Abstract
The ability to detect oxygen availability is a ubiquitous attribute of aerobic organisms. However, the mechanism(s) that transduce oxygen concentration or availability into appropriate physiological responses is less clear and often controversial. This review will make the case for oxygen-dependent metabolism of hydrogen sulfide (H2S) and polysulfides, collectively referred to as reactive sulfur species (RSS) as a physiologically relevant O2 sensing mechanism. This hypothesis is based on observations that H2S and RSS metabolism is inversely correlated with O2 tension, exogenous H2S elicits physiological responses identical to those produced by hypoxia, factors that affect H2S production or catabolism also affect tissue responses to hypoxia, and that RSS efficiently regulate downstream effectors of the hypoxic response in a manner consistent with a decrease in O2. H2S-mediated O2 sensing is then compared to the more generally accepted reactive oxygen species (ROS) mediated O2 sensing mechanism and a number of reasons are offered to resolve some of the confusion between the two.
1. Introduction: The Need for Oxygen Sensing
A key to survival for aerobic organisms is the ability to detect oxygen availability and make the necessary behavioral, physiological and/or metabolic adjustments to either ensure adequate oxygen delivery or to cope with what is available. These ‘oxygen sensing’ systems operate at various levels, external, internal, tissue-specific, and intracellular. While some form of oxygen sensing system is present in essentially all aerobic (and some anaerobic) organisms, in the interest of space this review is limited to those sensing systems employed by vertebrates to ensure adequate delivery of O2 to tissues.
External chemoreceptors monitor ambient oxygen and usually initiate behavioral and physiological responses. Ambient oxygen supply is adequate for most terrestrial organisms but for some it may be limited by high altitude or in poorly ventilated environments such as nests or burrows. Aquatic organisms are more susceptible to ambient hypoxia because the content of oxygen is only 1/30 of that in air, oxygen diffusivity is 200,000 times slower, and the medium is 60 times more viscous. While the percent of oxygen in air is a constant 21%, aquatic organisms can be subjected to wide swings in oxygen seasonally, daily and spatially due to variations in temperature (which affects solubility), photosynthesis (ponds can vary from oxygen saturation to near anoxia in 24 h) and convection (tidal pools, wind mixing, etc. [1]). Additional details on hypoxia in aquatic organisms can be found in Farrell and Brauner [2].
2. O2 Sensing Systems
Oxygen sensing systems that monitor ambient oxygen would appear to be the first line of defense against hypoxia, but these are relatively rare. The external surfaces of fish gills contain chemoreceptor neuroepithelial cells (NEC; [3,4]) that are anatomically and functionally similar to the neuroepithelial bodies (NEB) near airway bifurcations in lungs of newborn mammals. While gill NEC may continuously monitor O2 throughout the life of the fish, NEB appear to be more involved during and shortly after birth in the transition from the relatively hypoxic uterine environment [5]. Surprisingly, there are relatively few other instances of external O2 sensors in terrestrial vertebrates as these animals employ internal O2 sensors that are better suited to regulate O2 stores in the blood and adjust O2 delivery commensurate with the needs of the tissues.
Blood-monitoring O2-sensing cells are found in all vertebrates. Mammalian neuroepithelial cells and type I glomus cells in the carotid and aortic bodies are essentially identical to NEC cells that line blood vessels in fish gills [4]. This undoubtedly stems from their embryonic origins as the mammalian carotid body and the first gill arch arise from the third embryonic arch and the fourth embryonic aortic arch forms second gill arch and aortic bodies. Mammalian adrenal medullary cells are also homologous to chromaffin cells that line systemic veins in fish. Both cells secrete catecholamines in response to hypoxemia [4,6,7].
Arguably, the most extensively and intensively investigated O2-sensing tissues are the blood vessels. It has been generally accepted that hypoxia dilates systemic vessels to match perfusion with metabolism and it constricts pulmonary vessels to match lung perfusion and ventilation. However, this paradigm is not consistent among all vertebrates or even within mammals [8,9,10,11,12]. It may also vary along the length of a single vessel as in the case of the chick ductus arteriosus [13], or with stage of development [14,15], or even over minutes [16]. It was these inconsistencies that led my group to develop the novel hypothesis that the O2-dependent metabolism of hydrogen sulfide (H2S) was an effective and efficient mechanism to both detect O2 availability and to initiate the appropriate downstream effector responses.
3. Definition of an Oxygen Sensor
Oxygen is a requirement of all aerobic cells. How cells respond to an oxygen deficiency varies with the cells themselves and with the complexity of their organization into multicellular entities. Cells may respond to a decrease in oxygen availability by altering their utilization of metabolic substrates or decrease their metabolism to conserve resources; mechanisms that are usually more coupled to energy currency than to the actual detection of oxygen per se, e.g., AMP kinase [17].
In the context of this review, an oxygen ‘sensor’ must operate within the bounds of a classical proportional feedback control system in which the ‘sensor’ detects oxygen availability, or tension, and then transduces this information into a signal that can be transmitted by ‘mediators’ (or couplers; [18]) to the appropriate effectors. The strength of the response is proportional to the strength of the stimulus (error signal) and reciprocal mechanisms exist to restore the system as the error signal decreases. Oxygen sensing systems have been described where the ‘sensor’ is an actual oxygen-binding receptor, e.g., prolyl hydroxylases [19,20,21], and the hypoxia-inducible factors (HIFs) serve as mediators. Alternatively, the oxygen ‘sensor’ may be metabolically coupled to oxygen, but not directly (at least not initially) react with it. This is the case for the reactive oxygen species (ROS) theory of oxygen sensing as well as the H2S/reactive sulfur species, H2S/RSS theory of oxygen sensing. In both of these mechanisms, hypoxia decreases electron flow down the electron transport chain causing either a leak of electrons (ROS theory) or it prevents normal H2S catabolism (H2S/RSS theory). These ‘sensors’ are unique in that they neither possess an oxygen-binding receptor nor are they involved in general metabolism and they may be more appropriately termed ‘oxygen-coupled sensing systems’.
4. Metabolism of H2S as an O2 Sensing Mechanism
In 2006 we observed that hypoxia and H2S produced identical mechanical responses in systemic and respiratory vessels isolated from rats, cows and the most primitive vertebrates, the hagfish and lamprey. We also observed that the hypoxic responses could be inhibited or augmented by inhibitors of H2S biosynthesis or H2S donors, respectively [22]. Based on these observations we proposed that there was a close inverse metabolic coupling between H2S constitutively generated through sulfur metabolism and its O2-dependent catabolism, i.e., during hypoxia H2S oxidation can no longer keep pace with H2S production and H2S levels rise. This inexorably couples H2S to O2 availability. Since then, there has been considerable progress by our laboratory as well as others in developing this hypothesis and in elucidating the many downstream effectors involved in promulgating this O2-sensing mechanism. These are described in detail in the following sections.
4.1. H2S Production and O2-Dependent Catabolism
The canonical pathways for H2S production, mainly from cysteine, and its catabolism are shown in Figure 1. Essentially all H2S production by these pathways is independent of O2, whereas nearly all aspects of H2S catabolism are O2-dependent.

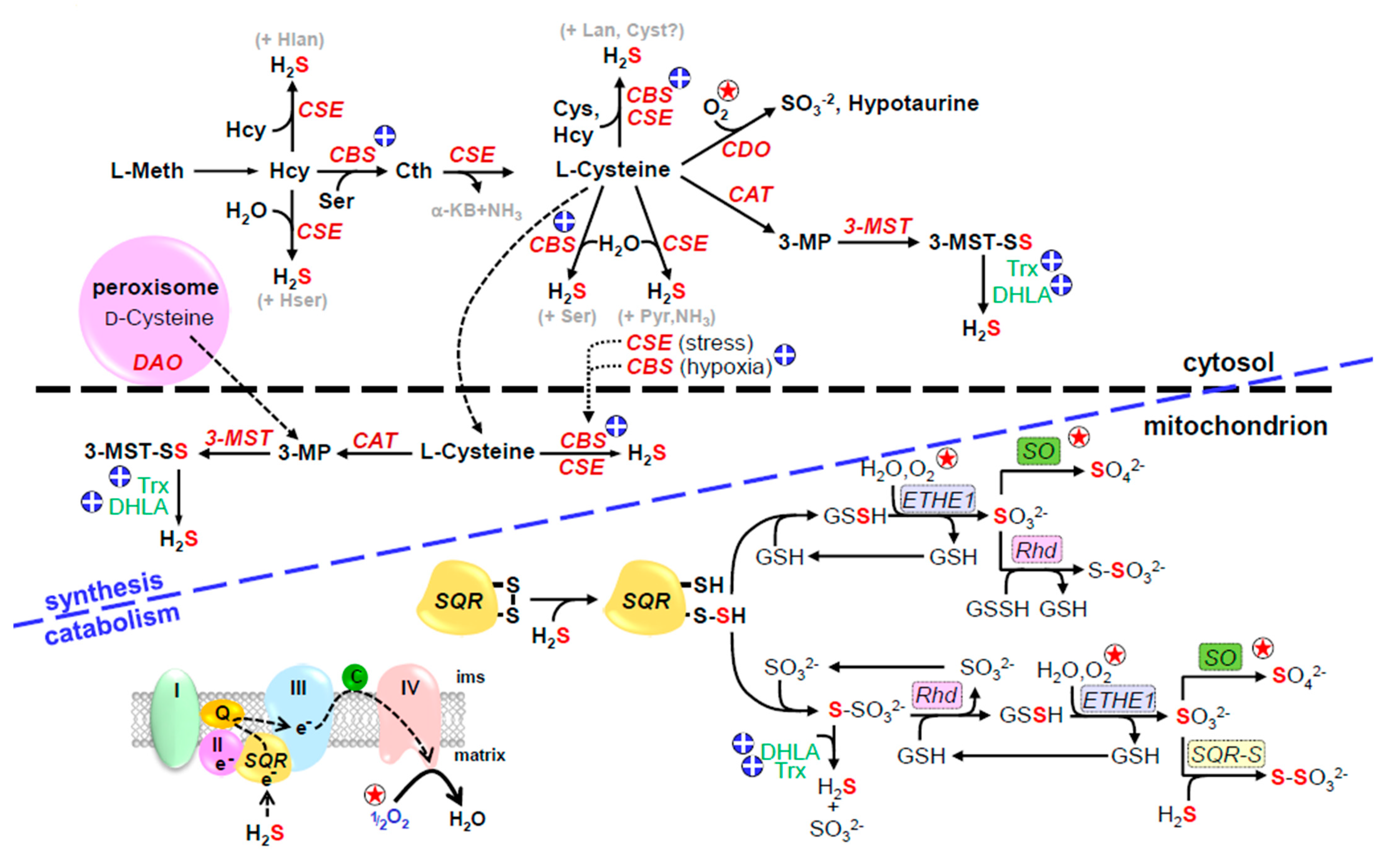
Figure 1.
Canonical pathways for H2S production and degradation; circled star indicates O2-sensitive reactions, circled plus indicates reactions affected by hypoxia-induced increased reducing environment. Cytosolic enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase generate H2S from homocysteine (Hcy) or cysteine. In addition, cysteine aminotransferase (CAT, also known as aspartate aminotransferase, AST, or glutamate transaminase, GOT) transfers sulfur from cysteine to α-ketoglutarate to form 3-mercaptopyruvate (3-MP) and the sulfur is then transferred to 3-mercaptopyruvate sulfur transferase (3-MST) to form a 3-MST persulfide (3-MST-SS). Both CAT and 3-MST are found in the cytosol and mitochondrion. H2S can be liberated from 3-MST-SS by intracellular reductants, dihydrolipoic acid (DHLA), or reduced thioredoxin (Trx). 3-mercaptopyruvate can also be produced from d-cysteine in the peroxisome by d-amino acid oxidase (DAO) which is shuttled to the mitochondrion. During hypoxia CBS migrates from the cytosol to the mitochondrial matrix and in the absence of Lon protease degradation this will generate H2S from abundant cysteine in the matrix. CBS activity is also increased as the cell becomes more reduced. General reaction of H2S oxidation in the mitochondrion is shown in lower left of figure, blue letters to right show specific reactions. H2S is initially oxidized by sulfide:quinone oxidoreductase (SQR) producing a SQR persulfide (SQR-S) and delivering two electrons to the electron transport chain at complex III (III) via coenzyme Q10 (Q). The persulfide sulfur is then transferred to a mobile sulfide carrier, either glutathione (GSH) or sulfite (SO32−) and to form glutathione persulfide (GSSH) or thiosulfate (S2O32−), respectively. The GSSH persulfide is oxidized to sulfite (SO32−) by the mitochondrial sulfur dioxygenase (ETHE1) and the sulfur may be further oxidized to sulfate (SO42−) by sulfite oxidase (SO). Rhodanese (Rhd, thiosulfate sulfur transferase) reversibly transfers sulfur between thiosulfate and GSSH. Under reducing conditions, H2S may be regenerated from thiosulfate by dihydrolipoic acid (DHLA) or reduced thioredoxin (Trx). Reproduced with permission from [23], copyright 2017 Elsevier.
4.1.1. H2S Production from Cysteine and Methionine
Four enzymes, cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE aka CGL), cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3-MST), and two substrates, l-cysteine (Cys) and l-homocysteine (Hcys), are generally assumed to be the major contributors to cellular H2S production. In the transsulfuration pathway, CBS catalyzes the β-replacement of homocysteine with serine which forms cystathionine and commits sulfur metabolism to this pathway [24]. CSE then catalyzes the α-elimination of cystathionine to form Cys, α-ketobutyrate and ammonia (NH3). Both CBS and CSE can then generate H2S from Cys via β-elimination reactions. CBS and CSE are relatively promiscuous and H2S can also be produced through a variety of reactions involving various combinations of Cys and Hcys [25,26,27,28]. CAT and 3-MST operate in tandem. CAT transfers the amine group from Cys to α-ketoglutarate producing 3-mercaptopyruvate (3-MP) and the sulfur is then transferred to 3-MST forming a persulfide on the enzyme. This sulfur may be then transferred to Cys or glutathione (GSH) forming Cys or GSH persulfides (Cys-S or GSH-S), or the 3-MST-persulfide may be reduced by endogenous reductants such as thioredoxin (Trx) or dihydrolipoic acid (DHLA), thereby liberating H2S [29,30,31]. H2S can also be derived from d-cysteine in peroxisomes. Here, d-amino acid oxidase (DAO) oxidizes d-cysteine to 3-MP which is then delivered to the mitochondrion by vesicular transport for further metabolism by 3-MST [32]. However, d-cysteine metabolism appears to be limited to the brain and kidney where it protects them from oxidative stress or re-perfusion injury, respectively [32,33].
CBS is predominantly identified in the brain and CSE in the cardiovascular system, although they may be found in other tissues including plasma [10,34,35]. CBS and CSE are generally considered to be cytosolic enzymes, however, CBS may be translocated to the mitochondrion by hypoxia [36] and CSE by stress [37]. CBS also contains two redox-sensitive vicinal Cys (Cys272 and Cys275) that oscillate between the disulfide and free thiols; reduction of the disulfide increases CBS activity 2-3-fold and increases H2S production in HEK293 cells [38]. The redox potential of these Cys (314 mV) are similar to that of cytosolic glutathione (300-320 mV; [38]). This would appear to provide another mechanism to increase H2S generation as the intracellular environment becomes more reduced during hypoxia. CBS contains a heme group that when exposed to carbon monoxide (CO) results in inhibition of the enzyme, whereas both nitric oxide (NO) and O2 do not appear to be effective at physiological concentrations [39]. Calcium inhibits CSE as well as cytosolic and mitochondrial CAT and this is independent of calmodulin [30,40].
