Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Research Material
2.3. Acid Value, Peroxide Value and p-Anisidine Value
2.4. Chlorophyll, Carotenoid Pigments and Color Determination
2.5. Smoke Point
2.6. FAMEs
2.7. Rancimat Test
2.8. Total Polar Compounds
2.9. Oxidizability (COX) Value and Nutritional Quality Indices of Oils
2.10. Calculations and Statistics
3. Results and Discussion
3.1. Acid Value, Peroxide Value, and p-Anisidine Value
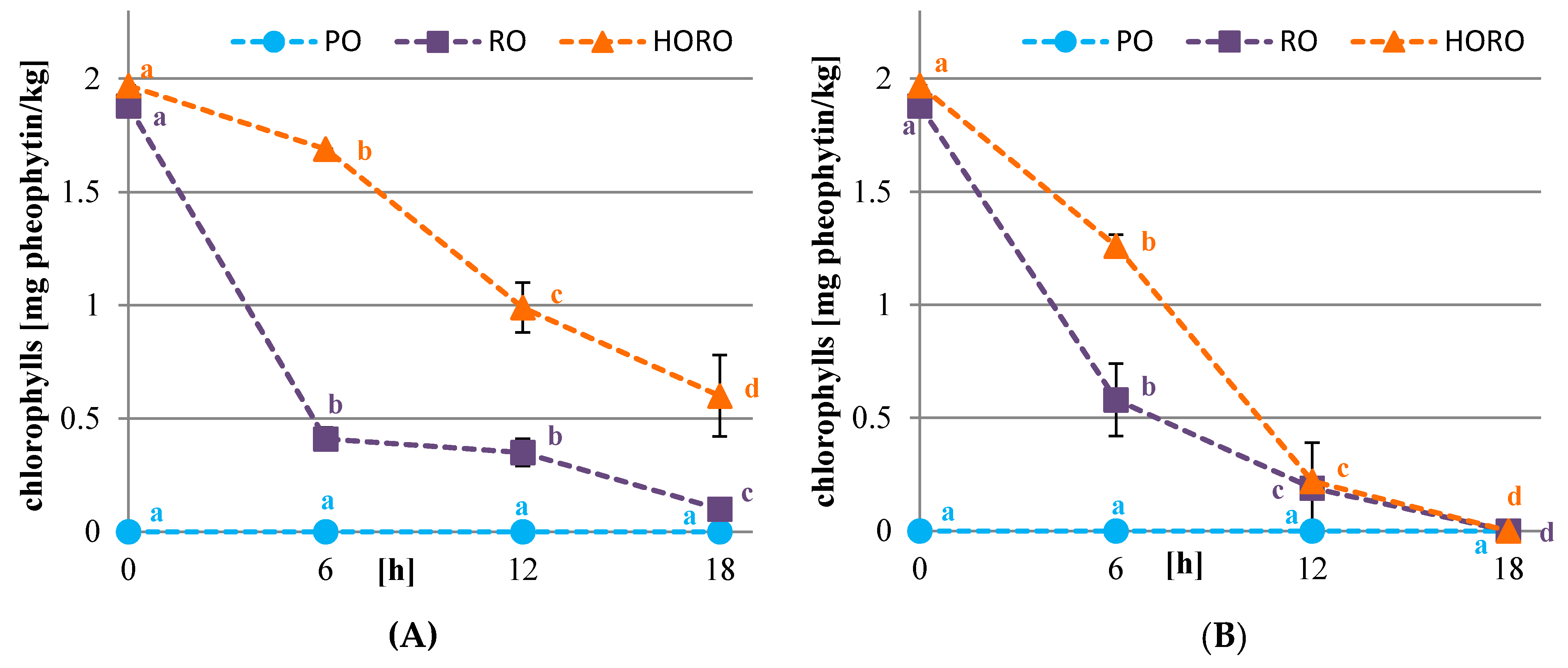
3.2. Chlorophyll, Carotenoid Pigments, and Color of Oils
3.3. Smoke Point
3.4. FAMEs
3.5. Rancimat Test
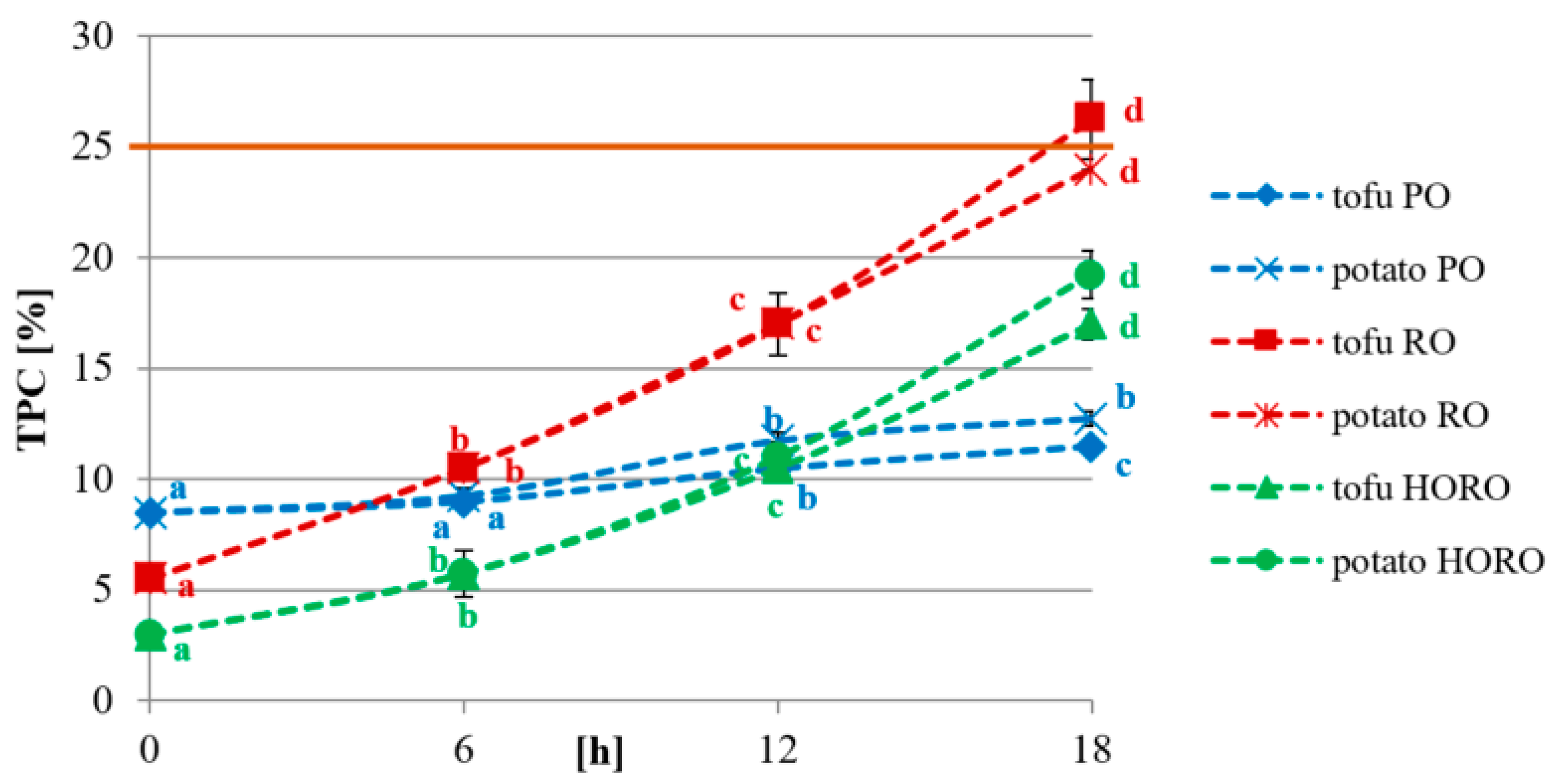
3.6. Total Polar Compounds
3.7. Oxidizability (COX) Value and Nutritional Quality Indices of Oils
3.8. PCA Analysis of Oil Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chew, S.C. Cold-pressed rapeseed (Brassica napus) oil: Chemistry and functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef]
- del Carmen Flores-Álvarez, M.; Molina-Hernández, E.F.; Hernández-Raya, J.C.; Sosa-Morales, M.E. The Effect of Food Type (Fish Nuggets or French Fries) on Oil Blend Degradation during Repeated Frying. J. Food Sci. 2012, 77, C1136–C1143. [Google Scholar] [CrossRef]
- Matthäus, B. Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. Eur. J. Lipid. Sci. Technol. 2006, 108, 200–211. [Google Scholar] [CrossRef]
