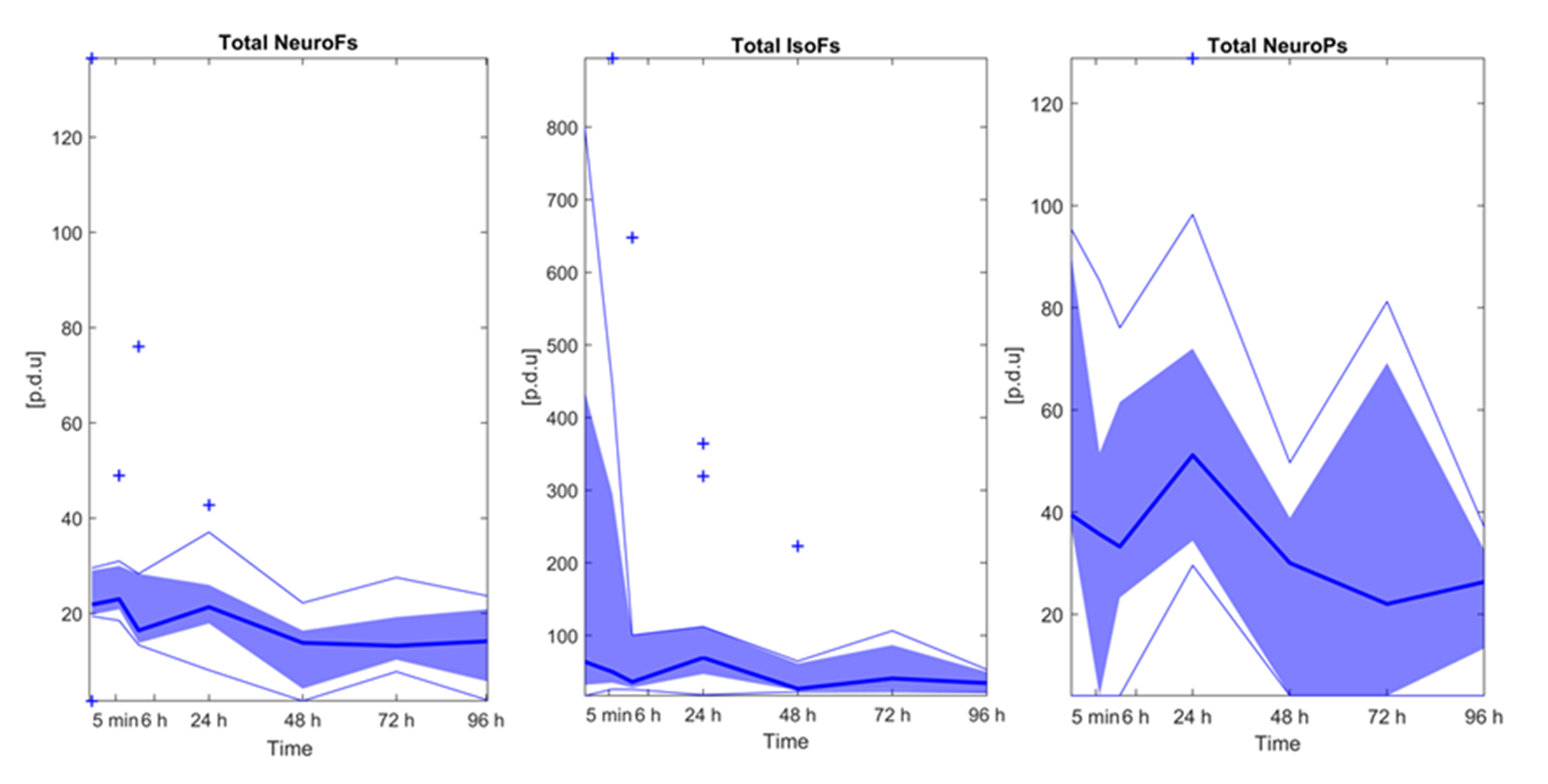
| Analyte [p.d.u.] | RT [min] | Parent Ion (m/z) | Daughter Ion (m/z) | CE (eV) | Cone Voltage (V) |
|---|---|---|---|---|---|
| IsoPs | 4.3–6.6 | 353.20 | 115.00 | 30 | 35 |
| Di-homo-IsoPs | 5.0–6.8 | 381.00 | 143.00 | 20 | 20 |
| Di-homo-IsoFs | 3.5–6.5 | 397.00 | 155.00 | 24 | 35 |
| NeuroFs | 2.70–6.50 | 393.00 | 193.00 | 20 | 35 |
| IsoFs | 2.1–6.60 | 369.20 | 115.00 | 20 | 45 |
| NeuroPs | 2.30–6.50 | 377.00 | 101.00 | 20 | 35 |
| Patients’ Demographics | All Patients | BAS Patients |
|---|---|---|
| Patients recruited, N (%) | 12 (100) | 9 (100) |
| Gender, N (%) | ||
| - Male | 10 (83) | 8 (89) |
| - Female | 2 (17) | 1 (11) |
| Balloon atrial septostomy, N (%) | 9 (75) | 9 (100) |
| Expired, N (%) | 1 (8.3) | 0 (0) |
| Prenatal diagnosis, N (%) | 6 (50) | 4 (44) |
| Gestational age, weeks (median, range) | 39.5 (37.0–41.3) | 39.9 (38.4–41.3) |
| Birth weight, kg (median, range) | 3.4 (2.0–4.0) | 3.5 (3.1–4.0) |
| Postnatal age, hours (median, range) | ||
| - At the time of the first measured peripheral saturation | 1.7 (0.5–13.2) | 2.3 (0.5–6.7) |
| - At time of BAS | 3.2 (2.0–9.1) | |
| Saturation, % (median, range) | ||
| - First peripheral saturation (SpO2) | 79 (51–91) | 77 (56–88) |
| - Lowest peripheral saturation (SpO2) | 65 (39–86) | 65 (39–86) |
| - First regional cerebral oxygen saturation (rcSO2) | 58 (37–79) | 53 (37–79) |
| - 5-min post septostomy peripheral saturation (SpO2) | 86 (62–91) | 86 (62–91) |
| Blood lactate, mmol/L (median, range) | ||
| - Before BAS | 3.2 (1.3–4.9) | 3.6 (1.3–4.9) |
| - 5-min after BAS | 2.7 (1.4–5.0) | 3.3 (1.4–5.0) |
| Pathway Name | Compound Code (KEGG) | Pathway ID | # Hits | # Sig Hits | p-Value |
|---|---|---|---|---|---|
| Pentose phosphate pathway | C01801; C00672; C00121; C00121; C00257; C00257; C00258 | map00030 | 7 | 7 | 0.00002 |