CAT and 3-MST are found in both the cytosol and mitochondria with 3-MST being especially abundant in the mitochondrial matrix [30,41]. The mitochondrial disposition of these enzymes comports well with the presumed mitochondrial locus of an oxygen sensor and the observation that the Cys concentration in the mitochondrion is three-fold greater than that in the cytosol [37] provides additional support for an effective H2S-mediated sensing system. In addition, a recent study found that approximately 20% of complex I of the yeast, Yarrowia lipolytica, contained an accessory sulfur transferase unit, ST1, whose amino acid sequence was consistent with 3-MST [42]. This subunit also generated H2S from 3-MST and the authors suggest its close association with sulfur:quinone oxidoreductase (SQR), the enzyme catalyzing the initial step in H2S catabolism (see below), allows for rapid H2S detoxification. This would also provide tight coupling between H2S and O2 in an oxygen sensing system. However, to the author’s knowledge ST1 has not yet been identified in vertebrates.
4.1.2. H2S Production from Thiosulfate and Polysulfides
There are a number of mechanisms capable of generating H2S independent of Cys, Hcy or methionine, where H2S is derived from thiosulfate (Figure 1) and polysulfides (Figure 2; reviewed in [23]). Thiosulfate produced as an intermediate in H2S oxidation may also release H2S when exposed to reductants such as dihydrolipoic acid (DHLA) and this may be catalyzed by thioredoxin (Trx), rhodanese (Rhd), thiosulfate sulfur transferase (TST) or thiosulfate reductase [30,41,43,44,45,46,47]. H2S release from thiosulfate has been suggested to serve as an additional source of H2S during hypoxia [45].

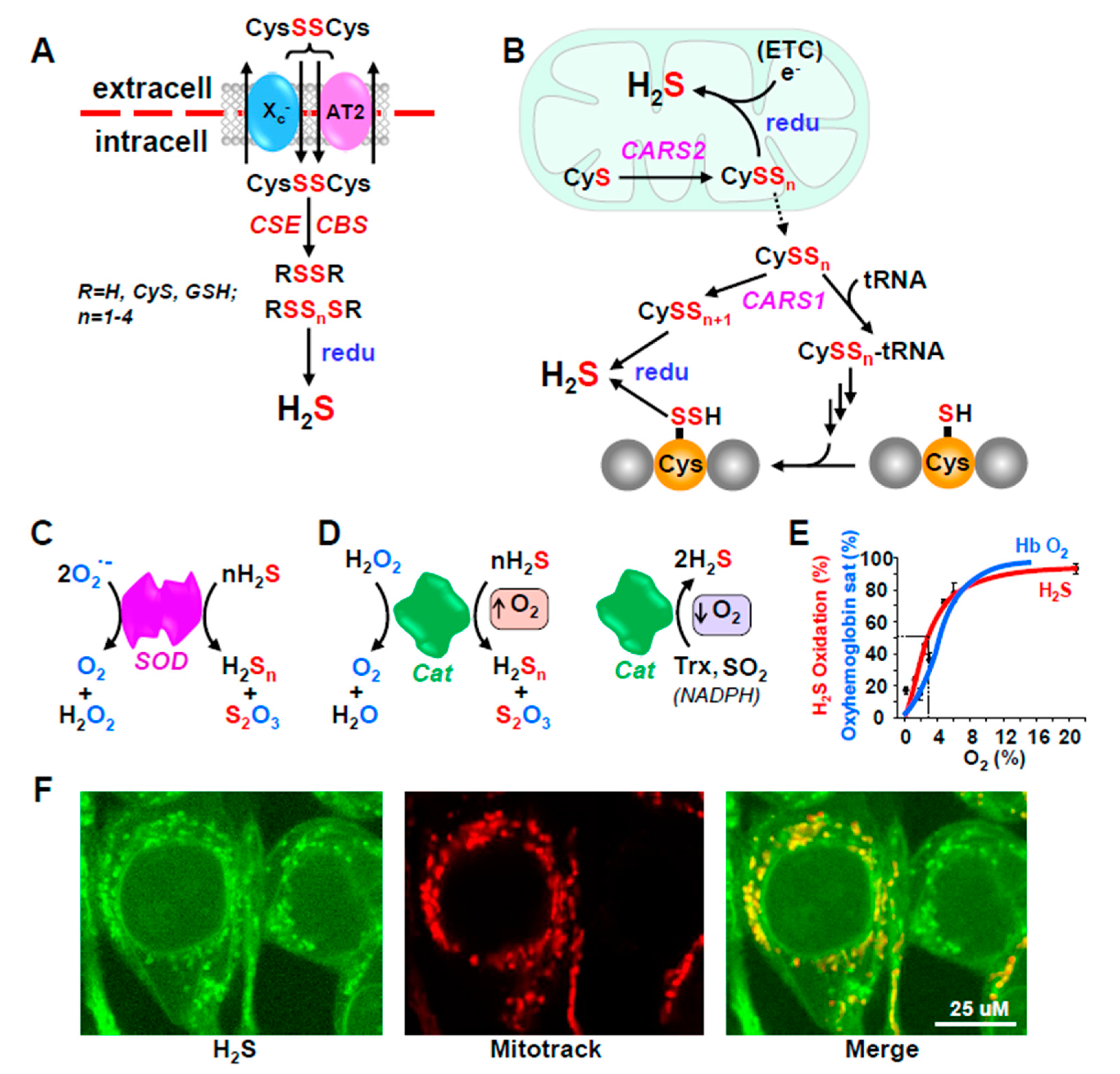
Figure 2.
Additional redox-dependent mechanisms for H2S production from polysulfides (A,B) and catabolism by antioxidant enzymes (C–F). (A) Cystine (CysSSCys) is transported into cells by the cystine/glutamate antiporter, system, Xc- or sodium-coupled neutral amino acid transporter (AT2). CSE and CBS then catalyze sulfur transfer to produce multiple polysulfides (RSSnSR) and H2S can be regenerated from these by intracellular reductants (redu) that are expected to increase under hypoxic conditions; the Xc- antiporter is also up-regulated by preconditioning with hypoxia. (B) Mitochondrial and cytoplasmic cysteinyl-tRNA synthetase (CARS2 and CARS1) catalyze sulfrur transfer from one cysteine to another producing cysteine per- and polysulfides (CysSSn; n = 1–3). CARS1 also catalyzes attachment of these persulfides to tRNA for subsequent incorporation into nascent proteins. H2S can be regenerated from any of these by reductants (redu). (C,D) Superoxide dismutase (SOD) dismutes superoxide (O2•−) to peroxide (H2O2) and water and catalase (Cat) then dismutes peroxide to oxygen and water. Both enzymes oxidize H2S to persulfide (H2S2) and thiosulfate (H2S2O3) in the presence of O2. In the absence of O2 Cat catalyzes the release of H2S from thioredoxin (Trx) or sulfite (SO32–) while consuming NADPH. (E) The switch of Cat from an oxidase to a reductase is O2-dependent with a P50 similar to the oxyhemoglobin dissociation curve. (F) Mitochondrial origin of H2S in HEK293 cells. H2S was monitored with the H2S-sensitive fluorophore, MeRho-Az and fluorescence co-localized with mitochondria (Reproduced with permission from [48], copyright 2019 John Wiley & Sons Ltd).
H2S may also be released from persulfides (RSSH; where R may be H, Cys, GSH or a protein thiol) or polysulfides (RSSnR; n typically equals 2–5) by DHLA, Trx, GSH and Cys [30,49,50], however, neither NADPH, NADH, GSH, cysteine nor CoA release H2S from a 3-MST persulfide [30]. Obviously, these per- and polysulfides could have initially been produced by oxidation of H2S, which would serve as a storage or recycling mechanism, but they could also be derived from other sources such as cystine (CysSSCys) or cysteine persulfide (CysSSH; where SH indicates the additional sulfur moiety).
Plasma is relatively more oxidized than cells [51] and most Cys circulates in the bloodstream as oxidized CSSC [52,53]. CSSC is taken up by cells by the cystine/glutamate antiporter, system Xc- [54] or by a sodium-coupled neutral amino acid transporter (AT2; [55]; Figure 2A). While conventionally thought of as a source of intracellular Cys, both CSE and CBS catalyze CSSC to multiple polysulfides such as Cys-SnH and Cys-Sn-Cys (where n = 1–4); these sulfane sulfur atoms may also be transferred to GSH forming GSH persulfides, e.g., GS-SnG ((n = 1–3); [55]). These, in turn can be reduced by glutathione reductase (GSR) to generate GS-SnH (n-1-3) in μM concentrations [55]. H2S can be regenerated from any one of these compounds, especially under reducing conditions. Perhaps as no coincidence, the Xc- antiporter is up-regulated in murine stem cells by preconditioning with hypoxia [56], suggestive of an O2-sensing process.
Akaike et al. [57], have shown that sulfur can be directly transferred from one Cys to another in a reaction catalyzed by cysteinyl-tRNA synthetase, an enzyme found mainly in the mitochondrion (CARS2) or to a lesser extent in the cytoplasm (CARS1; Figure 2B). This can produce Cys-SnH (n = 1–3). Cys-SnH can be exported from the mitochondria to the cytosol where CARS1 catalyzes its attachment to tRNA resulting in polysulfidation of nascent proteins. This can directly incorporate a redox-sensitive signaling element in a number of regulatory proteins. H2S can be released from either the Cys persulfides or persulfidated proteins by cellular reductant processes, as described above. Akaike et al. [57] also propose that electrons leaking from the electron transport chain (ETC) reduce mitochondrial cysteine persulfides thereby liberating H2S. This may provide an additional O2-sensitive process when O2 is low and electrons ‘back up’ in the ETC, although this has not been confirmed.
4.2. H2S Metabolism (Inactivation)
4.2.1. Conventional Pathways
H2S freely diffuses through cell membranes [58,59], and although diffusion out of cells could theoretically contribute to H2S inactivation, mitochondrial oxidation is far more efficient, it can be regulated, and it is O2-dependent [60]. There is a general consensus that H2S is oxidized in the mitochondrion [61]. The initial step in H2S oxidation is catalyzed by the flavoprotein, sulfide:quinone oxidoreductase (SQR), whose crystal structure and catalytic activity in humans has recently been identified [62].
SQR is a monomeric integral protein in the internal mitochondrial membrane, facing the matrix, and conveniently situated between complexes II and III of the ETC [62,63]. SQR contains two redox-active cysteines (Cys201 and Cys379) that are normally present as a disulfide. When H2S binds to SQR it is oxidized to sulfane (S0) forming a SQR-SH persulfide (SQR-S-SH). Two electrons are transferred via the flavin to ubiquinone (coenzyme Q10; CoQ10) and subsequently delivered to complex III and shuttled down the ETC. The oxygen-dependency of this process is evident; it inexorably links H2S catabolism to O2 availability and it can serve as a rapid O2 sensing system whose response is proportional to the degree of hypoxia.
The sulfane sulfur of SQR persulfide is then transferred to a mobile carrier, either GSH [64] forming GSH-persulfide (GSSH), or sulfite forming thiosulfate [43], the comparatively higher GSH concentrations favoring the former. GSSH is then oxidized by the persulfide dioxygenase, ETHE1, to sulfite (another O2-dependent reaction) and further oxidized to sulfate by sulfite oxidase (SO) or another sulfane sulfur from GSSH is transferred to sulfite by rhodanese (thiosulfate sulfur transferase) to form thiosulfate. With sulfite as the mobile carrier, rhodanese transfers the sulfane sulfur to GSH and many of the subsequent reactions are carried out as above. Electrons produced by the oxidation-reduction reaction of water and sulfite, catalyzed by SO, are also delivered to the ETC via cytochrome C, providing yet another pathway that is potentially affected by, and sensitive to, O2 availability. Reportedly, human SQR can also form H2S2 from H2S in the absence of a sulfane sulfur carrier [43].
SQR may also catalyze H2S oxidation by reverse electron transfer (RET). RET is normally thought to occur when the pool of coenzyme Q (CoQ) becomes over-reduced with electrons from respiratory complex II [65]. These electrons are then transferred retrograde to complex I where some then leak from complex I and reduce oxygen to superoxide which is then dismuted to peroxide thereby signaling elevated ROS and oxidative stress. Electrons from SQR-catalyzed H2S oxidation may also be delivered to complex I by RET [66,67]. This has also been proposed to induce superoxide-dependent mitochondrial uncoupling and downstream activation of adenosine monophosphate–activated protein kinase (AMPK; [66]). It also has the potential to increase polysulfide production from H2S.
4.2.2. Unconventional Pathways
There are a number of other mechanisms for H2S metabolism in addition to the ‘conventional’ pathways described above that could impart O2- or redox-sensing attributes. Both the cytoplasmic (Cu-Zn) and mitochondrial (Mn) superoxide dismutases (SOD1 and SOD2, respectively) as well as catalase (Cat) oxidize H2S to persulfide (H2S2) and thiosulfate (H2S2O3) in reactions that require O2 (Figure 2C–E; [68,69]. In addition, in the absence of O2, Cat catalyzes the release of H2S from thioredoxin (Trx) or sulfite (SO32−) while consuming NADPH. This switch of Cat from an oxidase to a reductase is O2-dependent with a P50 similar to the oxyhemoglobin dissociation curve. Catalase is notably abundant in red blood cells and the O2-sensitivity of H2S production is suggestive of an O2-sensing process designed to couple H2S-mediated vasoactivity to O2 availability.
We have also shown that numerous polyphenols commonly found in a variety of nutraceutical ‘antioxidants’ (e.g., green tea, berries, grapes and spices) readily oxidize H2S to polysulfides and thiosulfate. This activity is the result of O2-dependent catalytic redox cycling of the quinone in the B ring of the polyphenol [70,71,72]. Oxidized (ferric) hemoglobin and myoglobin will also oxidize H2S to Fe-bound polysulfides and thiosulfate [73,74].
5. Inverse and Po2-Dependent Relationship between O2 and H2S
Oxygen and H2S do not typically coexist, either in tissues or in the environment. Early studies showed that in a variety of organisms from the mussel, Geukensia demissa, to rats that H2S is either rapidly consumed in the presence of O2 or increases in its absence [75,76,77]. The use of rapid responding H2S-selective amperometric electrodes or H2S-sensitive fluorophores that provide a long historical perspective of cellular H2S production and metabolism has extended these observations to include a variety of tissues and cells from one or more species in all vertebrate classes. It is evident from these studies that the effects of O2 on cellular H2S can occur within seconds and they may persist for days (Figure 3; [10,48,78,79,80,81,82,83,84]).

