Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-κB Phosphorylation in Ovalbumin-Induced Asthma
Abstract
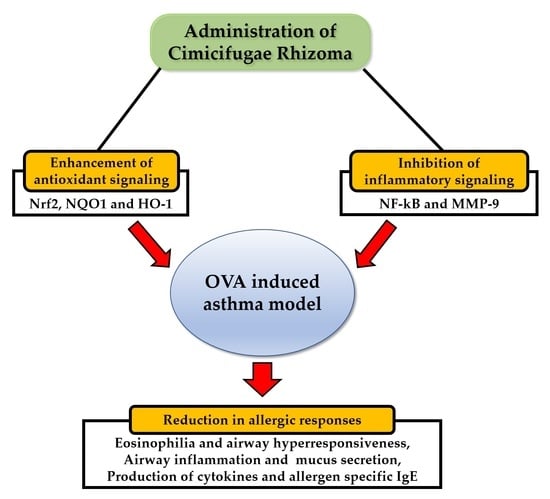
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Preparation of Sample and Standard Solution
2.4. HPLC Condition
2.5. Experimental Animals and Protocol
2.6. Bronchoalveolar Lavage Fluid (BALF) and Serum Analysis
2.7. Histological Examination of Lung Tissue
2.8. Western Blot
2.9. Gelatin Zymography
2.10. Statistical Analysis
3. Results
3.1. HPLC Analysis of CRE
3.2. CRE Inhibited Eosinophilia and Airway Hyperresponsiveness (AHR) in Animals with Asthma
3.3. CRE Decreased Inflammatory Cytokines and OVA-Specific IgE in Animals with Asthma
3.4. CRE Reduced Airway Inflammation and Mucus Secretion of Lung Tissue from Animals with Asthma
3.5. CRE Elevated the Expression of the Nrf2/HO-1/NQO1 in Animals with Asthma
3.6. CRE Suppressed NF-κB Phosphorylation and MMP-9 Expression in Animals with Asthma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambrecht, B.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Ci, X.; Chu, X.; Wei, M.; Hua, S.; Deng, X. Hesperidin suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Inflammation 2012, 35, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Banga, J.; Silveyra, P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol. Ther. 2018, 181, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Ichinose, M. Oxidative and nitrative stress in bronchial asthma. Antioxid. Redox Signal. 2008, 10, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cui, J.; Wang, W.; Tang, W.; Teng, F.; Zhu, X.; Qin, J.; Wuniqiemu, T.; Sun, J.; Wei, Y.; et al. Formononetin Attenuates Airway Inflammation and Oxidative Stress in Murine Allergic Asthma. Front. Pharmacol. 2020, 11, 533841. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.H.; Kwon, K.M.; Park, J.Y.; Abekura, F.; Lee, Y.C.; Chung, T.W.; Ha, K.T.; Chang, H.W.; Cho, S.H.; Kim, J.S.; et al. Esculentoside H inhibits colon cancer cell migration and growth through suppression of MMP-9 gene expression via NF-kB signaling pathway. J. Cell Biochem. 2019, 120, 9810–9819. [Google Scholar] [CrossRef]
- Han, Z.; Junxu; Zhong, N. Expression of matrix metalloproteinases MMP-9 within the airways in asthma. Respir. Med. 2003, 97, 563–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Hwang, I.; Lee, J.; Son, K.; Jeong, C.; Jung, J. Protective Effect of Cimicifuga heracleifolia Ethanol Extract and Its Constituents against Gastric Injury. J. Health Sci. 2011, 57, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Li, J.X.; Yu, Z.Y. Cimicifuga rhizoma: From origins, bioactive constituents to clinical outcomes. Curr. Med. Chem. 2006, 13, 2927–2951. [Google Scholar] [CrossRef]
- Ma, Y.; Cong, W.; Huang, H.; Sun, L.; Mai, A.H.; Boonen, K.; Maryam, W.; De Borggraeve, W.; Luo, G.; Liu, Q.; et al. Identification of fukinolic acid from Cimicifuga heracleifolia and its derivatives as novel antiviral compounds against enterovirus A71 infection. Int. J. Antimicrob. Agents 2019, 53, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.W.; You, J.R.; Kim, Y.S.; Cho, E.Y.; Kim, S.H.; Yoon, J.H.; Kwon, E.; Chung, D.H.; Kim, Y.T.; Jang, J.J.; et al. Pre-clinical in vitro and in vivo safety evaluation of Cimicifuga heracleifolia. Regul. Toxicol. Pharmacol. 2015, 73, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifuga. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Song, X.; Nagai, T.; Yamamoto, M.; Dai, Y.; He, L.; Kiyohara, H.; Yao, X.; Yao, Z. Chemical profile of Cimicifuga heracleifolia Kom. And immunomodulatory effect of its representative bioavailable component, cimigenoside on Poly(I:C)-induced airway inflammation. J. Ethnopharmacol. 2021, 267, 113615. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Qin, R.; Zhao, Y.; Han, L.; Lu, J.; Ly, C. Stimultaneous determination of 19 constituents in Cimicifugaee Rhizoma by HPLC-DAD and screening for antioxidants through DPPH free radical scavenging assay. Biomed. Chromatorgr. 2019, 33, e4624. [Google Scholar] [CrossRef]
- Jung, T.Y.; Lee, A.Y.; Song, J.H.; Lee, M.Y.; Lim, J.O.; Lee, S.J.; Ko, J.W.; Shin, N.R.; Kim, J.C.; Shin, I.S.; et al. Scrophularia koraiensis Nakai attenuates allergic airway inflammation via suppression of NF-kB and enhancement of Nrf2/HO-1 signaling. Antioxid 2020, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loutsios, C.; Farahi, N.; Porter, L.; Lok, L.S.; Peters, A.M.; Condliffe, A.M.; Chilvers, E.R. Biomarkers of eosinophilic inflammation in asthma. Expert. Rev. Respir. Med. 2014, 8, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Xiao, H.Q.; Breslin, L.M.; Bochner, B.S.; Schroeder, J.T. Enhanced antigen presenting and T cell functions during late-phase allergic responses in the lung. Clin. Exp. Allergy 2018, 48, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Montero-Pérez, O.; Contreras-Rey, M.B.; Sánchez-Gómez, E. Effectiveness and safety of mepolizumab in severe refractory eosinophilic asthma: Results in clinical practice. Drugs Context 2019, 8, 212584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, Ö. Oxidative stress in asthma: Part of the puzzle. Pediatr. Allergy Immunol. 2018, 29, 789–800. [Google Scholar] [CrossRef]
- Lou, Y.; Guo, Z.; Zhu, Y.; Kong, M.; Zhang, R.; Lu, L.; Wu, F.; Liu, Z.; Wu, J. Houttuynia cordata Thunb. and its bioactive compound 2-undecanone significantly suppress benzo(a)pyrene-induced lung tumorigenesis by activating the Nrf2-HO-1/NQO-1 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 242. [Google Scholar] [CrossRef] [PubMed]
- Athari, S.S. Targeting cell signaling in allergic asthma. Signal Transduct. Target. Ther. 2019, 4, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizumura, K.; Maruoka, S.; Shimizu, T.; Gon, Y. Role of Nrf2 in the pathogenesis of respiratory diseases. Respir. Investig. 2020, 58, 28–35. [Google Scholar] [CrossRef]
- Pan, Y.; Li, W.; Feng, Y.; Xu, J.; Cao, H. Edaravone attenuates experimental asthma in mice through induction of HO-1 and the Keap1/Nrf2 pathway. Exp. Ther. Med. 2020, 19, 1407–1416. [Google Scholar] [CrossRef]
- Tacon, C.E.; Wiehler, S.; Holden, N.S.; Newton, R.; Proud, D.; Leigh, R. Human rhinovirus infection up-regulates MMP-9 production in airway epithelial cells via NF-{kappa}B. Am. J. Respir. Cell Mol. Biol. 2010, 43, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattos, W.; Lim, S.; Russell, R.; Jatakanon, A.; Chung, K.F.; Barnes, P.J. Matrix metalloproteinase-9 expression in asthma: Effect of asthma severity, allergen challenge, and inhaled corticosteroids. Chest 2002, 122, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, J.; Zhu, R.; Gao, S.; Fan, J.; Xia, M.; Zhao, R.C.; Zhang, J. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-κB pathway. Aging (Albany NY) 2019, 11, 11391–11415. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Lee, A.Y.; Song, J.H.; Yang, S.; Park, I.; Lim, J.O.; Jung, T.Y.; Ko, J.W.; Kim, J.C.; Lim, K.S.; et al. Scrophularia buergeriana attenuates allergic inflammation by reducing NF-κB activation. Phytomedicine 2020, 67, 153159. [Google Scholar] [CrossRef]