| Pentose and glucuronate interconversions | C01068; C00181; C00259; C00310; C00312; C00379; C00532; C00379; C00532; C00379; C00379; C00532; C00257; C00257; C00029; C00052; C00191; C00618; C02266 | map00040 | 11 | 7 | 0.003 |
| Ascorbate and aldarate metabolism | C00137; C00029; C02670; C00191; C00800 | map00053 | 8 | 5 | 0.013 |
| Inositol phosphate metabolism | C00137; C00222; C00191 | map00562 | 4 | 3 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñeiro-Ramos, J.D.; Rahkonen, O.; Korpioja, V.; Quintás, G.; Pihkala, J.; Pitkänen-Argillander, O.; Rautiainen, P.; Andersson, S.; Kuligowski, J.; Vento, M. A Reductive Metabolic Switch Protects Infants with Transposition of Great Arteries Undergoing Atrial Septostomy against Oxidative Stress. Antioxidants 2021, 10, 1502. https://doi.org/10.3390/antiox10101502
Piñeiro-Ramos JD, Rahkonen O, Korpioja V, Quintás G, Pihkala J, Pitkänen-Argillander O, Rautiainen P, Andersson S, Kuligowski J, Vento M. A Reductive Metabolic Switch Protects Infants with Transposition of Great Arteries Undergoing Atrial Septostomy against Oxidative Stress. Antioxidants. 2021; 10(10):1502. https://doi.org/10.3390/antiox10101502
Chicago/Turabian StylePiñeiro-Ramos, José David, Otto Rahkonen, Virpi Korpioja, Guillermo Quintás, Jaana Pihkala, Olli Pitkänen-Argillander, Paula Rautiainen, Sture Andersson, Julia Kuligowski, and Máximo Vento. 2021. "A Reductive Metabolic Switch Protects Infants with Transposition of Great Arteries Undergoing Atrial Septostomy against Oxidative Stress" Antioxidants 10, no. 10: 1502. https://doi.org/10.3390/antiox10101502
APA StylePiñeiro-Ramos, J. D., Rahkonen, O., Korpioja, V., Quintás, G., Pihkala, J., Pitkänen-Argillander, O., Rautiainen, P., Andersson, S., Kuligowski, J., & Vento, M. (2021). A Reductive Metabolic Switch Protects Infants with Transposition of Great Arteries Undergoing Atrial Septostomy against Oxidative Stress. Antioxidants, 10(10), 1502. https://doi.org/10.3390/antiox10101502