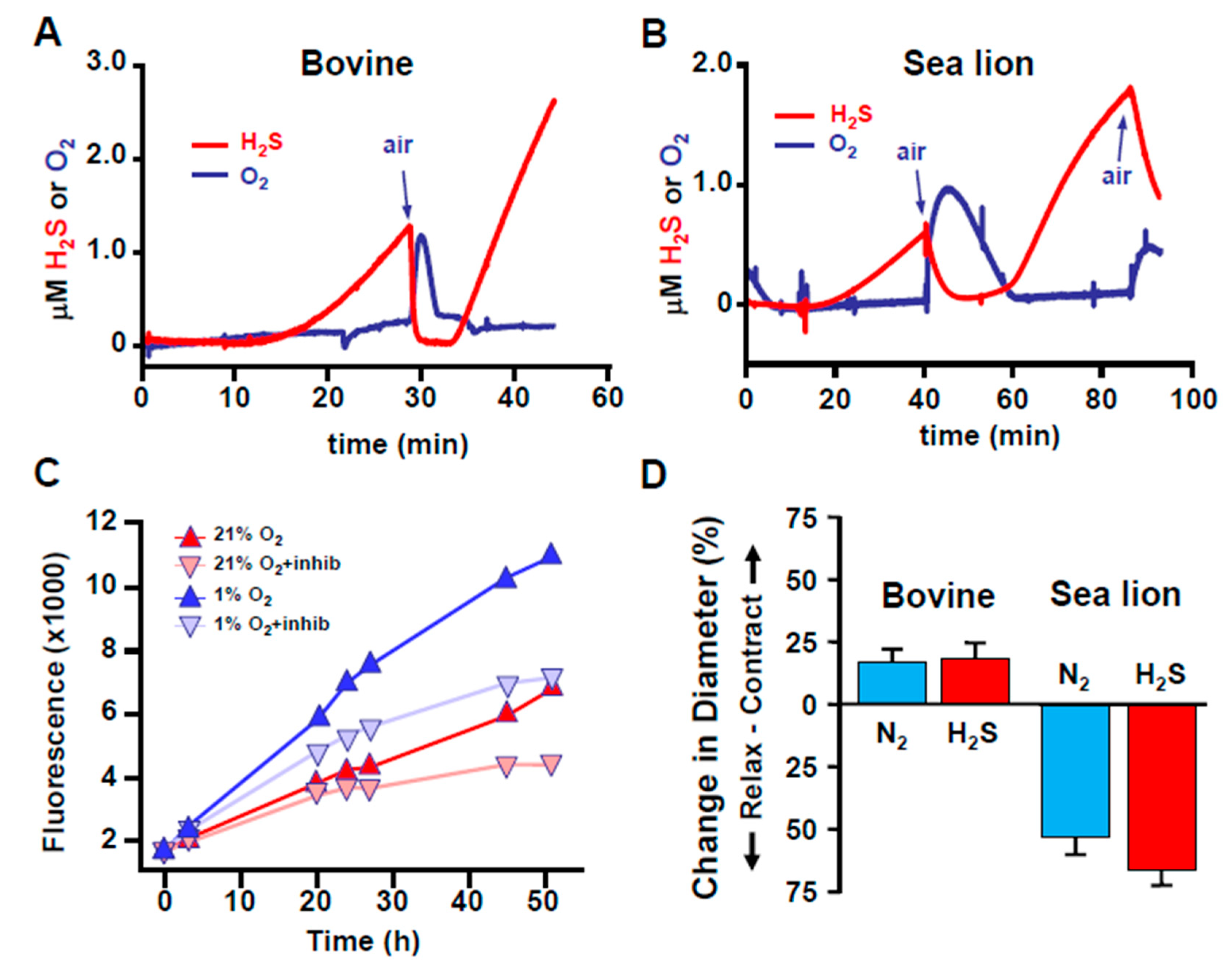
Figure 3.
H2S production is inversely related to O2 acutely and chronically. (A,B) Relationship between H2S and O2 in homogenized bovine and sea lion lungs measured in real-time with amperometric electrodes. H2S is spontaneously produced in hypoxia, rapidly disappears upon exposure to O2, and reappears after the O2 has been consumed. (C) Comparison of H2S production in HEK293 cells in 21% and 5% O2 over 52 h monitored with the H2S-sensitive fluorophore, AzMC. More H2S was produced by cells in 5% O2 over this period. Inhibition of H2S biosynthesis by cystathionine β-synthase, cystathionine γ-lyase and 3-mercaptopyrucate sulfur transferase with aminooxyacetate, propargyl glycine and compound 3, respectively (+inhibs) decreased but did not prevent H2S production in either environment. (D) Effects of hypoxia (N2) and H2S on the diameter of cannulated and pressurized bovine and sea lion pulmonary resistance arterioles. Vessels were slightly precontracted with the thromboxane A2 analog, U-46619 (10−6 M) and exposed to either hypoxia (N2) or 3 × 10−4 M H2S. Both N2 and H2S contracted bovine arterioles but relaxed sea lion vessels. A, B and D from [10]; C from [48], Reproduced with permission from Kenneth R. Olson et al. [10]. Reproduced with permission from [48], copyright 2019 John Wiley & Sons Ltd.
In order for an oxygen-sensing mechanism to be effective it must also be responsive to changes in oxygen tension (Po2) experienced by tissues under physiological conditions. Indeed, this appears to be the case as Po2-dependent inactivation of H2S appears to correlate better with hypoxic vasoconstriction and activation of carotid body chemoreceptors than does the more commonly accepted O2-sensing mechanisms (Figure 4). Using amperometric H2S sensors we measured the rate of H2S oxidation by bovine lung homogenate, bovine pulmonary arterial smooth muscle cells, or purified bovine heart mitochondria as a function of Po2 (Figure 4A; [10]). H2S oxidation in tissue homogenates begins to fail when Po2 falls below 30 mmHg and the Po2 at which oxidation is halved (P50) occurs in tissues around 4–7 mmHg and in isolated mitochondria below 1 mmHg. These P50s are physiologically relevant as they are encountered during hypoxia [85] and they are similar to the P50s for hypoxic pulmonary vasoconstriction. It is also noteworthy that the Po2 for H2S oxidation by isolated mitochondria is strongly left-shifted commensurate with their in-situ environment. The carotid body has a high metabolic rate and a commensurate oxygen sensitivity [86]. O2-dependent H2S production [87], a corollary of O2-dependent H2S metabolism, also correlates with O2 sensitivity (Figure 4B). Collectively, it is evident from these studies that the reciprocal relationship between O2 and H2S provides a convenient yin and yang mechanism for oxygen sensing with the caveats that it functions at physiological O2 tensions, it responds within seconds, and its effects can be sustained for days.

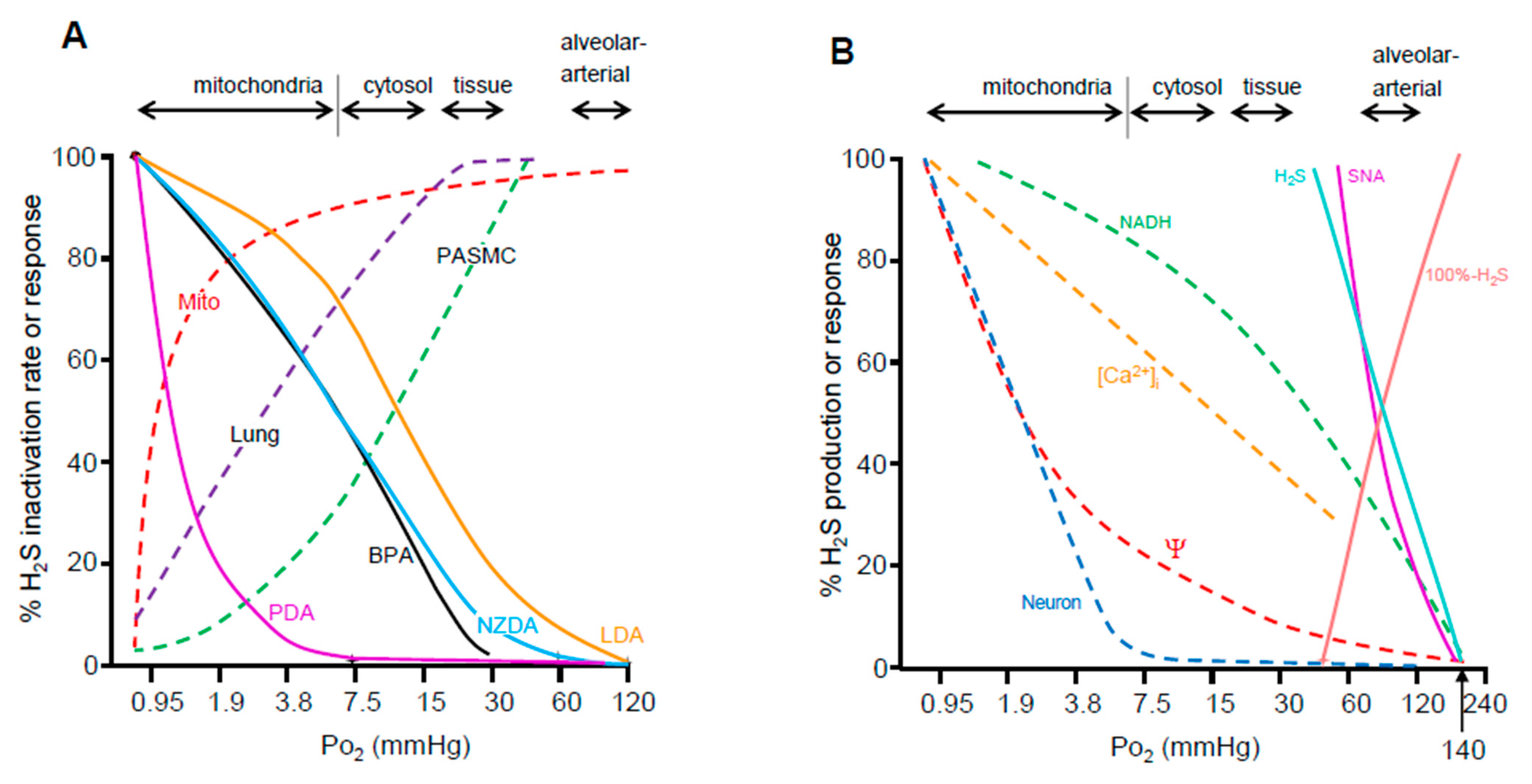
Figure 4.
(A) Oxygen sensitivity of putative O2-sensing systems as a function of approximate range of oxygen tension (Po2) in blood, tissues, and intracellular compartments (arrows). These are compared to O2 sensitivity of H2S oxidation (inactivation) by homogenized bovine lung (Lung), pulmonary arterial smooth muscle cells (PASMC) and bovine heart mitochondria (Mito) indicated by dashed lines. Solid colored lines show physiological responses (hypoxic vasoconstriction) of bovine pulmonary arteries (BPA), lamprey dorsal aorta (LDA), and dorsal aortas from New Zealand and Pacific hagfish (NZDA and PDA, respectively) as a function of Po2. The H2S oxidation and O2 sensitivity of tissue H2S consumption is similar to O2 sensitivity in vessels from oxygen sensitive vertebrates (bovine, lamprey and New Zealand hagfish), whereas O2 sensitivity in Pacific hagfish aortas is considerably lower commensurate with their tolerance to hypoxia. The Po2 values at which H2S metabolism is impaired are at the low end of cytosolic and mitochondrial Po2s and would be expected during hypoxia. It is evident that the efficacy of H2S oxidation mechanisms correlates well with physiological responses. (B) Comparison of the O2 sensitivity of afferent sinus nerve activity from the carotid body (SNA) to H2S production (H2S; or its calculated inverse, 100%-H2S) and components of intracellular signaling in the carotid body (solid lines). The Po2 of the half-maximal response (P50) for activation of the carotid is essentially identical to the P50 for H2S production which is more evident when the latter is expressed as the inverse (100%-H2S). The P50 for intracellular excitation events such as, mitochondrial NADH, intracellular calcium ([Ca2+]I), mitochondrial transmembrane potential (Ψ) or activation of sympathetic neurons (Neuron; dashed lines) are well below the P50s of the intact carotid body or H2S production. (A) Adapted from [10,88], with permission. (B) Adapted from [86], with permission and drawn from data in [87]. Reproduced with permission from Kenneth R. Olson et al. [10]. Reproduced with permission from Kenneth R. Olson et al. [88].
6. Multiple Effectors of H2S Metabolism and Signaling Provide a Broad Timeline for O2 Sensing
H2S and its numerous metabolites arguably comprise one of the most extensive and complex biological signaling systems. This is due in part to the high reactivity of sulfur with itself, as well as oxygen and nitrogen, and in part due to the central and extensive role that receptive cysteines play in a myriad of regulatory proteins that are susceptible to these S/N/O moieties. The chemistry and biology of these signaling process have been the subject of numerous and comprehensive reviews [89,90,91,92,93,94,95,96,97,98,99,100,101,102] and are only briefly summarized below.
6.1. H2S Signaling via Persulfidation
Cysteine sulfur is one of the most reactive sulfur-containing small molecules nucleophiles in the cell and as the most highly conserved amino acid, its function in protein (and peptide) structure, catalytic activity and signaling, i.e., the cysteine proteome, is well known [95]. Perhaps the broadest and most extensive mechanism of H2S signaling is through persulfidation (also known as S-sulfuration and sulfhydration) of thiols on regulatory protein cysteines. The sulfurs in both H2S and protein thiols (Prot-SH) are in their most reduced state and will not react unless one or the other is oxidized. This can occur through a direct reaction between H2S and protein cysteine sulfenic acids or protein disulfides, by oxidation of H2S to a polysulfide (the oxidized sulfur is referred to as sulfane) that then reacts with a reduced protein cysteine, or by transfer of a sulfane sulfur from a low molecular weight persulfide to the protein thiol (Figure 5A–D). Typically, these reactions inhibit protein function. There are numerous examples of protein persulfidation and it is estimated that at least 30% of cellular proteins are endogenously persulfidated, leaving the opportunity for activation of these proteins by removing the sulfane sulfur with cellular reductants [103]. As more than one sulfur may be attached to these persulfides some protein/peptides may also serve as sulfur reservoirs from which sulfane can be removed and transferred to other proteins by mobile carriers such as cysteine and glutathione. The extent of these processes is an active area of investigation. H2O2 signaling is essentially similar to persulfide signaling with the caveat that H2O is produced when the protein sulfenyl is reduced, whereas the sulfur can be transferred from the protein to another thiol (usually Cys or GSH) and it can be stored or recirculated or reduced to H2S which can also be recycled [104].

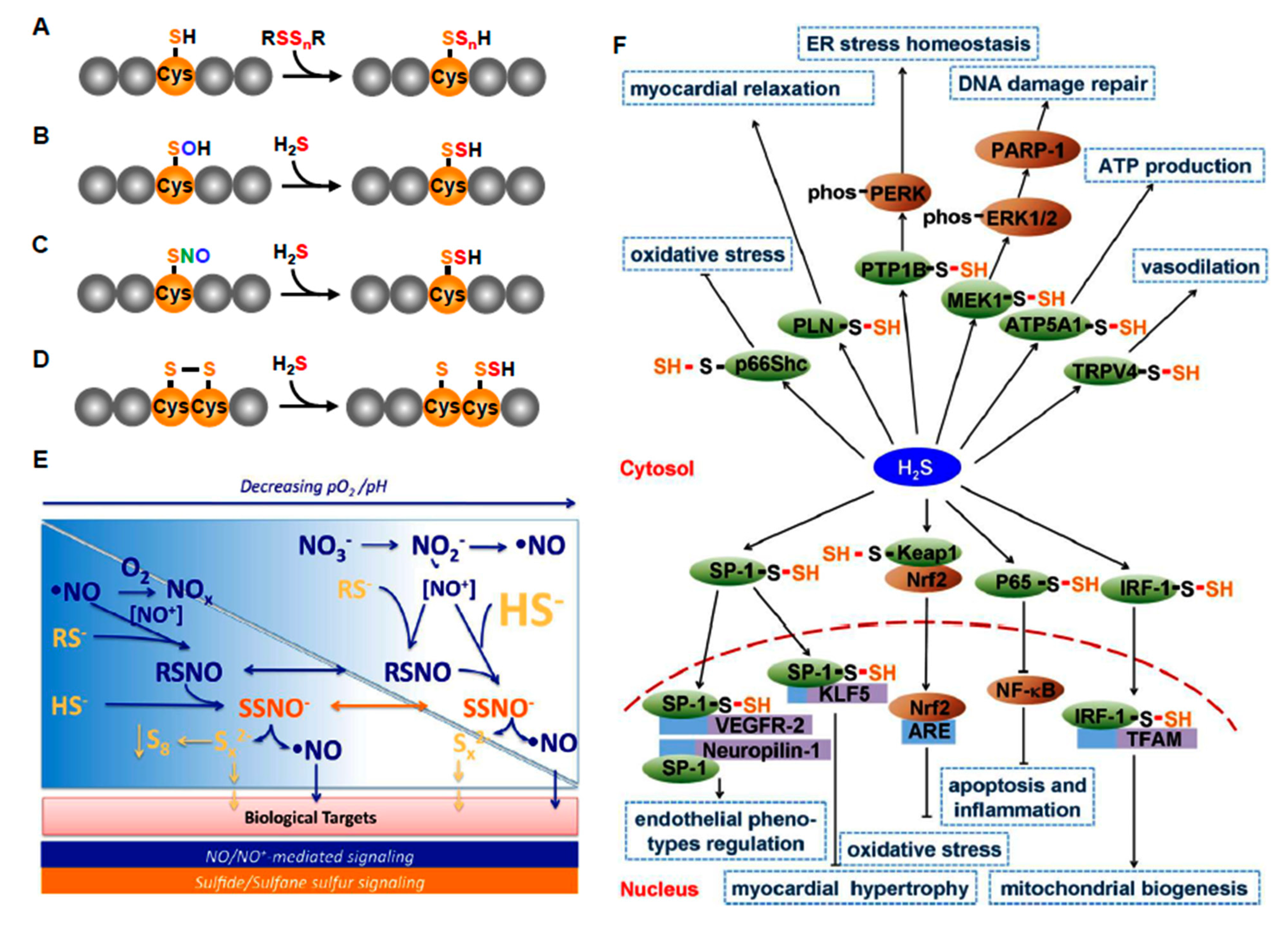
Figure 5.