- Rasep, Z.; Muhammad Yazid, M.N.A.W.; Samion, S. Lubrication of textured journal bearing by using vegetable oil: A review of approaches, challenges, and opportunities. Renew. Sustain. Energy Rev. 2021, 146, 111191. [Google Scholar] [CrossRef]
- Liberty, J.T.; Dehghannya, J.; Ngadi, M.O. Effective strategies for reduction of oil content in deep-fat fried foods: A review. Trends Food Sci. Technol. 2019, 92, 172–183. [Google Scholar] [CrossRef]
- Moreira, R.G. Vacuum frying versus conventional frying—An overview. Eur. J. Lipid Sci. Technol. 2014, 116, 723–734. [Google Scholar] [CrossRef]
- Iskandar, M.J.; Baharum, A.; Anuar, F.H.; Othaman, R. Palm oil industry in South East Asia and the effluent treatment technology—A review. Environ. Technol. Innov. 2018, 9, 169–185. [Google Scholar] [CrossRef]
- Przybylski, R.; Wu, J.; Eskin, N.A.M. A rapid method for determining the oxidative stability of oils suitable for breeder size samples. JAOCS J. Am. Oil Chem. Soc. 2013, 90, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Ouchon, P.B.; Pyle, D.L. Studying Oil Absorption in Restructured Potato Chips. J. Food Sci. 2004, 69, 115–121. [Google Scholar] [CrossRef]
- Moreno, M.C.; Brown, C.A.; Bouchon, P. Effect of food surface roughness on oil uptake by deep-fat fried products. J. Food Eng. 2010, 101, 179–186. