Pterostilbene Attenuates Particulate Matter-Induced Oxidative Stress, Inflammation and Aging in Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
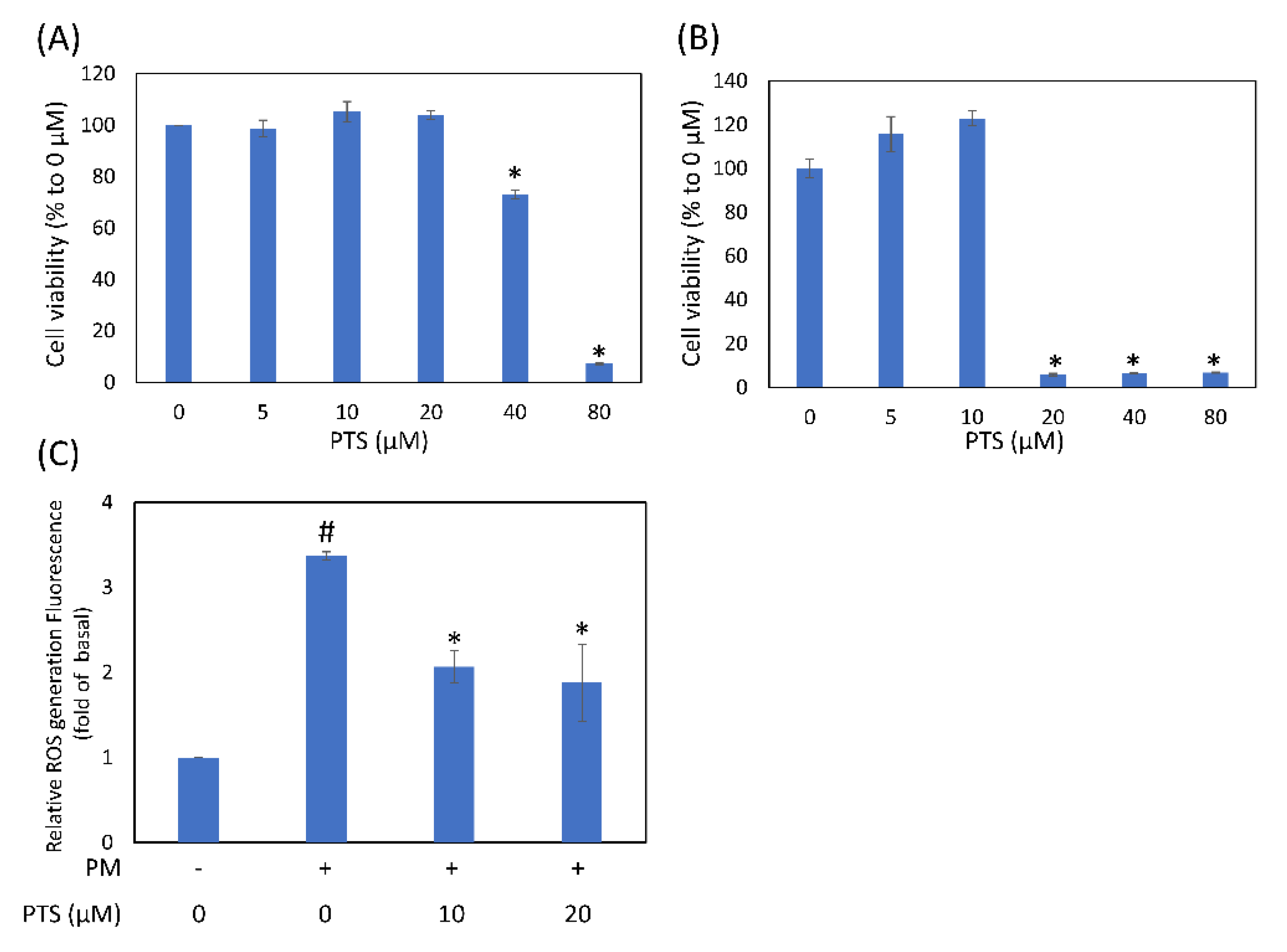
2.1. Cell Culture and Viability Assay
2.2. Intracellular Reactive Oxygen Species (ROS) Assay
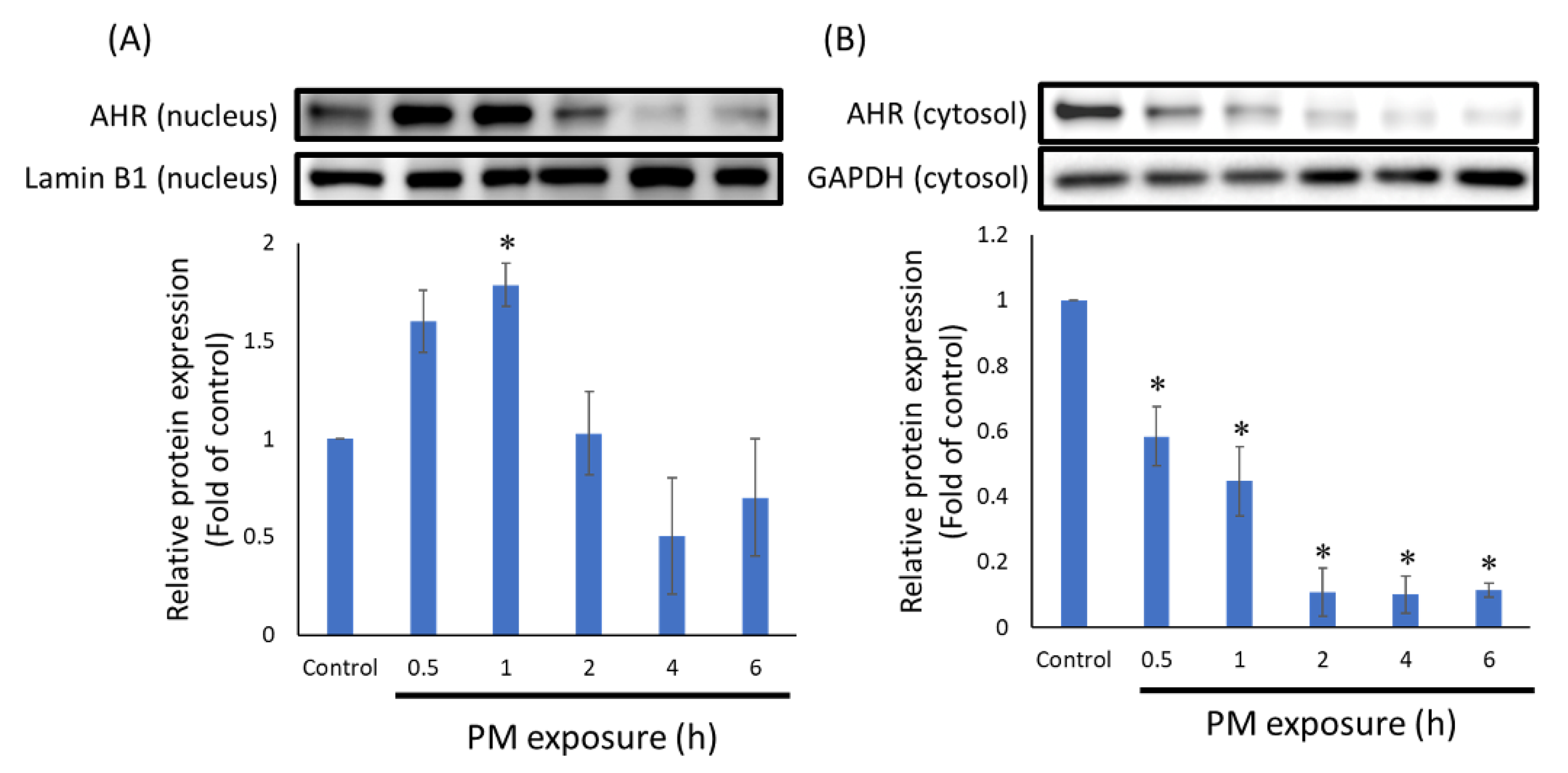
2.3. Western Blot
2.4. HaCaT Cells Nuclear Protein Extraction
2.5. Measurement of Pterostilbene Content in HaCaT Cells
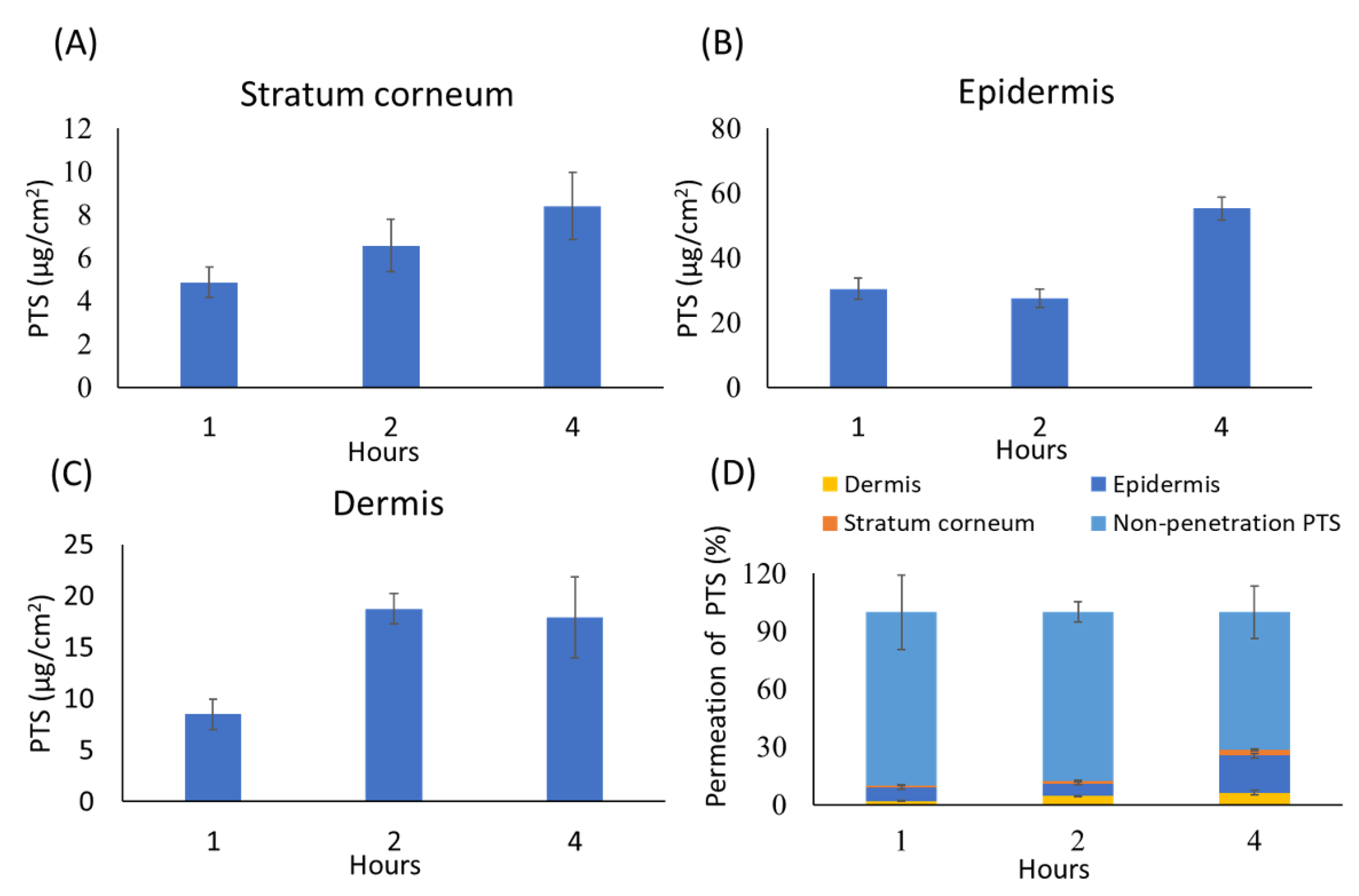
2.6. In Vitro Skin Penetration Study
2.7. Statistical Analysis
3. Results
3.1. Effects of Pterostilbene on Cell Viability and Intracellular ROS Generation in Human Keratinocyte Cells
3.2. PTS Inhibited the Translocation of AHR into Nucleus
3.3. PTS Inhibited PM-Induced MAPKs Protein Activation
3.4. PTS Inhibited PM-Induced Inflammatory, Aging and Moisturizing Proteins Expression in HaCaT Cells
3.5. The Distribution of Pterostilbene in the Intracellular and Extracellular Compartments
3.6. In Vitro Skin Penetration of Pterostilbene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemayehu, Y.A.; Asfaw, S.L.; Terfie, T.A. Exposure to urban particulate matter and its association with human health risks. Environ. Sci. Pollut. Res. Int. 2020, 27, 27491–27506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, Z.; Tian, Z.; Xie, Y.; Xin, F.; Jiang, R.; Kan, H.; Song, W. The biological effects of individual-level PM(2.5) exposure on systemic immunity and inflammatory response in traffic policemen. Occup. Environ. Med. 2013, 70, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, L.; Tang, M. Toxicity of inhaled particulate matter on the central nervous system: Neuroinflammation, neuropsychological effects and neurodegenerative disease. J. Appl. Toxicol. 