H2S and per- polysulfide signaling mechanisms. (A–D) Interactions of H2S or per- polysulfides (RSSnR, where R=H, Cys or GSH in various combinations and n = 1–4) with reactive protein Cys. (E) Sulfur and nitric oxide (NO) signaling. As oxygen falls, cellular pH decreases and H2S and NO increase leading to accumulation of a variety of bioactive products. (F) Effector pathways for H2S (more appropriately per- and polysulfides) signaling in the cardiovascular system. Abbreviations: ATP5A1, ATP synthase subunit α; Cys, cysteine; GSH, glutathione; IRF-1, interferon regulatory factor-1; Keap1, kelch-like ECH-associating protein 1; MEK1, mitogen-activated extracellular signal-regulated kinase 1; NF-κ, nuclear factor κB; Nrf2, nuclear factor E2-related factor 2; PTP1B, protein tyrosine phosphatase 1B; SNO, S-nitrosocysteine; SOH, sulfenyl cysteine; SP-1, specific protein-1; TFAM, mitochondrial transcription factor A; VEGFR, VEGF receptor. (E) from [105], with permission. (F) From [98], with permission. Reproduced with permission from [98], copyright 2017 The British Pharmacological Society.
6.2. H2S Signaling via Reactions with Nitrogenous Compounds
H2S reacts with nitric oxide (NO) to produce a variety of bioactive compounds, some with and some without sulfur including polysulfides, S-nitrosothiols (RSNO, where R = H, Cys, GSH), nitrosopersulfide (SSNO−), and nitroxyl (HNO; Figure 5E). Other compounds are likely produced as well, the identity of which is still being actively investigated and debated. NO can also be released from nitrosothiols by H2S thereby initiating the NO signaling cascade [106]. Peroxynitrite reaction with H2S can form thionitrite (HSNO2) and under anaerobic conditions produce HSO and NO or under aerobic conditions, sulfinyl nitrite (HS(O)NO; [107]). H2S can react with sulfinyl nitrite to form thiosulfate and nitrite (NO2−) and the latter may then be reduced by H2S, catalyzed by heme iron, and produce NO and nitroxyl (HNO). H2S may also directly activate endothelial nitric oxide synthase (eNOS). The production and/or activity of many of these compounds becomes increasingly apparent in hypoxia or as pH falls (Figure 5E), the latter being a common feature of cellular hypoxia [105]. Furthermore, H2S and the polysulfide, garlic derivative diallyl trisulfide (DATS) stimulates xanthine oxidoreductase conversion to nitrite reductase with subsequent formation of NO [108]. H2S also potentiates the response of soluble guanylyl cyclases to NO and it inhibits phosphodiesterase activity [109].
6.3. H2S Signaling via Carbon Monoxide
Carbon monoxide (CO) has been shown to inhibit CSE via protein kinase G (PKG)-dependent phosphorylation of Ser377 on CSE, thereby inhibiting the production of H2S [110]. Hypoxic inhibition of hemeoxygenase-2 (HO-2) can relieve this inhibition and increase H2S. In this mechanism H2S is proposed to serve as a downstream mediator of the hypoxic response in the carotid body glomus cells, adrenal medulla, and cerebral cortical vessels [111,112,113,114].
6.4. Timescale of H2S/O2 Signaling
As described above, the timescale for H2S-mediated stimulus-effector coupling ranges from seconds to days. These responses will depend on factors that are directly involved in affecting cellular H2S metabolism as well as those that depend on activating downstream effectors of the hypoxic response. The following sections describe metabolic regulation of H2S signaling as well as a few of the downstream effectors with various response rates. In the context of this discussion ‘responders’ refers to those factors that are influenced by hypoxia, ‘regulators’ are those factors that regulate the response and ‘effectors’ are the downstream factors that bring about the desired homeostatic effect.
6.4.1. Rapid Responders
The most rapid H2S signaling is expected to be achieved through factors that directly affect H2S metabolism and downstream effectors that mediate ion channels.
H2S Metabolism
(1) Arguably, the fastest of these affect electron transport down the ETC, i.e., decreased availability of the terminal electron acceptor, O2 which slows the removal of the constitutively produced H2S; our hypothesis of H2S-mediated O2 sensing [22].
(2) ETHE1, the mitochondrial dioxygenase, ETHE1, catalyzes the oxidization of the mobile persulfide from SQR to form sulfite in a reaction that uses O2 and H2O. Inhibition of this pathway prevents H2S binding to SQR and allows H2S and thiosulfate to accumulate [115,116,117].
(3) The other O2-dependent reaction, catalyzed by sulfite oxidase (SO), transfers an atom of oxygen from water to sulfite, forming sulfate and reducing SO [118]. The two electrons from SO are delivered to the ETC at cytochrome c thereby inversely coupling sulfite concentration to O2 availability. Elevated urinary thiosulfate is common in humans with SO deficiencies [119].
(4) H2S can also be ‘regenerated’ from thiosulfate by endogenous reductants in reactions catalyzed by 3-MST or thiosulfate reductase [30,46]. This has been demonstrated in a variety of vertebrate tissues [45]. H2S would be expected to increase during hypoxia when thiosulfate accumulates in the mitochondria and the mitochondrial matrix becomes reduced [120]. Thiosulfate also produces KATP channel-mediated vasodilation which is consistent with the effector of H2S vasodilation as discussed below.
Rapid Responding Effectors
The variety of effectors of H2S signaling is only beginning to be unraveled. Arguably, the best known rapid responding systems are numerous ion channels found in the cardiovascular system and elsewhere. As with most instances of H2S signaling, most of the effects are produced by polysulfides. Zhao et al. [121] were the first to demonstrate that H2S opened ATP-sensitive potassium (KATP) channels resulting in vasodilation. Subsequent studies have shown that H2S also dilates by opening voltage gated Kv7 potassium channels [122]. We initially reported that H2S has concentration-dependent multiphasic effects on a variety of blood vessels that are virtually identical to the effects of hypoxia [22]. Furthermore, the degree of hypoxia can further affect H2S responsiveness [123]. This effect appears to be explained in part by different effectors, at low concentrations H2S constricts systemic vessels by activating Na+-K+-2Cl− cotransport and opening L-type Ca2+ channels, whereas at higher concentrations vasodilation is mediated by K+ channels [124]. H2S has also been shown to affect a variety of other channels (Figure 5F) including large conductance calcium channels (BKCa) and smooth muscle Ca2+ sparks [125,126]. Other effectors include phosphodiesterase inhibition which augments NO responses [127] and decreasing intracellular pH by activation of the Cl−/HCO3− exchanger [128]. Many of these systems are found in vascular smooth muscle and/or endothelial cells and can result in complex vasoactive effects.
Interactions between H2S and NO, described above provide additional examples of rapid activation responses such as activation of TRPA1 channels [129]. These, and other actions of H2S in the cardiovascular system have been extensively reviewed [130,131,132,133,134,135].
6.4.2. Medium and Long-Term Responders
Medium to long-term responses include enzymes involved in H2S metabolism, effectors that require subsequent catalytic activity. Many also exert action via genomic effectors.
H2S Metabolism
CBS is transported from the cytosol into the mitochondrial matrix by mitochondrial heat shock protein (mtHsp 70). Under normoxic conditions the prosthetic heme group in CBS is oxygenated which targets it for degradation by Lon protease [36]. Hypoxia prevents this and CBS concentration can double within 10 min and increase sixfold in one hour. CBS is also restored to control levels within 5 min of normoxia. Mitochondrial cysteine concentration is three times that of the cytosol [37]. This CBS-generated H2S prevents Ca2+-mediated cytochrome C release from mitochondria and prevents mitochondrial swelling while decreasing ROS production. Teng et al. [36] proposed this as a protective effect of H2S in myocardial and hepatic ischemia/reperfusion injury.
CSE translocation from the cytosol into the mitochondria is also stimulated by hypoxia in vascular smooth muscle cells. This has been proposed to provide protection from hypoxia by increasing ATP generation from H2S [37]. However, this hypothesis is problematic as hypoxia will also decrease electron flux from H2S down the ETC, as described above, and vascular smooth muscle can obtain sufficient energy from anaerobic metabolism [136]. Nevertheless, this process could contribute to O2 sensing and the resultant hypoxic vasodilation.
Cytosolic cysteine dioxygenase (CDO) uses O2 to irreversibly catalyze cysteine oxidation to cysteine sulfinate which prevents cysteine from entering the transsulfuration pathway [137]. Although not directly examined, it is likely that hypoxia will also impair cysteine oxidation and favor H2S production. The corollary, CDO deficiency, does, in fact redirect cysteine through this pathway and increases thiosulfate and H2S production [138,139], providing some anecdotal support to this hypothesis.
Delivery of substrate may also be affected by hypoxia. Hypoxic preconditioning upregulates the cystine/glutamate antiporter, system Xc-, in murine neural stem cells, a process that may take from 45 min to 4 h [56].
Slow-Responding Effectors
Some of the known reactive thiols on regulatory proteins in the cardiovascular system and their downstream effectors are shown in Figure 5F. Many of them could potentially be affected by per- and polysulfides that result from hypoxia, but this is yet to be thoroughly examined.
7. Evidence for H2S Mediated O2 Sensing in Various Organ Systems and Tissues
Technical note: There are numerous reports of H2S concentration exceeding 1 μM in body fluids and tissues. These are unphysiologically high [140]. The reader is urged to use caution in interpreting results and conclusions from these studies.
7.1. Cardiovascular System
7.1.1. Blood Vessels
Numerous studies on all classes of vertebrates have shown that mono- and multiphasic responses of blood vessels, perfused organs, and intact animals to exogenous H2S are similar, if not identical, to those initiated by hypoxia [10,22,81,88,141,142,143,144]. Arguably, the strongest argument for this association, and H2S as an oxygen-sensing mechanism, is the comparison of the vasoactive responses of bovine and sea lion pulmonary arteries to hypoxia and H2S. Both treatments constrict bovine vessels, but they dilate sea lion vessels, whereas hypoxia increases H2S production by both tissues [10].
The effects of compounds that augment or inhibit H2S production on vascular responses to hypoxia have had mixed results. Sulfur donors, such as l-cysteine, d-cysteine, 3-mercaptopyruvate and glutathione are reported to augment hypoxic responses of rat aortas [127,145,146], bovine pulmonary arteries [10,22] lamprey aortas [22] and the perfused rat lung [81] and perfused trout gills [143]. Cysteine plus α-ketoglutarate (presumably via the CAT/3-MST pathway) also increases hypoxic vasoconstriction in bovine pulmonary arteries [10,81].
Although inhibitors of H2S biosynthesis have inherent problems [147,148], in a number of studies they have been shown to inhibit hypoxic responses in a variety of vessels including the lamprey and rat aorta, bovine pulmonary arteries, perfused trout gills and perfused rat lungs [22,81,143,145,146]. The major pathway for H2S production by systemic vessels appears to involve CSE, whereas CBS and CAT/3-MST are involved in H2S production in bovine pulmonary vessels; CSE may also be involved in the rat lung [81].
There are a number of studies that suggest additional levels of hypoxic regulation of H2S and homeostasis in the vasculature. Intermittent hypoxia associated with sleep apnea decreases CSE and H2S in resistance arterioles resulting in increased vascular resistance [149]. Administration of H2S to rats with hypoxic pulmonary hypertension (HPH) inhibits the expression of elastin in its extracellular matrix, which also has remarkable regulatory function in forming HPH and remodeling hypoxic pulmonary vascular structure [150]. Furthermore, inhibition of various components of the electron transport chain may also inhibit both hypoxia- and H2S-mediated response. The roles of H2S in regulation of vascular tone have been recently reviewed [151,152].
7.1.2. Heart
Using a newly developed mitochondria-targeted mass spectrometry probe, Arndt et al. [153] demonstrated that mitochondrial H2S was increased in murine hearts after 30 min occlusion of the left anterior descending coronary artery followed by 45 min of reperfusion. Similar results were observed in ischemic liver, supporting the hypothesis that hypoxia increases cellular H2S in a variety of tissues and that this has a mitochondrial origin.
CSE has long been presumed to be the primary enzymatic mechanism for H2S biosynthesis in the heart [154]. However, recent work suggests important roles for 3-MST. 3-MST is more abundant in cardiomyocytes and smooth muscle than CSE and, curiously, deletion of 3-MST protects young mice from reperfusion injury but exacerbates in in older 3-MST-/- mice and predisposes them to hypertension and cardiac hypertrophy [155]. Additional information can be found in recent reviews [156,157,158].
7.1.3. Central Cardiovascular Regulation
A number of studies have shown that H2S modulates the brain cardiovascular centers, and these effects are exacerbated in spontaneously hypertensive animals (e.g., [158,159,160,161]). Several studies have shown an association between H2S and brain oxygenation. Sabino et al., [162] reported that microinjection of aminooxyacetate (AOA) into the fourth ventricle of Wis-tar normotensive rats (WNR) did not affect the cardiovascular responses to hypoxia (30 min of 10% inspired O2), whereas it blunted the ventilatory and cardiovascular responses in SHR rats. The same group also demonstrated that H2S modulates hypoxia-induced hypothermia in rats, and this is also exacerbated in spontaneously hypertensive (SHR) rats due to excess H2S production in the caudal nucleus of the solitary tract [163]. These findings support a link between H2S and O2.
7.1.4. Ischemia/Reperfusion Injury
There is an extensive body of literature on the protective effects of H2S and H2S donors against ischemia and the efficacy of these compounds to pre- and post-condition the myocardium as well as the central nervous system, liver, kidney and other organs and tissues. While this implies that ischemic conditioning increases cellular H2S, which then initiates appropriate effector responses, this is rarely examined and results are on occasion contradictory (e.g., [164,165]). Nevertheless, these studies are indicative of the importance of the O2/H2S axis in health and disease and are suggestive of the role of H2S in O2 sensing. The connection between O2 and H2S is especially important in ischemia and reperfusion injury in the central nervous system, and this is examined in Section 7. Pathophysiological consequences of the H2S/O2 axis. Additional details can be found in a number of recent reviews [166,167,168,169,170,171,172,173,174,175,176,177].
7.2. Respiratory System
7.2.1. General Effects on Respiration
It is well known that high levels of H2S inhibit respiration in vertebrates, intravascular injection or inhalation of lower levels of H2S will mimic hypoxic hyperventilation in fish, birds and mammals [82,178,179,180,181,182,183,184,185]. These responses appear to be mediated by both central and peripheral mechanisms.
7.2.2. H2S and Central Respiratory Centers
H2S injected into cerebral ventricles produces a concentration-dependent bradycardia and hypotension, mimicking the diving reflex in mammals [186]. A number of respiratory centers including the pre-Bötzinger (pB) dorsal inspiratory respiratory group, the parafacial respiratory group and hypoglossal rootlets are stimulated by H2S [187,188] and H2S helps protect the medullary respiratory centers from hypoxic injury [189,190]. 3MST mRNA and protein are expressed in neurons of pre-Bötzinger complex (pre-BotC), hypoglossal nucleus (12N), ambiguous nucleus (Amb) in rats. These 3MST-positive neurons are significantly increased in animals exposed to chronic intermittent hypoxia (CIH) suggesting that adaption to CIH is mediated, at least in part, by H2S [191]). Conversely, it has also been reported that microinjection of AOA into the rostral ventrolateral complex (RVLM)/Bötzinger complex increases hypoxia-induced hyperventilation and mitigates hypoxic hyperthermia while injection of H2S does not affect ventilation; hypoxia also decreases H2S production in rat medullary homogenates [192,193]. Clearly, additional studies are necessary to resolve these issues.