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Aro, A.; Willett, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutr. 2009, 63, S5–S21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.D.; Jahreis, G.; Busch-Stockfisch, M.; Fritsche, J. Chemical and sensory assessment of deep-frying oil alternatives for the processing of French fries. Eur. J. Lipid Sci. Technol. 2013, 115, 935–945. [Google Scholar] [CrossRef]
- Petersen, K.D.; Kleeberg, K.K.; Jahreis, G.; Busch-Stockfisch, M.; Fritsche, J. Comparison of analytical and sensory lipid oxidation parameters in conventional and high-oleic rapeseed oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1193–1203. [Google Scholar] [CrossRef]
- Romano, R.; Giordano, A.; Vitiello, S.; Le Grottaglie, L.; Musso, S.S. Comparison of the Frying Performance of Olive Oil and Palm Superolein. J. Food Sci. 2012, 77, C519–C531. [Google Scholar] [CrossRef]
- Official Methods and Recommended Practices of the AOCS 7th ed. AOCS Official Method Cd 3d-63. Acid Value of Fats and Oils. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details/productId/111545 (accessed on 12 October 2021).
- Official Methods of Analysis AOAC INTERNATIONAL 21st Ed. AOAC Official Method 965.33. Peroxide Value of Oils and Fats. Titration Method. Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 12 October 2021).
- Official Methods and Recommended Practices of the AOCS 7th ed. AOCS Official Method Cd 18-90. p-Anisidine Value. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details?productId=111529 (accessed on 12 October 2021).