2017, 37, 644–667. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Dijkhoff, I.M.; Drasler, B.; Karakocak, B.B.; Petri-Fink, A.; Valacchi, G.; Eeman, M.; Rothen-Rutishauser, B. Impact of airborne particulate matter on skin: A systematic review from epidemiology to in vitro studies. Part. Fibre Toxicol. 2020, 17, 35. [Google Scholar] [CrossRef]
- Esser, C.; Bargen, I.; Weighardt, H.; Haarmann-Stemmann, T.; Krutmann, J. Functions of the aryl hydrocarbon receptor in the skin. Semin. Immunopathol. 2013, 35, 677–691. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Bossis, G.; Hyun, Y.M.; Park, C.O.; Hyun, J.W. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Byun, E.J.; Lee, J.D.; Kim, S.; Kim, H.S. Air Pollution, Autophagy, and Skin Aging: Impact of Particulate Matter (PM10) on Human Dermal Fibroblasts. Int. J. Mol. Sci. 2018, 19, 2727. [Google Scholar] [CrossRef] [Green Version]
- Morita, A.; Torii, K.; Maeda, A.; Yamaguchi, Y. Molecular basis of tobacco smoke-induced premature skin aging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Sardy, M. Role of matrix metalloproteinases in skin ageing. Connect. Tissue Res. 2009, 50, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.H.; Jeong, G.J.; Park, K.Y.; Lee, M.K.; Seo, S.J. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp. Dermatol. 2019, 28, 809–815. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hsu, L.F.; Fang, J.Y.; Chiang, Y.C.; Tsai, M.H.; Lee, M.H.; Li, S.Y.; Hu, S.C.; Lee, I.T.; et al. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants-exposed human keratinocytes through ROS/MAPKs pathways. J. Ethnopharmacol. 2016, 189, 300–309. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hu, S.C.; Chiang, Y.C.; Hsu, L.F.; Lin, Y.C.; Lee, I.T.; Tsai, M.H.; Fang, J.Y. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016, 6, 27995. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Li, Q.; Du, H.Y.; Wang, Q.W.; Huang, Y.; Liu, W. Airborne polycyclic aromatic hydrocarbons trigger human skin cells aging through aryl hydrocarbon receptor. Biochem. Biophys. Res. Commun. 2017, 488, 445–452. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ito, T.; Tsuji, G.; Furue, M. Baicalein Inhibits Benzo[a]pyrene-Induced Toxic Response by Downregulating Src Phosphorylation and by Upregulating NRF2-HMOX1 System. Antioxidants 2020, 9, 507. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.H.; Yoo, J.A.; Kwon, K.; Kim, J.W.; Oh, S.W.; Park, S.J.; Kim, J.; Yu, E.; Han, B.S.; et al. Antagonizing Effects of Clematis apiifolia DC. Extract against Benzo[a]pyrene-Induced Damage to Human Keratinocytes. Oxidative Med. Cell. Longev. 2019, 2019, 2386163. [Google Scholar] [CrossRef]
- Uchi, H.; Yasumatsu, M.; Morino-Koga, S.; Mitoma, C.; Furue, M. Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 2017, 85, 36–43. [Google Scholar] [CrossRef]
- Liu, Y.; You, Y.; Lu, J.; Chen, X.; Yang, Z. Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol. Molecules 2020, 25, 5166. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Chiou, Y.S.; Ho, C.T.; Pan, M.H. Chemoprevention by resveratrol and pterostilbene: Targeting on epigenetic regulation. Biofactors 2018, 44, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, N.; Liang, B.; Liu, Q.; Zhang, E.; Peng, L.; Deng, H.; Li, R.; Li, Z.; Zhu, H. Pterostilbene protects against UVB-induced photo-damage through a phosphatidylinositol-3-kinase-dependent Nrf2/ARE pathway in human keratinocytes. Redox Rep. 2017, 22, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.J.; Chiu, H.W.; Lee, Y.L.; Li, C.Y.; Wang, Y.J.; Lee, Y.H. Pterostilbene Attenuates Hexavalent Chromium-Induced Allergic Contact Dermatitis by Preventing Cell Apoptosis and Inhibiting IL-1beta-Related NLRP3 Inflammasome Activation. J. Clin. Med. 2018, 7, 489. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.H.; Tseng, C.H.; Lin, C.Y.; Lee, C.W.; Yen, F.L. Preparation, characterizations and anti-pollutant activity of 7,3’,4’-trihydroxyisoflavone nanoparticles in particulate matter-induced HaCaT keratinocytes. Int. J. Nanomed. 2018, 13, 3279–3293. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.H.; Hu, S.C.S.; Yen, F.L.; Tseng, C.H. Improvement of Skin Penetration, Antipollutant Activity and Skin Hydration of 7,3’,4’-Trihydroxyisoflavone Cyclodextrin Inclusion Complex. Pharmaceutics 2019, 11, 399. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Guo, M.; Yu, H.; Liu, D.; Lu, Z.; Lu, Y. Propionibacterium acnes related anti-inflammation and skin hydration activities of madecassoside, a pentacyclic triterpene saponin from Centella asiatica. Biosci. Biotechnol. Biochem. 2019, 83, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Jain, R.; Mundkur, L.; Rajalakshmi, H.R.; Lad, P.S.; Neupane, P. An Open-Label Single-Arm, Monocentric Study Assessing the Efficacy and Safety of Natural Pterostilbene (Pterocarpus marsupium) for Skin Brightening and Antiaging Effects. Clin. Cosmet. Investig. Dermatol. 2020, 13, 105–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors-Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Soares-Silva, M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef]