The first pair of gill arches are peripheral chemoreceptors in fish, and they are sensitive to H2S (see below). Hypoxic bradycardia is inhibited by removing these arches, but their removal does not affect hypoxic hyperventilation, and inhibitors of H2S production, AOA or propargyl glycine (PPG) are also ineffective [82]. This suggests that central chemoreceptors are involved, but it remains to be determined if H2S contributes to the O2 sensing process in the fish central nervous system.
7.2.3. H2S Mediation of Peripheral Chemoreceptors, Carotid Body and Neuroepithelial Cells
Exogenous H2S depolarizes carotid glomus cells, it increases afferent nerve activity from the carotid, and it mimics or augments hypoxic hyperventilation while accelerating sinus nerve response to hypoxia [87,194,195,196,197]. Although excess exogenous H2S has been reported to inhibit hypoxic responses and to inhibit acetylcholine and ATP release by the carotid body [195,196,198], these are likely to be toxicological, rather than physiological effects.
CBS and CSE immunoreactivity have been identified in glomus cells from cats, rats and mice [87,195,196,198]. In vitro and in vivo studies have provided evidence for CSE, CBS and 3MST-mediation of hypoxia-induced release of H2S [87,195,196,197] and hypoxia (Po2 ~ 30 mmHg) increases H2S production rat carotid bodies [87,196]. Breathing 100% O2 will suppress H2S-induced hyperventilation suggesting that enhanced O2 increases H2S metabolism by the glomus cells [185].
Arguably the most extensive studies on H2S and O2 sensing by the carotid body have been done by Prabhakar’s group (reviewed in; [199,200,201,202]. These authors make the case that H2S is a downstream effector of the O2 sensing process. They describe two mechanisms, both of which involve relieving tonic inhibition of CSE by carbon monoxide (CO) or nitric oxide (NO) in normoxic conditions. In the primary mechanism, hypoxia inhibits heme oxygenase-2 (HO-2) thereby decreasing carbon dioxide (CO) which relieves CSE inhibition and increases H2S synthesis. A backup mechanism has also been proposed where, in the absence of HO-2, neuronal nitric oxide synthase (nNOS) is upregulated and CSE is now inhibited by nitric oxide (NO). As NO synthesis is also O2-dependent, H2S production will increase as O2 falls. The effects of H2S on glomus cells have been attributed to inhibition of BKCa channels [195,203,204], or TASK channels [194], KATP channels do not seem to be involved [87]. Inhibiting K channels depolarizes the glomus cells, and the resulting influx of calcium initiates release of neurotransmitters. Conversely, it should be noted that other studies have found that chemical inhibition of CSE or genetic deletion of the enzyme had no effect on the hypoxic response [205,206] and clearly additional work is needed.
Peripheral chemoreceptor cells (neuroepithelial cells; NEC), especially prevalent on the first pair of gill arches in many fish, are the antecedents of mammalian carotid glomus cells [4]. NEC contain both CBS and CSE [207]. H2S injected into the buccal cavity (mouth) stimulates NEC in the trout gill and produces classic hypoxic bradycardia which is prevented by ablation of the first pair of arches. Both hypoxia and H2S depolarize NEC isolated from zebrafish gills, as does hypoxia [82]. NEC are also found on the skin of larval (4-day post hatching) zebrafish, where the gills are relatively undeveloped. Hypoxic responses can be attenuated by chemical inhibition of CBS and CSE in adult zebrafish or by morpholino knockdown of these enzymes in larval forms [207], further implicating H2S in O2 sensing.
7.2.4. H2S Mediation of O2 Sensing by Adrenal Medulla
Mammalian adrenal medullary chromaffin cells function as O2 sensors during neonatal development [4]. CSE immunoreactivity has been identified neonatal chromaffin cells in both rats and mice and H2S appears to mediate hypoxic stimulation of catecholamine secretion in these animals. This likely involves HO-2/CO/CSE and BKCa channels, similar to those described in the carotid glomus cells [87,111,208].
Fish do not have adrenal glands; however, homologous chromaffin cells line the posterior cardinal vein and anterior kidney, and these cells possess both CBS and CSE; they also release H2S and epinephrine into the systemic circulation in response to hypoxia by a CBS-mediated mechanism [209]. Catecholamines are also released by exogenous H2S, a process that requires extracellular calcium indicative of chromaffin cell depolarization. It has not been determined if HO-2 and CO are involved in the O2 sensing process in these cells.
7.2.5. Airway Receptors
Neuroepithelial bodies in airways sense changes in inspired O2 and initiate appropriate cardiorespiratory reflexes [5,210]. These responses are initiated by inhibition of potassium channels, suggestive of a H2S-activated response. This is supported by observations that H2S enhances airway reflex responses in part, through action on TRPA1 receptors [211]. Low concentrations (0.2%) of inhaled H2S also stimulate ventilation in chickens, which has been proposed to be mediated in part by airway receptors [178]. However, a hypoxia-mediated increase in H2S in airway receptors has yet to be demonstrated.
7.2.6. Mechanical Effects on Airway Smooth Muscle
Hypoxia and H2S relax tracheal and bronchiolar airway smooth muscle, which is mediated, at least in part, by BKCa channels [212,213,214]. Small airways appear considerably more sensitive to H2S than larger ones [215]. Conversely, guinea pig main bronchi and distal trachea are contracted by high concentrations of H2S, a response that appears to be mediated by activation of vanilloid neurons [216]. As with airway receptors the direct coupling between hypoxia and an increase in H2S remains to be demonstrated.
7.3. Kidney
The physiological activities of H2S in the kidney have been extensively reviewed [174,217,218]. Low oxygen tensions in the renal medulla have been proposed to necessitate H2S responses to help maintain renal blood blow and reduce energy requirements for tubular transport [219] and this is supported by beneficial effects of H2S observed in acute kidney injury which occurs during hypoxia or ischemia-reperfusion injury [174]. Additional work on H2S/O2 coupling is needed to confirm these hypotheses.
7.4. Genitourinary Tract
H2S has multiple functions in the genitourinary tract [220,221,222]. There are numerous examples where hypoxia and H2S relax non-vascular smooth muscle in the genitourinary systems of mammalian and non-mammalian vertebrates [78,223,224,225]. We have recently shown that hypoxia initiates both transient and long-term (days) increases in H2S production in HTC116 human colonic epithelial cells [48], suggestive of H2S/O2 coupled signaling.
7.5. H2S-HIF Interactions
It is not surprising that H2S would interact with hypoxia inducible factors (HIF) in oxygen sensing mechanisms, although the extent and nature of these interactions are still being resolved. H2S has been shown to both inhibit HIF-1α expression and stabilization [56,226,227,228,229] and augment it [230,231,232,233,234,235,236]. HIF-1α stabilization also inhibits colonic H2S production and may represent a negative feedback mechanism to prevent prolonged HIF-1α stabilization [231]. Recently, polysulfides were shown to inhibit HIF-1α gene expression, protein accumulation and subsequent stabilization with the potency correlated with the number of sulfur molecules, i.e., S4 > S3 > S2; with S1 (H2S) showing relatively low activity. The main effect of these polysulfides appeared to enhance degradation rather than affect synthesis. The study by Uba et al. [237] is key to our further understanding of H2S-HIF regulation as most physiological effects of H2S are only initiated after H2S is oxidized to polysulfides.
8. Pathophysiological Consequences of the H2S/O2 Axis
Too much of a good thing is not necessarily a good thing and there are a number of instances where hypoxia-initiated increases in H2S may be detrimental. The following are three such examples.
8.1. Cerebral Ischemia and Stroke
It is well known that the central nervous system is especially sensitive to hypoxic insult. A number of studies have shown that administration of exogenous H2S or H2S donors or upregulation of endogenous H2S production can protect the central nervous system from ischemia and ischemia-reperfusion injury and that the latter is exacerbated by inhibition of endogenous H2S production [238,239,240,241,242,243,244,245,246,247]. This would imply that both ischemia and reperfusion injury are associated with a decrease in endogenous H2S production. This is supported by several studies that have shown that hypoxia decreases CBS [245,246].
Conversely, other studies have shown that H2S worsens the ischemic insult [248,249] and that this is the cause of neuronal death. Although a low Po2 has been assumed to be the direct cause of the collapse of the electron transport chain and energy production, Marutani et al. posit that the collapse occurs long before Po2 falls to levels that jeopardize O2 binding to cytochrome c oxidase (CCO; [249]). Furthermore, they suggested that the relative inability of neurons to metabolize H2S may explain this conundrum and, indeed, this seems to be the case. SQR levels in neurons are extremely low, or even non-existent [67,80,250] and CCO is especially sensitive to inhibition by H2S with a Ki of ~0.2 μM [251]. In a series of elegant experiments Marutani et al. [249] demonstrated in mice, rats, and naturally hypoxia-tolerant ground squirrels that increasing expression of neuronal SQR decreased H2S accumulation in the hypoxic brain, sustained energy production and prevented ischemic brain injury. Similar results were observed after administration of exogenous H2S scavengers. Conversely, decreasing SQR expression in the brain, heart and liver exacerbated the sensitivity of these tissues to hypoxia. These findings show why the brain is comparatively more sensitive to hypoxia than other tissues and they may offer a therapeutic opportunity in ischemic injury and RPI.
These somewhat contradictory observations may be due to the level and/or the duration of hypoxia or other methodological differences. Clearly, sorting this out and targeting the appropriate H2S-metabolizing pathways will have considerable therapeutic value.
8.2. High Altitude Pulmonary Edema (HAPE)
High altitude pulmonary edema (HAPE) occurs in un-acclimatized individuals upon rapid ascent to altitudes over 2500 m. HAPE is noncardiogenic and generally attributed to pulmonary vasoconstriction-mediated exudate as a result of sympathetic stimulation, reduced nitric oxide (NO) bioavailability or increased endothelin; inflammatory mediators such as C-reactive protein (CRP) and interleukin (IL-6) may further modulate the disease but do not appear to be the cause [252,253].
Fluid balance across a healthy respiratory epithelium is governed by the rate of salt secretion and reabsorption. Epithelial sodium channels (ENAC) and a basolateral Na+, K+ ATPase create a alveolar lumen-to-blood trans-epithelial osmotic gradient that reabsorbs fluid which keeps the lung ‘dry’ and ensures a relatively minimal diffusion barrier for respiratory gases. H2S inhibits fluid reabsorption by alveolar cells by inhibiting both ENAC and Na+, K+ ATPase and produces pulmonary edema [254,255,256]. As hypoxia also increases H2S production by the respiratory epithelium [254,256], it seems reasonable to assume that this contributes to HAPE. Not surprisingly, pulmonary edema is also a hallmark of H2S poisoning in humans (https://www.osha.gov/hydrogen-sulfide/hazards, accessed on date/month/year, accessed on 18 October 2021).
8.3. HAPE and Down Syndrome
Down syndrome (DS), the result of trisomy of chromosome 21, results in over-expression of CBS, one of the enzymes encoded on this chromosome [257]. This results in increased H2S production in these individuals. In a meta-analysis, Pecze et al. [258] found that there were significantly decreased levels of ATP, CoQ10, homocysteine, serine, arginine and tyrosine; slightly decreased ADP; significantly increased uric acid, succinate, lactate and cysteine; slightly increased phosphate, pyruvate and citrate in DS individuals. They concluded that the levels of metabolites involved in bioenergetic pathways was suggestive of a “pseudohypoxic state” even though arterial gases were normal. With an already elevated titer of H2S, one might expect that DS individuals would be especially susceptible to HAPE, at even moderate altitudes, and, indeed, this appears to be the case [259,260].
9. Resolving Differences between Competitive Theories of O2 Sensing; Reactive Oxygen Species (ROS) vs. Reactive Sulfur Species (RSS)
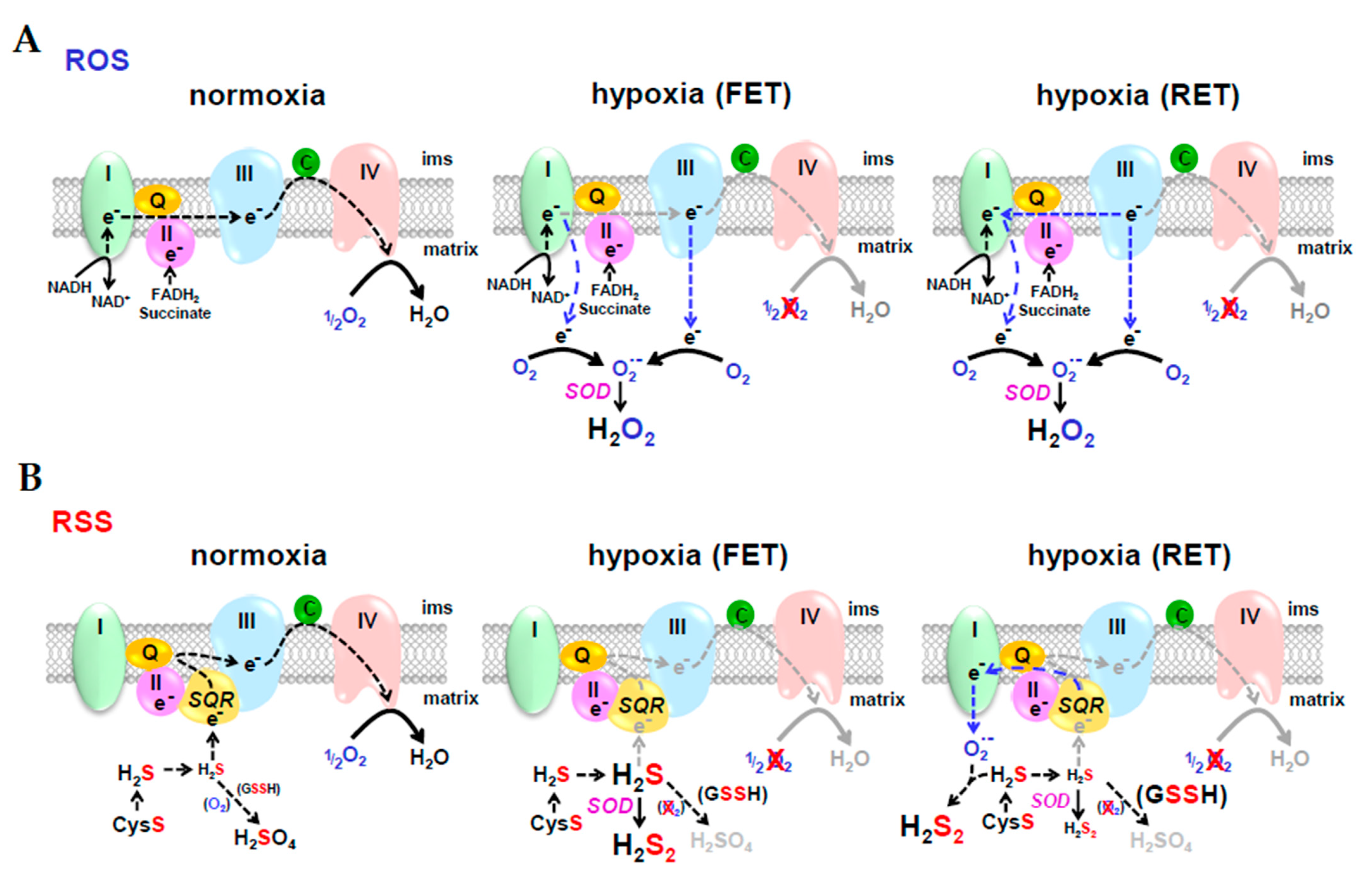
O2 sensing by ROS is arguably the most prevalent theory of an O2 sensing mechanism, especially in the case of hypoxic pulmonary vasoconstriction (HPV; [261]). In the ROS hypothesis (Figure 6A), hypoxia decreases forward electron transport (FET) down the electron transport chain and as electrons begin to build up they leak from complexes I and III and reduce O2 to superoxide (O2•−). Dismutation of superoxide, either spontaneously, or catalyzed by superoxide dismutase (SOD), produces hydrogen peroxide (H2O2) which then diffuses out of the mitochondrion and oxidizes cysteine residues on the appropriate regulatory proteins [262]. In reverse electron transport (RET), electrons may be delivered retrograde from complex III to complex I, however, there is little evidence that that this contributes to HPV [263]. In the RSS hypothesis (Figure 6B), hypoxia also decreases FET, but this decreases H2S oxidation by (SQR) which then allows mitochondrial H2S concentrations to increase. This excess H2S is oxidized by SOD, or by electrons from RET, to hydrogen persulfide, H2S2, which then diffuses out of the mitochondrion and persulfidates cysteine residues on regulatory proteins that are essentially identical to those oxidized by H2O2.