- Raczyk, M.; Popis, E.; Kruszewski, B.; Ratusz, K.; Rudzińska, M. Physicochemical quality and oxidative stability of linseed (Linum usitatissimum) and camelina (camelina sativa) cold-pressed oils from retail outlets. Eur. J. Lipid Sci. Technol. 2016, 118, 834–839. [Google Scholar] [CrossRef]
- Official Methods and Recommended Practices of the AOCS 7th ed. AOCS Official Method Cc 13i-96. Determination of Chlorophyll Pigments in Crude Vegetable Oils. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details?productId=111501 (accessed on 12 October 2021).
- Rękas, A.; Wroniak, M.; Siger, A.; Ścibisz, I. Chemical composition and resistance to oxidation of high-oleic rapeseed oil pressed from microwave pre-treated intact and de-hulled seeds. Grasas Aceites 2017, 68, 1–9. [Google Scholar] [CrossRef] [Green Version]
- British Standards Institution (BSI) 76th ed. BS 684-2.20. Methods of analysis of Fats and Fatty Oils—Part 2: Other Methods—Section 2.20: Determination of Carotene in Vegetable oils. Available online: https://global.ihs.com/doc_detail.cfm?document_name=BS%20684%2D2%2E20&item_s_key=00130822 (accessed on 12 October 2021).
- Official Methods and Recommended Practices of the AOCS 7th ed. AOCS Official Method Cc 9a-48. Smoke, Flash, and Fire Points, Cleveland Open Cup Method. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details?productId=111517 (accessed on 12 October 2021).
- Official Methods and Recommended Practices of the AOCS 7th ed. AOCS Official Method Ce 2-66. Preparation of Methyl Esters of Fatty Acids. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details?productId=111788 (accessed on 12 October 2021).
- Official Methods and Recommended Practices of the AOCS 7th ed. AOCS Official Method Cd 12b-92. Oil Stability Index. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details/productId/111524 (accessed on 12 October 2021).
- Fatemi, S.H.; Hammond, E.G. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Mendes, I.A.; Portugal, P.V.; Bessa, R.J.B. Effect of particle size and soybean oil supplementation on growth performance, carcass and meat quality and fatty acid composition of intramuscular lipids of lambs. Livest. Prod. Sci. 2004, 90, 79–88. [Google Scholar] [CrossRef]
- Kruszewski, B.; Zawada, K.; Karpiński, P. Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice. Molecules 2021, 26, 1802. [Google Scholar] [CrossRef]
- Codex Alimentarius CXS 19-1981. Standard for Edible Fats and Oils Not Covered by Individual Standards. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 12 October 2021).
- Farhoosh, R.; Moosavi, S.M.R. Rancimat test for the assessment of used frying oils quality. J. Food Lipids 2007, 14, 263–271. [Google Scholar] [CrossRef]
- Ismail, R. Palm oil and palm olein frying applications. Asia Pac. J. Clin. Nutr. 2005, 14, 414–419. [Google Scholar]
- Nederal, S.; Škevin, D.; Kraljić, K.; Obranović, M.; Papeša, S.; Bataljaku, A. Chemical composition and oxidative stability of roasted and cold pressed pumpkin seed oils. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 1763–1770. [Google Scholar] [CrossRef]