- Zhen, A.X.; Hyun, Y.J.; Piao, M.J.; Fernando, P.; Kang, K.A.; Ahn, M.J.; Yi, J.M.; Kang, H.K.; Koh, Y.S.; Lee, N.H.; et al. Eckol Inhibits Particulate Matter 2.5-Induced Skin Keratinocyte Damage via MAPK Signaling Pathway. Mar. Drugs 2019, 17, 444. [Google Scholar] [CrossRef] [Green Version]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxidative Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Li, S.; Lin, C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Boury-Jamot, M.; Sougrat, R.; Tailhardat, M.; Le Varlet, B.; Bonte, F.; Dumas, M.; Verbavatz, J.M. Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter? Biochim. Biophys. Acta 2006, 1758, 1034–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seleit, I.; Bakry, O.A.; El Rebey, H.S.; El-Akabawy, G.; Hamza, G. Is Aquaporin-3 a Determinant Factor of Intrinsic and Extrinsic Aging? An Immunohistochemical and Morphometric Study. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Tang, L.C.; Liu, C.H.; Liao, P.Y.; Lai, J.C.; Yang, J.H. Glycolic acid attenuates UVB-induced aquaporin-3, matrix metalloproteinase-9 expression, and collagen degradation in keratinocytes and mouse skin. Biochem. J. 2019, 476, 1387–1400. [Google Scholar] [CrossRef]
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Di, L.; Kerns, E.H. The effect of plasma protein binding on in vivo efficacy: Misconceptions in drug discovery. Nat. Rev. Drug Discov. 2010, 9, 929–939. [Google Scholar] [CrossRef]
- Gordon, L.J.; Allen, M.; Artursson, P.; Hann, M.M.; Leavens, B.J.; Mateus, A.; Readshaw, S.; Valko, K.; Wayne, G.J.; West, A. Direct Measurement of Intracellular Compound Concentration by RapidFire Mass Spectrometry Offers Insights into Cell Permeability. J. Biomol. Screen 2016, 21, 156–164. [Google Scholar] [CrossRef] [Green Version]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- Cheruvu, H.S.; Liu, X.; Grice, J.E.; Roberts, M.S. Modeling percutaneous absorption for successful drug discovery and development. Expert Opin. Drug Discov. 2020, 15, 1181–1198. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]