Figure 6.
Reactive oxygen species (ROS) and reactive sulfur species (RSS) hypotheses for O2 sensing. In the ROS hypothesis (A), hypoxia decreases forward electron transport (FET) down the electron transport chain and electrons leak from complexes I and III; in reverse electron transport (RET), electrons are delivered retrograde from complex III to complex I. As electrons leak from the complexes, they reduce O2 to superoxide (O2•−). Dismutation of superoxide, either spontaneously, or catalyzed by superoxide dismutase (SOD), produces hydrogen peroxide (H2O2) which then diffuses out of the mitochondrion and oxidizes cysteine residues on the appropriate regulatory proteins. In the RSS hypothesis (B), hypoxia also decreases FET, but this decreases H2S oxidation by sulfide quinone oxidoreductase (SQR) thereby increasing mitochondrial H2S concentration. Excess H2S is oxidized by SOD, or by electrons from RET, to hydrogen persulfide H2S2 which diffuses out of the mitochondrion and persulfidates cysteine residues on the appropriate regulatory proteins.
Many of the arguments in support of ROS in biological signaling can also be made for RSS and it is becoming increasingly difficult to distinguish between the two. Comparisons and distinctions between ROS and RSS have detailed in a recent review [104] and references therein and are only briefly described in the following paragraphs.
9.1. Chemical Similarities between ROS and RSS
Both oxygen and sulfur have six valence electrons, but sulfur’s electrons are farther from the positive nucleus which favors electron transfer reactions. Single-electron reduction of O2 produces superoxide (O2•−), hydrogen peroxide (H2O2), the hydroxyl radical (HO•) and water (Equation (1)), whereas single electron oxidation of H2S produces a thiyl radical (HS•), hydrogen persulfide (H2S2) and a persulfide “supersulfide” radical (S2•−) before terminating in elemental sulfur (S2; Equation (2)), the latter often cyclizes to S8.
H2O < +e− HO• < +e− H2O2 < +e− O2•− < +e− O2
H2S −e− > HS• −e− > H2S2 −e− > S2•− −e− > S2
Most ROS and much, but certainly not all, RSS signaling is mediated by H2O2 and H2S2, respectively; the advantages of the latter over the former were described in Section 6.1 H2S signaling via persulfidation.
9.2. ROS or RSS?
In addition to their redox similarities there are a number of other disconcerting aspects that hamper evaluation of the relative contributions of ROS and RSS in biological signaling. (1) It can be difficult to analytically distinguish between the two. The redox-sensitive green fluorescent protein (roGFP), arguably, the gold standard for intracellular ROS measurement, is up to 200-fold more sensitive to RSS than ROS; H2O2 amperometric electrodes are 25 times more sensitive to RSS as well. Other fluorescent ROS probes may also respond to RSS. (2) Most biochemical and physiological experiments are conducted in room air (21% O2, or 18.5% O2 for cell culture). These conditions well above physiological O2 tensions in cells (physioxia) and greatly exceed those in the mitochondrion. This undoubtedly favors ROS over RSS. (3) Life originated in an anoxic and sulfidic world and much of subsequent evolution occurred in these conditions. Many homeostatic pathways were developed under these conditions and likely will become more evident as more experimentation is performed under more physioxic conditions. The reader is referred to the excellent monograph Martin et al. [264] on the evolution of anaerobic energy metabolism in eukaryotic mitochondria.
10. Conclusions
H2S and polysufide signaling is a relatively new, yet rapidly expanding field. Because of the lability of these sulfur moieties in oxic environments, many challenges remain in identifying relevant signaling species and their metabolism under physioxic conditions. Nevertheless, it is this nearly mutually exclusive relationship between H2S and O2 that forms the basis for an O2 sensing mechanism that is exquisitely tuned to respond to O2 availability without the need for complex, highly evolved, and sophisticated sensors. This bespeaks of a mechanism that likely appeared early in evolution and one that has persisted up to the present because of its simplicity and utility.
Funding
The author’s work has been supported by National Science Foundation Grants: IBN 0235223, IOS 0641436, IOS 1051627 and IOS 2012106.
Acknowledgments
The author wishes to acknowledge the numerous colleagues that contributed to this research.
Conflicts of Interest
No competing financial interest exist.
Abbreviations
AMP—adenosine monophosphate; AOA—Aminooxyacetate; BKCa—large-conductance Ca2+-dependent K+ channels; BPA—bovine pulmonary arteries; CBS—cystathionine β-synthase; CCO—cytochrome c oxidase; CIH—chronic intermittent hypoxia; CO—carbon monoxide; CoA—coenzyme A; CSE—cystathionine λ-lyase; DAO—d-amino acid oxidase; DHLA—dihydrolipoic acid; eNOS—endothelial nitric oxide synthase; ETHE1—mitochondrial sulfur dioxygenase; GSH—reduced glutathione; H2S—hydrogen sulfide; HIF—hypoxia inducible factor; HNO—nitroxyl; HO-2—hemeoxygenase-2; HS•—thiyl radical; HSNO2—thionitrite; HS(O)NO—sulfinyl nitrite; Katp—ATP-dependent potassium channels; KCa—Ca2+-dependent K+ channels; Kv—voltage-gated potassium channels; LDA—lamprey dorsal aorta; MEK-ERK—mitogen-activated protein kinase kinase-extracellular signal-related kinase; Mito—mitochondria; 3-MST—3-mercaptopyruvate sulfurtransferase; mtHsp 70—mitochondrial heat shock protein; NADPH—nicotinamide adenine dinucleotide phosphate; NEB—neuroepithelial body; NEC—neuroepithelial cell; NO2−—nitrite; NOS—nitric oxide synthase; NZDA—New Zealand hagfish dorsal aorta; PASMC—pulmonary arterial smooth muscle cells; pB—pre-Bötzinger dorsal inspiratory respiratory group; PDA—Pacific hagfish dorsal aorta; Po2—partial pressure of oxygen; PPG—propargyl glycine; ROS—reactive oxygen species; SNA—sinus nerve activity; SO—sulfite oxidase; SQR—sulfide-quinone oxidoreductase; TASK—TWIK-related acid-sensitive potassium channels; TRP—transient receptor potential; Trx—thioredoxin; Ψ—mitochondrial transmembrane potential.
References
- Bickler, P.E.; Buck, L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Farrell, A.; Brauner, C. Hypoxia; Academic Press: London, UK, 2009. [Google Scholar]
- Milsom, W.K.; Burleson, M.L. Peripheral arterial chemoreceptors and the evolution of the carotid body. Respir. Physiol. Neurobiol. 2007, 157, 4–11. [Google Scholar] [CrossRef]
- Jonz, M.G.; Nurse, C.A. Peripheral chemoreceptors in air- versus water- breathers. Adv. Exp. Med. Biol. 2012, 758, 19–27. [Google Scholar] [PubMed]
- Kemp, P.J.; Lewis, A.; Hartness, M.E.; Searle, G.J.; Miller, P.; O’Kelly, I.; Peers, C. Airway chemotransduction: From oxygen sensor to cellular effector. Am. J. Respir. Crit. Care Med. 2002, 166, S17–S24. [Google Scholar] [CrossRef]
- Nurse, C.A.; Buttigieg, J.; Thompson, R.; Zhang, M.; Cutz, E. Oxygen sensing in neuroepithelial and adrenal chromaffin cells. Novartis. Found. Symp. 2006, 272, 106–114. [Google Scholar]
- Perry, S.F.; Montpetit, C.J.; Borowska, M. The effects of acute hypoxia on chemically or neuronally induced catecholamine secretion in rainbow trout (Oncorhynchus mykiss) in situ and in vivo. J. Exp. Biol. 2000, 203, 1487–1495. [Google Scholar] [CrossRef]
- Brinks, L.; Moonen, R.M.; Moral-Sanz, J.; Barreira, B.; Kessels, L.; Perez-Vizcaino, F.; Cogolludo, A.; Villamor, E. Hypoxia-induced contraction of chicken embryo mesenteric arteries: Mechanisms and developmental changes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R858–R869. [Google Scholar] [CrossRef]
- Mohammed, R.; Salinas, C.E.; Giussani, D.A.; Blanco, C.E.; Cogolludo, A.L.; Villamor, E. Acute hypoxia-reoxygenation and vascular oxygen sensing in the chicken embryo. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef]
- Olson, K.R.; Whitfield, N.L.; Bearden, S.E.; St. Leger, J.; Nilson, E.; Gao, Y.; Madden, J.A. Hypoxic pulmonary vasodilation: A paradigm shift with a hydrogen sulfide mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R51–R60. [Google Scholar] [CrossRef]
- Russell, M.J.; Dombkowski, R.A.; Olson, K.R. Effects of hypoxia on vertebrate blood vessels. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2008, 309, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, N.; Abe, A.S.; Andrade, D.V.; Wang, T. Hypoxic pulmonary vasoconstriction in reptiles: A comparative study of four species with different lung structures and pulmonary blood pressures. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1280–R1288. [Google Scholar] [CrossRef]
- Villamor, E.; Moreno, L.; Mohammed, R.; Perez-Vizcaino, F.; Cogolludo, A. Reactive oxygen species as mediators of oxygen signaling during fetal-to-neonatal circulatory transition. Free Radic. Biol. Med. 2019, 142, 82–96. [Google Scholar] [CrossRef]
- Agren, P.; Cogolludo, A.L.; Kessels, C.G.; Perez-Vizcaino, F.; de Mey, J.G.; Blanco, C.E.; Villamor, E. Ontogeny of chicken ductus arteriosus response to oxygen and vasoconstrictors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R485–R496. [Google Scholar] [CrossRef] [PubMed]
- Crossley, D.A., 2nd; Altimiras, J. Cardiovascular development in embryos of the American alligator Alligator mississippiensis: Effects of chronic and acute hypoxia. J. Exp. Biol. 2005, 208, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bennie, R.E.; Packer, C.S.; Powell, D.R.; Jin, N.; Rhoades, R.A. Biphasic contractile response of pulmonary artery to hypoxia. Am. J. Physiol. 1991, 261, L156–L163. [Google Scholar] [CrossRef]
- Evans, A.M.; Hardie, D.G.; Peers, C.; Mahmoud, A. Hypoxic pulmonary vasoconstriction: Mechanisms of oxygen-sensing. Curr. Opin. Anaesthesiol. 2011, 24, 13–20. [Google Scholar] [CrossRef]
- Clanton, T.L.; Hogan, M.C.; Gladden, L.B. Regulation of cellular gas exchange, oxygen sensing, and metabolic control. Compr. Physiol. 2013, 3, 1135–1190. [Google Scholar] [PubMed]
- Kennel, K.B.; Burmeister, J.; Schneider, M.; Taylor, C.T. The PHD1 oxygen sensor in health and disease. J. Physiol. 2018, 596, 3899–3913. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Pharmacologic Targeting of Hypoxia-Inducible Factors. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 379–403. [Google Scholar] [CrossRef]
- Strowitzki, M.J.; Cummins, E.P.; Taylor, C.T. Protein Hydroxylation by Hypoxia-Inducible Factor (HIF) Hydroxylases: Unique or Ubiquitous? Cells 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Dombkowski, R.A.; Russell, M.J.; Doellman, M.M.; Head, S.K.; Whitfield, N.L.; Madden, J.A. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 2006, 209, 4011–4023. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. H2S and polysulfide metabolism: Conventional and unconventional pathways. Biochem. Pharmacol. 2018, 149, 77–90. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef]
- Banerjee, R. Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Curr. Opin. Chem. Biol. 2017, 37, 115–121. [Google Scholar] [CrossRef]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Banerjee, R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox. Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Ogasawara, Y.; Kimura, H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011, 439, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox. Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.K.; Araujo, T.S.; Sousa, N.A.; Sousa, F.B.; Nogueira, K.M.; Nicolau, L.A.; Medeiros, J.V. Evidence that d-cysteine protects mice from gastric damage via hydrogen sulfide produced by d-amino acid oxidase. Nitric. Oxide. 2017, 64, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bearden, S.E.; Beard, R.S., Jr.; Pfau, J.C. Extracellular Transsulfuration Generates Hydrogen Sulfide from Homocysteine and Protects Endothelium from Redox Stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1568–H1576. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen Sulfide: From Brain to Gut. Antioxid. Redox. Signal. 2010, 12, 1111–1123. [Google Scholar] [CrossRef]
- Teng, H.; Wu, B.; Zhao, K.; Yang, G.; Wu, L.; Wang, R. Oxygen-sensitive mitochondrial accumulation of cystathionine beta-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA 2013, 110, 12679–12684. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef]
- Niu, W.; Wang, J.; Qian, J.; Wang, M.; Wu, P.; Chen, F.; Yan, S. Allosteric control of human cystathionine beta-synthase activity by a redox active disulfide bond. J. Biol. Chem. 2018, 293, 2523–2533. [Google Scholar] [CrossRef]
- Banerjee, R.; Zou, C.G. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: A PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 2005, 433, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Ogasawara, Y.; Kimura, H. Hydrogen sulfide is produced by cystathionine gamma-lyase at the steady-state low intracellular Ca(2+) concentrations. Biochem. Biophys. Res. Commun. 2013, 431, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 2004, 26, 243–254. [Google Scholar] [CrossRef]
- D’Imprima, E.; Mills, D.J.; Parey, K.; Brandt, U.; Kuhlbrandt, W.; Zickermann, V.; Vonck, J. Cryo-EM structure of respiratory complex I reveals a link to mitochondrial sulfur metabolism. Biochim. Biophys. Acta 2016, 1857, 1935–1942. [Google Scholar] [CrossRef]
- Jackson, M.R.; Melideo, S.L.; Jorns, M.S. Human Sulfide:Quinone Oxidoreductase Catalyzes the First Step in Hydrogen Sulfide Metabolism and Produces a Sulfane Sulfur Metabolite. Biochemistry 2012, 51, 6804–6815. [Google Scholar] [CrossRef]
- Koj, A.; Frendo, J.; Wojtczak, L. Subcellular distribution and intramitochondrial localization of three sulfurtransferases in rat liver. FEBS Lett. 1975, 57, 42–466. [Google Scholar] [CrossRef]
- Olson, K.R.; DeLeon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: A Readily Accessible Source of Hydrogen Sulfide in Oxygen Sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R592–R603. [Google Scholar] [CrossRef]
- Villarejo, M.; Westley, J. Mechanism of rhodanase catalysis of thiosulfate-lipoate oxidation-reduction. J. Biol. Chem. 1963, 238, 4016–4020. [Google Scholar] [CrossRef]
- Villarejo, M.; Westley, J. Rhodanese-catalyzed reduction of thiosulfate by reduced lipoic acid. J. Biol. Chem. 1963, 238, 1185–1186. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Markel, T.A.; Drucker, N.; Boone, D.; Whiteman, M.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; et al. Stahelin, Extended hypoxia-mediated H2 S production provides for long-term oxygen sensing. Acta Physiol. 2020, 228, e13368. [Google Scholar] [CrossRef] [PubMed]