- Yan, G.H.; Choi, Y.H. Phellinus linteus Extract Exerts Anti-asthmatic Effects by Suppressing NF-κB and p38 MAPK Activity in an OVA-induced Mouse Model of Asthma. Immune Netw. 2014, 14, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.; Pauli, G.F.; Zheng, B.; Wang, H.; Bai, N.; Peng, T.; Roller, M.; Zheng, Q. Cimicifuga species identification by high performance liquid chromatography-photodiode array/mass spectrometric/evaporative light scattering detection for quality control of black cohosh products. J. Chromatogr. A 2006, 1112, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Moon, D.C.; Son, K.H.; Son, J.K.; Min, B.S.; Woo, M.H. Quantitative and pattern recognition analyses for the quality evaluation of Cimicifuga Rhizoma by HPLC. Bull. Korean Chem. Soc. 2011, 32, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Wang, C.C.; Huang, H.M.; Lin, C.L.; Leu, S.J.; Lee, Y.L. Ferulic acid induces Th1 responses by modulating the function of dendritic cells and ameliorates Th2-meidated allergic airway inflammation in mice. Evid. Based Complement. Alternat. Med. 2015, 2015, 678487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Jeong, M.; Park, S.; Ryu, S.M.; Lee, J.; Song, Z.; Guo, Y.; Choi, J.H.; Lee, D.; Jang, D.S. Chemical constitutents of the leaves of Butterbur (Petasites japonicas) and their anti-inflammatory effects. Biomolecules 2019, 9, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.K.; Lee, D.Y.; Choi, Y.H.; Yea, S.S.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Kim, S.K.; et al. Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced astham. Life Sci. 2008, 82, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Woehs, F.; Svoboda, M.; Thalhammer, T.; Chiba, P.; Moeslinger, T. Aqueous extracts of Cimicifuga racemosa and phenolcarboxylic constituents inhibit production of proinflammatory cytokines in LPS-stimulated human whole blood. Can. J. Physiol. Pharmacol. 2009, 87, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Meeprom, A.; Sumgong, W.; Suantawee, T.; Thilavech, T.; Chan, C.B.; Adisakwattana, S. Isoferulic acid prevents methylglyoxal-induced protein glycation and DNA damage by free radical scavenging activity. BMC Complement. Altern. Med. 2015, 15, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Compound | Contents (Mean ± SD, n = 3) | |
|---|---|---|
| mg/g | % | |
| Caffeic acid | 0.62 ± 0.01 | 0.06 |
| Ferulic acid | 1.35 ± 0.04 | 0.14 |
| Isoferulic acid | 8.09 ± 0.24 | 0.81 |
| Cimicifugic acid B | 1.86 ± 0.03 | 0.19 |
| Cimicifugic acid F | 10.93 ± 0.14 | 1.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-O.; Song, K.H.; Lee, I.S.; Lee, S.-J.; Kim, W.-I.; Pak, S.-W.; Shin, I.-S.; Kim, T. Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-κB Phosphorylation in Ovalbumin-Induced Asthma. Antioxidants 2021, 10, 1626. https://doi.org/10.3390/antiox10101626
Lim J-O, Song KH, Lee IS, Lee S-J, Kim W-I, Pak S-W, Shin I-S, Kim T. Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-κB Phosphorylation in Ovalbumin-Induced Asthma. Antioxidants. 2021; 10(10):1626. https://doi.org/10.3390/antiox10101626
Chicago/Turabian StyleLim, Je-Oh, Kwang Hoon Song, Ik Soo Lee, Se-Jin Lee, Woong-Il Kim, So-Won Pak, In-Sik Shin, and Taesoo Kim. 2021. "Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-κB Phosphorylation in Ovalbumin-Induced Asthma" Antioxidants 10, no. 10: 1626. https://doi.org/10.3390/antiox10101626