- Koski, A.; Psomiadou, E.; Tsimidou, M.; Hopia, A.; Kefalas, P.; Wähälä, K.; Heinonen, M. Oxidative stability and minor constituents of virgin olive oil and cold-pressed rapeseed oil. Eur. Food Res. Technol. 2002, 214, 294–298. [Google Scholar] [CrossRef]
- Kowalski, B.; Ratusz, K.; Kowalska, D.; Bekas, W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. Eur. J. Lipid Sci. Technol. 2004, 106, 165–169. [Google Scholar] [CrossRef]
- Hosseini, H.; Ghorbani, M.; Meshginfar, N.; Mahoonak, A.S. A Review on Frying: Procedure, Fat, Deterioration Progress and Health Hazards. JAOCS J. Am. Oil Chem. Soc. 2016, 93, 445–466. [Google Scholar] [CrossRef]
- Adjonu, R.; Zhou, Z.; Prenzler, P.D.; Ayton, J.; Blanchard, C.L. Different processing practices and the frying life of refined canola oil. Foods 2019, 8, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulkarim, S.M.; Long, K.; Lai, O.M.; Muhammad, S.K.S.; Ghazali, H.M. Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem. 2007, 105, 1382–1389. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Barthet, V.J.; Daun, J.K. Seed Morphology, Composition, and Quality. In Canola: Chemistry, Production, Processing, and Utilization; Daun, J.K., Eskin, N.A.M., Hickling, D., Eds.; AOCS Press: Urbana, IL, USA, 2011; pp. 119–162. [Google Scholar]
- Symoniuk, E.; Ratusz, K.; Krygier, K. Evaluation of the Oxidative Stability of Cold-Pressed Rapeseed Oil by Rancimat and Pressure Differential Scanning Calorimetry Measurements. Eur. J. Lipid Sci. Technol. 2019, 121, 1–8. [Google Scholar] [CrossRef]
- Wroniak, M.; Rekas, A.; Ratusz, K. Influence of impurities in raw material on sensory and physicochemical properties of cold-pressed rapeseed oil produced from conventionally and ecologically grown seeds. Acta Sci. Pol. Technol. Aliment. 2016, 15, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the relationship between vegetable oil composition and oxidative stability: A multifactorial approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Kristott, J. High–oleic oils—How good are they for frying? Lipid Technol. 2003, 3, 29–32. [Google Scholar]
- Fan, H.Y.; Sharifudin, M.S.; Hasmadi, M.; Chew, H.M. Frying stability of rice bran oil and palm olein. Int. Food Res. J. 2013, 20, 403–407. [Google Scholar]
- Tarmizi, A.H.A.; Ismail, R.; Kuntom, A. Effect of frying on the palm oil quality attributes—A review. J. Oil Palm Res. 2016, 28, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Gao, T.; Song, L.; Zhang, L.; Jiang, Y.; Li, J.; Zhang, X.; Gao, F.; Zhou, G. Effects of oil-water mixed frying and pure-oil frying on the quality characteristics of soybean oil and chicken chop. Food Sci. Technol. 2016, 36, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Ye, Z.; Li, Y.; Li, J.; Liu, Y. Comparative study of the oxidation stability of high oleic oils and palm oil during thermal treatment. J. Oleo Sci. 2020, 69, 573–584. [Google Scholar] [CrossRef]