- Doka, E.; Pader, I.; Biro, A.; Johansson, K.; Cheng, Q.; Ballago, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E.; et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, e1500968. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Doka, E.; Fabian, I.; Nagy, P. Kinetic and thermodynamic studies on the disulfide-bond reducing potential of hydrogen sulfide. Nitric. Oxide 2015, 46, 93–101. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Jamurtas, A.Z. Blood as a reactive species generator and redox status regulator during exercise. Arch. Biochem. Biophys. 2009, 490, 77–84. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef]
- Wlodek, P.J.; Iciek, M.B.; Milkowski, A.; Smolenski, O.B. Various forms of plasma cysteine and its metabolites in patients undergoing hemodialysis. Clin. Chim. Acta 2001, 304, 9–18. [Google Scholar] [CrossRef]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc(−) cystine/glutamate antiporter: An update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef]
- Sims, B.; Clarke, M.; Francillion, L.; Kindred, E.; Hopkins, E.S.; Sontheimer, H. Hypoxic preconditioning involves system Xc− regulation in mouse neural stem cells. Stem Cell Res. 2012, 8, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017, 8, 1177. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.L. Transport of H2S and HS(−) across the human red blood cell membrane: Rapid H2S diffusion and AE1-mediated Cl(−)/HS(−) exchange. Am. J. Physiol. Cell Physiol. 2013, 305, C941–C950. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.C.; Missner, A.; Kugler, P.; Saparov, S.M.; Zeidel, M.L.; Lee, J.K.; Pohl, P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2009, 106, 16633–16638. [Google Scholar] [CrossRef]
- Olson, K.R. A theoretical examination of hydrogen sulfide metabolism and its potential in autocrine/paracrine oxygen sensing. Respir. Physiol. Neurobiol. 2013, 186, 173–179. [Google Scholar] [CrossRef]
- Kohl, J.B.; Mellis, A.T.; Schwarz, G. Homeostatic impact of sulfite and hydrogen sulfide on cysteine catabolism. Br. J. Pharmacol. 2019, 176, 554–570. [Google Scholar] [CrossRef]
- Jackson, M.R.; Loll, P.J.; Jorns, M.S. X-Ray Structure of Human Sulfide:Quinone Oxidoreductase: Insights into the Mechanism of Mitochondrial Hydrogen Sulfide Oxidation. Structure 2019, 27, 794–805.e794. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef]
- Libiad, M.; Yadav, P.K.; Vitvitsky, V.; Martinov, M.; Banerjee, R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 2014, 289, 30901–30910. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Fernandez-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Z.; Zhang, M.; Huang, C.; Song, Y.; Xu, F.; Zhang, J.; Li, J.; He, M.; Li, Y.; et al. SQR mediates therapeutic effects of H2S by targeting mitochondrial electron transport to induce mitochondrial uncoupling. Sci. Adv. 2020, 6, eaaz5752. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, E.; Mimoun, S.; Andriamihaja, M.; Chaumontet, C.; Blachier, F.; Bouillaud, F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim. Biophys. Acta 2010, 1797, 1500–1511. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Arora, K.; Patel, S.; DeLeon, E.R.; Sutton, T.R.; Feelisch, M.; Cortese-Krott, M.M.; Straub, K.D. Metabolism of hydrogen sulfide (H2S) and Production of Reactive Sulfur Species (RSS) by superoxide dismutase. Redox. Biol. 2017, 15, 74–85. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Arif, M.; Arif, F.; Arora, N.; Straub, K.D. Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox. Biol. 2017, 12, 325–339. [Google Scholar] [CrossRef]
- Olson, K.R.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Skora, N.C.; Whelan, J.; Villa, B.P.; Yuan, X.; Mannam, V.; Howard, S.; et al. Green tea polyphenolic antioxidants oxidize hydrogen sulfide to thiosulfate and polysulfides: A possible new mechanism underpinning their biological action. Redox Biol. 2020, 37, 101731. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Licursi, M.; Skora, N.C.; Whelan, J.; Villa, B.P.; Straub, K.D. ‘Antioxidant’ berries, anthocyanins, resveratrol and rosmarinic acid oxidize hydrogen sulfide to polysulfides and thiosulfate: A novel mechanism underlying their biological actions. Free Radic. Biol. Med. 2021, 165, 67–78. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Straub, K.D. Oxidation of Hydrogen Sulfide by Quinones: How Polyphenols Initiate Their Cytoprotective Effects. Int. J. Mol. Sci. 2021, 22, 961. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Miljkovic, J.L.; Bostelaar, T.; Adhikari, B.; Yadav, P.K.; Steiger, A.K.; Torregrossa, R.; Pluth, M.D.; Whiteman, M.; Banerjee, R.; et al. Cytochrome c Reduction by H2S Potentiates Sulfide Signaling. ACS Chem. Biol. 2018, 13, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Bostelaar, T.; Vitvitsky, V.; Kumutima, J.; Lewis, B.E.; Yadav, P.K.; Brunold, T.C.; Filipovic, M.; Lehnert, N.; Stemmler, T.L.; Banerjee, R. Hydrogen Sulfide Oxidation by Myoglobin. J. Am. Chem. Soc. 2016, 138, 8476–8488. [Google Scholar] [CrossRef]
- Doeller, J.E.; Isbell, T.S.; Benavides, G.; Koenitzer, J.; Patel, H.; Patel, R.P.; Lancaster, J.R., Jr.; Darley-Usmar, V.M.; Kraus, D.W. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal. Biochem. 2005, 341, 40–51. [Google Scholar] [CrossRef]
- Kraus, D.W.; Doeller, J.E. Sulfide consumption by mussel gill mitochondria is not strictly tied to oxygen reduction: Measurements using a novel polarographic sulfide sensor. J. Exp. Biol. 2004, 207, 3667–3679. [Google Scholar] [CrossRef] [PubMed]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef] [PubMed]
- Dombkowski, R.A.; Naylor, M.G.; Shoemaker, E.; Smith, M.; DeLeon, E.R.; Stoy, G.F.; Gao, Y.; Olson, K.R. Hydrogen sulfide (H2S) and hypoxia inhibit salmonid gastrointestinal motility: Evidence for H2S as an oxygen sensor. J. Exp. Biol. 2011, 214, 4030–4040. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drucker, N.A.; Winkel, J.P.T.; Shelley, W.C.; Olson, K.R.; Markel, T.A. Inhibiting hydrogen sulfide production in umbilical stem cells reduces their protective effects during experimental necrotizing enterocolitis. J. Pediatric Surg. 2019, 54, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R.; Furne, J.; Stoltz, G.J.; Abdel-Rehim, M.S.; Levitt, M.D.; Szurszewski, J.H. Sulfide quinone reductase contributes to hydrogen sulfide metabolism in murine peripheral tissues but not in the central nervous system. Br. J. Pharmacol. 2011, 165, 2178–2190. [Google Scholar] [CrossRef]
- Madden, J.A.; Ahlf, S.B.; Dantuma, M.W.; Olson, K.R.; Roerig, D.L. Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung. J. Appl. Physiol. 2012, 112, 411–418. [Google Scholar] [CrossRef]
- Olson, K.R.; Healy, M.J.; Qin, Z.; Skovgaard, N.; Vulesevic, B.; Duff, D.W.; Whitfield, N.L.; Yang, G.; Wang, R.; Perry, S.F. Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R669–R680. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Whitfield, N.L. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid. Redox Signal. 2010, 12, 1219–1234. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, N.L.; Kreimier, E.L.; Verdial, F.C.; Skovgaard, N.; Olson, K.R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1930–R1937. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.P. Oxygen sensors in context. Biochim. Biophys. Acta 2008, 1777, 1–14. [Google Scholar] [CrossRef]
- Buckler, K.J.; Turner, P.J. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J. Physiol. 2013, 591, 3549–3563. [Google Scholar] [CrossRef]
- Peng, Y.J.; Nanduri, J.; Raghuraman, G.; Souvannakitti, D.; Gadalla, M.M.; Kumar, G.K.; Snyder, S.H.; Prabhakar, N.R. H2S mediates O2 sensing in the carotid body. Proc. Natl. Acad. Sci. USA 2010, 107, 10719–10724. [Google Scholar] [CrossRef]
- Olson, K.R.; Russell, M.J.; Forster, M.E. Hypoxic vasoconstriction of cyclostome systemic vessels: The antecedent of hypoxic pulmonary vasoconstriction? Am. J. Physiol Regul. Integr. Comp. Physiol. 2001, 280, R198–R206. [Google Scholar] [CrossRef]
- Benchoam, D.; Cuevasanta, E.; Moller, M.N.; Alvarez, B. Hydrogen Sulfide and Persulfides Oxidation by Biologically Relevant Oxidizing Species. Antioxidants 2019, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Butler, A.R.; Woollins, J.D.; Feelisch, M. Inorganic sulfur-nitrogen compounds: From gunpowder chemistry to the forefront of biological signaling. Dalton. Trans. 2016, 45, 5908–5919. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef]
- Go, Y.M.; Chandler, J.D.; Jones, D.P. The cysteine proteome. Free Radic. Biol. Med. 2015, 84, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Signalling by hydrogen sulfide and polysulfides via protein S-sulfuration. Br. J. Pharmacol. 2020, 177, 720–733. [Google Scholar] [CrossRef]
- Kuschman, H.P.; Palczewski, M.B.; Thomas, D.D. Nitric oxide and hydrogen sulfide: Sibling rivalry in the family of epigenetic regulators. Free Radic. Biol. Med. 2021, 170, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zhao, S.; Xie, L.; Han, Y.; Ji, Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. Br. J. Pharmacol. 2018, 175, 1146–1156. [Google Scholar] [CrossRef]
- Miller, C.G.; Schmidt, E.E. Sulfur Metabolism Under Stress. Antioxid. Redox Signal. 2020, 33, 1158–1173. [Google Scholar] [CrossRef]
- Paul, D.B.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol 2021, 38, 101772. [Google Scholar] [CrossRef]
- Suarez, S.A.; Vargas, P.; Doctorovich, F.A. Updating NO(*)/HNO interconversion under physiological conditions: A biological implication overview. J. Inorg. Biochem. 2021, 216, 111333. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wu, Z.Y.; Cao, L.; Zhu, M.Y.; Nie, X.W.; Huang, D.J.; Sun, M.T.; Bian, J.S. Role of nitroxyl (HNO) in cardiovascular system: From biochemistry to pharmacology. Pharmacol. Res. 2020, 159, 104961. [Google Scholar] [CrossRef] [PubMed]
- Doka, E.; Ida, T.; Dagnell, M.; Abiko, Y.; Luong, N.C.; Balog, N.; Takata, T.; Espinosa, B.; Nishimura, A.; Cheng, Q.; et al. Control of protein function through oxidation and reduction of persulfidated states. Sci. Adv. 2020, 6, eaax8358. [Google Scholar] [CrossRef]
- Olson, K.R. Are Reactive Sulfur Species the New Reactive Oxygen Species? Antioxid. Redox Signal. 2020, 33, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Fernandez, B.O.; Kelm, M.; Butler, A.R.; Feelisch, M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 2015, 46, 14–24. [Google Scholar] [CrossRef]
- Ondrias, K.; Stasko, A.; Cacanyiova, S.; Sulova, Z.; Krizanova, O.; Kristek, F.; Malekova, L.; Knezl, V.; Breier, A. H2S and HS(−) donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflug. Arch. 2008, 457, 271–279. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Miljkovic, J.; Allgauer, A.; Chaurio, R.; Shubina, T.; Herrmann, M.; Ivanovic-Burmazovic, I. Biochemical insight into physiological effects of H2S: Reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem. J. 2012, 441, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Pardue, S.; Kolluru, G.K.; Shen, X.; Lewis, S.E.; Saffle, C.B.; Kelley, E.E.; Kevil, C.G. Hydrogen sulfide stimulates xanthine oxidoreductase conversion to nitrite reductase and formation of NO. Redox Biol. 2020, 34, 101447. [Google Scholar] [CrossRef]
- Cao, X.; Wu, Z.; Xiong, S.; Cao, L.; Sethi, G.; Bian, J.S. The role of hydrogen sulfide in cyclic nucleotide signaling. Biochem. Pharmacol. 2018, 149, 20–28. [Google Scholar] [CrossRef]
- Yuan, G.; Vasavda, C.; Peng, Y.J.; Makarenko, V.V.; Raghuraman, G.; Nanduri, J.; Gadalla, M.M.; Semenza, G.L.; Kumar, G.K.; Snyder, S.H.; et al. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci. Signal. 2015, 8, ra37. [Google Scholar] [CrossRef]
- Gridina, A.; Su, X.; Khan, S.A.; Peng, Y.J.; Wang, B.; Nanduri, J.; Fox, A.P.; Prabhakar, N.R. Gaseous transmitter regulation of hypoxia-evoked catecholamine secretion from murine adrenal chromaffin cells. J. Neurophysiol. 2021, 125, 1533–1542. [Google Scholar] [CrossRef]
- Peng, Y.J.; Makarenko, V.V.; Gridina, A.; Chupikova, I.; Zhang, X.; Kumar, G.K.; Fox, A.P.; Prabhakar, N.R. H2S mediates carotid body response to hypoxia but not anoxia. Respir. Physiol. Neurobiol. 2019, 259, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.J.; Yuan, G.; Nanduri, J. Reactive oxygen radicals and gaseous transmitters in carotid body activation by intermittent hypoxia. Cell Tissue Res. 2018, 372, 427–431. [Google Scholar] [CrossRef]
- Prabhakar, N.R. Sensing hypoxia: Physiology, genetics and epigenetics. J. Physiol. 2013, 591, 2245–2257. [Google Scholar] [CrossRef] [PubMed]
- Drousiotou, A.; DiMeo, I.; Mineri, R.; Georgiou, T.; Stylianidou, G.; Tiranti, V. Ethylmalonic encephalopathy: Application of improved biochemical and molecular diagnostic approaches. Clin. Genet. 2011, 79, 385–390. [Google Scholar] [CrossRef]
- Giordano, C.; Viscomi, C.; Orlandi, M.; Papoff, P.; Spalice, A.; Burlina, A.; Di, M.I.; Tiranti, V.; Leuzzi, V.; d’Amati, G.; et al. Morphologic evidence of diffuse vascular damage in human and in the experimental model of ethylmalonic encephalopathy. J. Inherit. Metab. Dis. 2011, 35, 451–458. [Google Scholar] [CrossRef]
- Tiranti, V.; Viscomi, C.; Hildebrandt, T.; Di, M.I.; Mineri, R.; Tiveron, C.; Levitt, M.D.; Prelle, A.; Fagiolari, G.; Rimoldi, M.; et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009, 15, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Rajapakshe, A.; Tollin, G.; Enemark, J.H. Kinetic and thermodynamic effects of mutations of human sulfite oxidase. Chem. Biodivers. 2012, 9, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H.; Irreverre, F.; Laster, L. Sulfite oxidase deficiency in man: Demonstration of the enzymatic defect. Science 1967, 156, 1599–1602. [Google Scholar] [CrossRef] [PubMed]
- Waypa, G.B.; Marks, J.D.; Guzy, R.; Mungai, P.T.; Schriewer, J.; Dokic, D.; Schumacker, P.T. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ. Res. 2010, 106, 526–535. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Breschi, M.C.; Lawson, K.; McKay, N.G.; Miceli, F.; Taglialatela, M.; Calderone, V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol. Res. 2013, 70, 27–34. [Google Scholar] [CrossRef]