| Protein | Time of PTS Administration (min) | Intracellular PTS Concentration (μM) | |||
|---|---|---|---|---|---|
| * Pre-Treat | PM Exposure | Total | PM Exposure | End of PM Exposure | |
| AHR (nucleus) | 60 | 30 | 90 | 4.3 | between 4.3 and 3.5 |
| CYP1A1 | 180 | 120 | 300 | 3.8 | 2.0 |
| p-p38 | 180 | 120 | 300 | 3.8 | 2.0 |
| p-ERK | 180 | 120 | 300 | 3.8 | 2.0 |
| p-JNK | 180 | 120 | 300 | 3.8 | 2.0 |
| COX-2 | 180 | 360 | 540 | 3.8 | 1.8 |
| MMPs | 180 | 360 | 540 | 3.8 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, W.-L.; Huang, P.-H.; Wang, H.-C.; Tseng, C.-H.; Yen, F.-L. Pterostilbene Attenuates Particulate Matter-Induced Oxidative Stress, Inflammation and Aging in Keratinocytes. Antioxidants 2021, 10, 1552. https://doi.org/10.3390/antiox10101552
Teng W-L, Huang P-H, Wang H-C, Tseng C-H, Yen F-L. Pterostilbene Attenuates Particulate Matter-Induced Oxidative Stress, Inflammation and Aging in Keratinocytes. Antioxidants. 2021; 10(10):1552. https://doi.org/10.3390/antiox10101552
Chicago/Turabian StyleTeng, Wei-Lin, Pao-Hsien Huang, Hui-Chun Wang, Chih-Hua Tseng, and Feng-Lin Yen. 2021. "Pterostilbene Attenuates Particulate Matter-Induced Oxidative Stress, Inflammation and Aging in Keratinocytes" Antioxidants 10, no. 10: 1552. https://doi.org/10.3390/antiox10101552
APA StyleTeng, W.-L., Huang, P.-H., Wang, H.-C., Tseng, C.-H., & Yen, F.-L. (2021). Pterostilbene Attenuates Particulate Matter-Induced Oxidative Stress, Inflammation and Aging in Keratinocytes. Antioxidants, 10(10), 1552. https://doi.org/10.3390/antiox10101552