- Alireza, S.; Tan, C.P.; Hamed, M.; Che Man, Y.B. Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. Int. Food Res. J. 2010, 17, 295–302. [Google Scholar]
- Romano, R.; Manzo, N.; Le Grottaglie, L.; Giordano, A.; Romano, A.; Masi, P. Comparison of the frying performance of high oleic oils subjected to discontinuous and prolonged thermal treatment. JAOCS J. Am. Oil Chem. Soc. 2013, 90, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Kmiecik, D.; Kobus-Cisowska, J.; Kulczyński, B. Thermal Decomposition of Partially Hydrogenated Rapeseed Oil During Repeated Frying Traditional and Fast French Fries. JAOCS J. Am. Oil Chem. Soc. 2018, 95, 473–483. [Google Scholar] [CrossRef]
- Xu, T.T.; Li, J.; Fan, Y.W.; Zheng, T.W.; Deng, Z.Y. Comparison of oxidative stability among edible oils under continuous frying conditions. Int. J. Food Prop. 2015, 18, 1478–1490. [Google Scholar] [CrossRef]
- Abril, D.; Mirabal-Gallardo, Y.; González, A.; Marican, A.; Durán-Lara, E.F.; Santos, L.S.; Valdés, O. Comparison of the oxidative stability and antioxidant activity of extra-virgin olive oil and oils extracted from seeds of Colliguaya integerrima and Cynara cardunculus under normal conditions and after thermal treatment. Antioxidants 2019, 8, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzak, M.; Rudzińska, M.; Przybylski, R.; Kmiecik, D.; Siger, A.; Olejnik, A. The degradation of bioactive compounds and formation of their oxidation derivatives in refined rapeseed oil during heating in model system. LWT 2020, 123, 109078. [Google Scholar] [CrossRef]





| PO | RO | HORO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Frying | After Frying Potatoes | After Frying Tofu | Before Frying | After Frying Potatoes | After Frying Tofu | Before Frying | After Frying Potatoes | After Frying Tofu | |
| C12:0 | 0.58 ± 0.04 | 0.59 ± 0.06 | 0.59 ± 0.06 | nd | nd | nd | nd | nd | nd |
| C14:0 | 0.97 ± 0.07 | 1.00 ± 0.02 | 1.01 ± 0.04 | nd | nd | nd | nd | nd | nd |
| C16:0 | 41.35 ± 0.14 | 41.45 ± 0.51 | 41.45 ± 0.51 | 4.13 ± 0.02 | 4.14 ± 0.04 | 4.13 ± 0.02 | 3.01 ± 0.04 | 3.19 ± 0.08 | 3.21 ± 0.05 |
| C16:1 | 0.20 ± 0.00 | 0.19 ± 0.01 | 0.19 ± 0.00 | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.00 | 0.20 ± 0.00 |
| C18:0 | 4.01 ± 0.00 | 4.03 ± 0.02 | 4.00 ± 0.00 | 1.82 ± 0.01 | 1.88 ± 0.08 | 1.90 ± 0.04 | 1.99 ± 0.01 | 2.02 ± 0.01 | 1.99 ± 0.01 |
| C18:1 | 41.41 ± 0.14 | 41.39 ± 0.23 | 41.29 ± 0.28 | 63.60 ± 0.14 | 61.80 ± 0.72 | 62.55 ± 0.83 | 76.96 ± 1.03 | 75.25 ± 1.23 | 75.71 ± 1.94 |
| C18:2 | 10.67 ± 0.14 a | 9.87 ± 0.01 b | 9.83 ± 0.05 b | 18.21 ± 0.11 a | 15.68 ± 0.73 b | 15.52 ± 0.66 b | 8.71 ± 0.11 a | 7.90 ± 0.02 b | 8.60 ± 0.08 a |
| C18:3 | 0.23 ± 0.00 | 0.20 ± 0.00 | 0.21 ± 0.01 | 8.56 ± 0.07 a | 6.21 ± 0.16 b | 6.16 ± 0.45 b | 2.92 ± 0.11 a | 1.72 ± 0.07 b | 2.31 ± 0.30 a |
| C20:0 | 0.37 ± 0.01 a | 0.42 ± 0.00 b | 0.44 ± 0.03 b | 0.55 ± 0.01 | 0.46 ± 0.04 | 0.51 ± 0.07 | 0.66 ± 0.02 | 0.60 ± 0.04 | 0.62 ± 0.01 |
| C20:1 | nd | nd | nd | 1.28 ± 0.04 | 1.27 ± 0.01 | 1.27 ± 0.03 | 1.28 ± 0.02 a | 1.30 ± 0.02 a | 1.00 ± 0.19 b |
| C22:0 | nd | nd | nd | 0.27 ± 0.04 | 0.26 ± 0.06 | 0.29 ± 0.01 | 0.58 ± 0.08 a | 0.32 ± 0.04 b | 0.27 ± 0.04 b |
| Σ SFA | 47.27 ± 0.03 | 47.48 ± 0.45 | 47.49 ± 0.45 | 6.76 ± 0.06 | 6.73 ± 0.06 | 6.82 ± 0.01 | 6.24 ± 0.01 | 6.12 ± 0.14 | 6.19 ± 0.07 |
| Σ MUFA | 41.61 ± 0.14 | 41.58 ± 0.23 | 41.48 ± 0.28 | 65.09 ± 0.19 a | 63.27 ± 0.72 b | 65.06 ± 0.81 a | 78.43 ± 1.06 | 76.76 ± 1.25 | 76.90 ± 2.14 |
| Σ PUFA | 10.90 ± 0.14 a | 10.07 ± 0.01 b | 10.03 ± 0.06 b | 26.77 ± 0.18 a | 21.88 ± 0.89 b | 21.68 ± 1.12 b | 11.62 ± 0.00 a | 9.62 ± 0.09 c | 10.90 ± 0.37 b |
| n − 6/n − 3 | 46.39 ± 0.61 b | 49.33 ± 0.04 a | 47.95 ± 1.41 ab | 2.13 ± 0.01 b | 2.53 ± 0.05 a | 2.52 ± 0.08 a | 2.99 ± 0.15 c | 4.59 ± 0.18 a | 3.75 ± 0.45 b |
| Type of Fried Product | Time of Frying [h] | PO | RO | HORO |
|---|---|---|---|---|
| potatoes | 0 | 13.05 ± 0.10 a | 3.22 ± 0.11 a | 10.26 ± 0.22 a |
| 6 | 10.95 ± 0.27 b | 2.03 ± 0.26 b | 7.16 ± 0.27 b | |
| 12 | 10.09 ± 0.41 b | 1.37 ± 0.05 c | 1.30 ± 0.07 c | |
| 18 | 7.76 ± 0.31 c | 0.73 ± 0.07 d | 0.72 ± 0.23 d | |
| tofu | 0 | 13.05 ± 0.10 a | 3.22 ± 0.11 a | 10.26 ± 0.22 a |
| 6 | 11.23 ± 0.33 b | 2.59 ± 0.25 b | 7.22 ± 0.18 b | |
| 12 | 9.61 ± 0.29 c | 1.03 ± 0.09 c | 2.39 ± 0.25 c | |
| 18 | 6.80 ± 0.39 d | 0.90 ± 0.29 c | 1.02 ± 0.38 d |
| PO | RO | HORO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Frying | After Frying Potatoes | After Frying Tofu | Before Frying | After Frying Potatoes | After Frying Tofu | Before Frying | After Frying Potatoes | After Frying Tofu | |
| COX | 1.56 ± 0.01 a | 1.47 ± 0.00 b | 1.47 ± 0.01 b | 4.36 ± 0.03 a | 3.57 ± 0.12 b | 3.56 ± 0.16 b | 2.30 ± 0.00 a | 1.94 ± 0.02 b | 2.14 ± 0.09 ab |
| AI | 0.87 ± 0.00 | 0.89 ± 0.00 | 0.89 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 |
| TI | 1.73 ± 0.00 | 1.76 ± 0.01 | 1.77 ± 0.03 | 0.09 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.11 ± 0.00 | 0.11 ± 0.00 |
| hH | 1.24 ± 0.00 | 1.21 ± 0.01 | 1.21 ± 0.02 | 21.91 ± 0.03 a | 20.24 ± 0.56 b | 20.66 ± 0.04 ab | 29.43 ± 0.76 a | 26.61 ± 0.67 b | 26.20 ± 0.53 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wroniak, M.; Raczyk, M.; Kruszewski, B.; Symoniuk, E.; Dach, D. Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein. Antioxidants 2021, 10, 1637. https://doi.org/10.3390/antiox10101637
Wroniak M, Raczyk M, Kruszewski B, Symoniuk E, Dach D. Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein. Antioxidants. 2021; 10(10):1637. https://doi.org/10.3390/antiox10101637
Chicago/Turabian StyleWroniak, Małgorzata, Marianna Raczyk, Bartosz Kruszewski, Edyta Symoniuk, and Dominika Dach. 2021. "Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein" Antioxidants 10, no. 10: 1637. https://doi.org/10.3390/antiox10101637
APA StyleWroniak, M., Raczyk, M., Kruszewski, B., Symoniuk, E., & Dach, D. (2021). Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein. Antioxidants, 10(10), 1637. https://doi.org/10.3390/antiox10101637