- Koenitzer, J.R.; Isbell, T.S.; Patel, H.D.; Benavides, G.A.; Dickinson, D.A.; Patel, R.P.; Darley-Usmar, V.M.; Lancaster, J.R., Jr.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2007, 297, H1953–H1960. [Google Scholar] [CrossRef] [PubMed]
- Orlov, S.N.; Gusakova, S.V.; Smaglii, L.V.; Koltsova, S.V.; Sidorenko, S.V. Vasoconstriction triggered by hydrogen sulfide: Evidence for Na(+),K(+),2Cl(-)cotransport and L-type Ca(2+) channel-mediated pathway. Biochem. Biophys. Rep. 2017, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Weaver, O.; Paredes, D.A.; Bosc, L.V.G.; Walker, B.R.; Kanagy, N.L. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ. Res. 2011, 108, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Weaver, O.; Osmond, J.M.; Riddle, M.A.; Naik, J.S.; Bosc, L.V.G.; Walker, B.R.; Kanagy, N.L. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca(2)(+)-activated K(+) channels and smooth muscle Ca(2)(+) sparks. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1446–H1454. [Google Scholar] [CrossRef]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef]
- Lee, S.W.; Cheng, Y.; Moore, P.K.; Bian, J.S. Hydrogen sulphide regulates intracellular pH in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2007, 358, 1142–1147. [Google Scholar] [CrossRef]
- Miyamoto, R.; Koike, S.; Takano, Y.; Shibuya, N.; Kimura, Y.; Hanaoka, K.; Urano, Y.; Ogasawara, Y.; Kimura, H. Polysulfides (H2Sn) produced from the interaction of hydrogen sulfide (H2S) and nitric oxide (NO) activate TRPA1 channels. Sci. Rep. 2017, 7, 45995. [Google Scholar] [CrossRef]
- Dunn, W.R.; Alexander, S.P.; Ralevic, V.; Roberts, R.E. Effects of hydrogen sulphide in smooth muscle. Pharmacol. Ther. 2016, 158, 101–113. [Google Scholar] [CrossRef]
- Nagpure, B.V.; Bian, J.S. Interaction of Hydrogen Sulfide with Nitric Oxide in the Cardiovascular System. Oxid. Med. Cell Longev. 2016, 2016, 6904327. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Q.; Zhu, D. An Update on Hydrogen Sulfide and Nitric Oxide Interactions in the Cardiovascular System. Oxid. Med. Cell Longev. 2018, 2018, 4579140. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jin, H.; Tang, C.; Du, J.; Zhang, Z. Sulfur-containing gaseous signal molecules, ion channels and cardiovascular diseases. Br. J. Pharmacol. 2018, 175, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Shen, X.; Kevil, C.G. Beyond a Gasotransmitter: Hydrogen Sulfide and Polysulfide in Cardiovascular Health and Immune Response. Antioxid. Redox Signal. 2017, 27, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Dromparis, P.; Michelakis, E.D. Mitochondria in vascular health and disease. Annu. Rev. Physiol. 2013, 75, 95–126. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I.; Dominy, J.E., Jr.; Simmons, C.R.; Hirschberger, L.L. Cysteine dioxygenase: A robust system for regulation of cellular cysteine levels. Amino. Acids 2009, 37, 55–63. [Google Scholar] [CrossRef]
- Ueki, I.; Roman, H.B.; Valli, A.; Fieselmann, K.; Lam, J.; Peters, R.; Hirschberger, L.L.; Stipanuk, M.H. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am. J. Physiol Endocrinol. Metab. 2011, 301, E668–E684. [Google Scholar] [CrossRef]
- Roman, H.B.; Hirschberger, L.L.; Krijt, J.; Valli, A.; Kozich, V.; Stipanuk, M.H. The Cysteine Dioxgenase Knockout Mouse: Altered Cysteine Metabolism in Nonhepatic Tissues Leads to Excess H2S/HS Production and Evidence of Pancreatic and Lung Toxicity. Antioxid. Redox. Signal. 2013, 19, 1321–1336. [Google Scholar] [CrossRef]
- Olson, K.R.; DeLeon, E.R.; Liu, F. Controversies and conundrums in hydrogen sulfide biology. Nitric. Oxide 2014, 41, 11–26. [Google Scholar] [CrossRef]
- Baragatti, B.; Ciofini, E.; Sodini, D.; Luin, S.; Scebba, F.; Coceani, F. Hydrogen sulfide in the mouse ductus arteriosus: A naturally occurring relaxant with potential EDHF function. Am. J. Physiol Heart Circ. Physiol. 2013, 304, H927–H934. [Google Scholar] [CrossRef]
- Derwall, M.; Francis, R.C.; Kida, K.; Bougaki, M.; Crimi, E.; Adrie, C.; Zapol, W.M.; Ichinose, F. Administration of hydrogen sulfide via extracorporeal membrane lung ventilation in sheep with partial cardiopulmonary bypass perfusion: A proof of concept study on metabolic and vasomotor effects. Crit. Care 2011, 15, R51. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, N.; Olson, K.R. Hydrogen sulfide mediates hypoxic vasoconstriction through a production of mitochondrial ROS in trout gills. Am. J. Physiol Regul. Integr. Comp. Physiol. 2012, 303, R487–R494. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.; Swathi, Y.; Yeo, A.L.; Shoon, M.L.; Moore, P.K.; Bhatia, M. Hydrogen sulfide: Regulatory role on blood pressure in hyperhomocysteinemia. Vascul. Pharmacol. 2010, 53, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Lloret, J.; Aaronson, P.I. Potentiation of Hypoxic Pulmonary Vasoconstriction by Hydrogen Sulfide Precursors 3-Mercaptopyruvate and D-Cysteine Is Blocked by the Cystathionine gamma Lyase Inhibitor Propargylglycine. Adv. Exp. Med. Biol. 2015, 860, 81–87. [Google Scholar] [PubMed]
- Prieto-Lloret, J.; Shaifta, Y.; Ward, J.P.; Aaronson, P.I. Hypoxic pulmonary vasoconstriction in isolated rat pulmonary arteries is not inhibited by antagonists of H2S-synthesizing pathways. J. Physiol. 2015, 593, 385–401. [Google Scholar] [CrossRef]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Panopoulos, P.; Chasapis, C.T.; Coletta, C.; Zhou, Z.; Cirino, G.; Giannis, A.; Szabo, C.; Spyroulias, G.A.; Papapetropoulos, A. Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br. J. Pharmacol. 2013, 169, 922–932. [Google Scholar] [CrossRef]
- Barrera, A.; Morales-Loredo, H.; Garcia, J.M.; Fregoso, G.; Pace, C.E.; Mendiola, P.J.; Naik, J.S.; Bosc, L.V.G.; Kanagy, N.L. Simulated sleep apnea alters hydrogen sulfide regulation of blood flow and pressure. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H511–H519. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Yu, W.; Chen, L.; Wang, Z.; Zhang, T. Expression of pulmonary arterial elastin in rats with hypoxic pulmonary hypertension using H2S. J. Recept. Signal. Transduct. Res. 2020, 40, 383–387. [Google Scholar] [CrossRef]
- Dongo, E.; Beliczai-Marosi, G.; Dybvig, A.S.; Kiss, L. The mechanism of action and role of hydrogen sulfide in the control of vascular tone. Nitric Oxide. 2018, 81, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Chen, S.; Tang, C.; Jin, H.; Du, J.; Huang, Y. Hydrogen sulfide and vascular regulation—An update. J. Adv. Res. 2021, 27, 85–97. [Google Scholar] [CrossRef]
- Arndt, S.; Baeza-Garza, C.D.; Logan, A.; Rosa, T.; Wedmann, R.; Prime, T.A.; Martin, J.L.; Saeb-Parsy, K.; Krieg, T.; Filipovic, M.R.; et al. Assessment of H2S in vivo using the newly developed mitochondria-targeted mass spectrometry probe Mito A. J. Biol. Chem. 2017, 292, 7761–7773. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr. Opin. Nephrol. Hypertens 2011, 20, 107–112. [Google Scholar] [CrossRef]
- Peleli, M.; Bibli, S.I.; Li, Z.; Chatzianastasiou, A.; Varela, A.; Katsouda, A.; Zukunft, S.; Bucci, M.; Vellecco, V.; Davos, C.H.; et al. Cardiovascular phenotype of mice lacking 3-mercaptopyruvate sulfurtransferase. Biochem. Pharmacol. 2020, 176, 113833. [Google Scholar] [CrossRef]
- LaPenna, K.B.; Polhemus, D.J.; Doiron, J.E.; Hidalgo, H.A.; Li, Z.; Lefer, D.J. Hydrogen Sulfide as a Potential Therapy for Heart Failure-Past, Present, and Future. Antioxidants 2021, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Polhemus, D.J.; Lefer, D.J. Evolution of Hydrogen Sulfide Therapeutics to Treat Cardiovascular Disease. Circ. Res. 2018, 123, 590–600. [Google Scholar] [CrossRef]
- Ufnal, M.; Nowinski, A. Central Administration of H2S Donors for Studying Cardiovascular Effects of H2S in Rats. Methods Mol. Biol. 2019, 2007, 167–172. [Google Scholar]
- Duan, X.C.; Liu, S.Y.; Guo, R.; Xiao, L.; Xue, H.M.; Guo, Q.; Jin, S.; Wu, Y.M. Cystathionine-beta-Synthase Gene Transfer into Rostral Ventrolateral Medulla Exacerbates Hypertension via Nitric Oxide in Spontaneously Hypertensive Rats. Am. J. Hypertens. 2015, 28, 1106–1113. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Liu, L.; Li, X.; Li, X.Y.; Sun, X.; Li, K.X.; Zha, R.R.; Wang, H.D.; Zhang, M.D.; et al. The baroreflex afferent pathway plays a critical role in H2S-mediated autonomic control of blood pressure regulation under physiological and hypertensive conditions. Acta Pharmacol. Sin. 2021, 42, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Sikora, M. The role of brain gaseous transmitters in the regulation of the circulatory system. Curr. Pharm. Biotechnol. 2011, 12, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Sabino, J.P.; Traslavina, G.A.; Branco, L.G. Role of central hydrogen sulfide on ventilatory and cardiovascular responses to hypoxia in spontaneous hypertensive rats. Respir. Physiol. Neurobiol. 2016, 231, 21–27. [Google Scholar] [CrossRef]
- Sabino, J.P.; Soriano, R.N.; Donatti, A.F.; Fernandez, R.R.; Kwiatkoski, M.; Francescato, H.D.; Coimbra, T.M.; Branco, L.G. Involvement of endogenous central hydrogen sulfide (H2S) in hypoxia-induced hypothermia in spontaneously hypertensive rats. Can. J. Physiol. Pharmacol. 2017, 95, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tang, S.; Hu, K.; Zhang, Z.; Liu, P.; Luo, Y.; Kang, J.; Xu, L. DL-Propargylglycine protects against myocardial injury induced by chronic intermittent hypoxia through inhibition of endoplasmic reticulum stress. Sleep Breath 2018, 22, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Zicola, E.; Arrigo, E.; Mancardi, D. H2S Pretreatment Is Promigratory and Decreases Ischemia/Reperfusion Injury in Human Microvascular Endothelial Cells. Oxid. Med. Cell Longev. 2021, 2021, 8886666. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Kishore, R. Potential role of hydrogen sulfide in diabetes-impaired angiogenesis and ischemic tissue repair. Redox. Biol. 2020, 37, 101704. [Google Scholar] [CrossRef]
- Citi, V.; Piragine, E.; Testai, L.; Breschi, M.C.; Calderone, V.; Martelli, A. The Role of Hydrogen Sulfide and H2S-donors in Myocardial Protection Against Ischemia/Reperfusion Injury. Curr. Med. Chem. 2018, 25, 4380–4401. [Google Scholar] [CrossRef]
- Ertugrul, I.A.; van Suylen, V.; Damman, K.; de Koning, M.L.Y.; van Goor, H.; Erasmus, M.E. Donor Heart Preservation with Hydrogen Sulfide: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 5737. [Google Scholar] [CrossRef]
- Jensen, A.R.; Drucker, N.A.; Khaneki, S.; Ferkowicz, M.J.; Yoder, M.C.; DeLeon, E.R.; Olson, K.R.; Markel, T.A. Hydrogen Sulfide: A Potential Novel Therapy for the Treatment of Ischemia. Shock 2017, 48, 511–524. [Google Scholar] [CrossRef]
- Jia, J.; Li, J.; Cheng, J. H2S-based therapies for ischaemic stroke: Opportunities and challenges. Stroke Vasc. Neurol. 2019, 4, 63–66. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Bice, J.S.; Baxter, G.F. Pre- and postconditioning the heart with hydrogen sulfide (H2S) against ischemia/reperfusion injury in vivo: A systematic review and meta-analysis. Basic Res. Cardiol. 2018, 113, 6. [Google Scholar] [CrossRef]
- Lv, S.; Wang, Z.; Wang, J.; Wang, H. Exogenous Hydrogen Sulfide Plays an Important Role Through Regulating Autophagy in Ischemia/Reperfusion Injury. Front. Mol. Biosci. 2021, 8, 681676. [Google Scholar] [CrossRef]
- Narne, P.; Pandey, V.; Phanithi, P.B. Role of Nitric Oxide and Hydrogen Sulfide in Ischemic Stroke and the Emergent Epigenetic Underpinnings. Mol. Neurobiol. 2019, 56, 1749–1769. [Google Scholar] [CrossRef]
- Roorda, M.; Miljkovic, J.L.; van Goor, H.; Henning, R.H.; Bouma, H.R. Spatiotemporal regulation of hydrogen sulfide signaling in the kidney. Redox. Biol. 2021, 43, 101961. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Wang, X.Y.; Bian, J.S. Implications of hydrogen sulfide in liver pathophysiology: Mechanistic insights and therapeutic potential. J. Adv. Res. 2021, 27, 127–135. [Google Scholar] [CrossRef]
- Wang, W.L.; Ge, T.Y.; Chen, X.; Mao, Y.; Zhu, Y.Z. Advances in the Protective Mechanism of NO, H2S, and H2 in Myocardial Ischemic Injury. Front. Cardiovasc. Med. 2020, 7, 588206. [Google Scholar] [CrossRef]
- Zhang, M.L.; Peng, W.; Ni, J.Q.; Chen, G. Recent advances in the protective role of hydrogen sulfide in myocardial ischemia/reperfusion injury: A narrative review. Med. Gas. Res. 2021, 11, 83–87. [Google Scholar]
- Klentz, R.D.; Fedde, M.R. Hydrogen sulfide: Effects on avian respiratory control and intrapulmonary CO2 receptors. Respir. Physiol. 1978, 32, 355–367. [Google Scholar] [CrossRef]
- Beauchamp, R.O., Jr.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxico. 1984, 13, 25–97. [Google Scholar] [CrossRef] [PubMed]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Haggard, H.W.; Henderson, Y. The influence of hydrogensulphide upon respiration. Am. J. Physiol. 1922, 61, 289–297. [Google Scholar] [CrossRef]
- Haouzi, P.; Bell, H.J.; Notet, V.; Bihain, B. Comparison of the metabolic and ventilatory response to hypoxia and H2S in unsedated mice and rats. Respir. Physiol. Neurobiol. 2009, 167, 316–322. [Google Scholar] [CrossRef]
- Haouzi, P. Ventilatory and metabolic effects of exogenous hydrogen sulfide. Respir. Physiol. Neurobiol. 2012, 184, 170–177. [Google Scholar] [CrossRef]
- Haouzi, P.; Bell, H.; Philmon, M. Hydrogen sulfide oxidation and the arterial chemoreflex: Effect of methemoglobin. Respir. Physiol. Neurobiol. 2011, 177, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Van de Louw, A.; Haouzi, P. Inhibitory effects of hyperoxia and methemoglobinemia on H2S induced ventilatory stimulation in the rat. Respir. Physiol. Neurobiol. 2012, 181, 326–334. [Google Scholar] [CrossRef]
- Liu, W.Q.; Chai, C.; Li, X.Y.; Yuan, W.J.; Wang, W.Z.; Lu, Y. The cardiovascular effects of central hydrogen sulphide are related to K(ATP) channels activation. Physiol. Res. 2011, 60, 729–738. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Ding, Y.; Li, H.; Nie, L.; Zhou, H.; Tang, Y.; Zheng, Y. Site-specific hydrogen sulfide-mediated central regulation of respiratory rhythm in medullary slices of neonatal rats. Neuroscience 2013, 233, 118–126. [Google Scholar] [CrossRef]
- Hu, H.; Shi, Y.; Chen, Q.; Yang, W.; Zhou, H.; Chen, L.; Tang, Y.; Zheng, Y. Endogenous hydrogen sulfide is involved in regulation of respiration in medullary slice of neonatal rats. Neuroscience 2008, 156, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.G.; Hu, H.Y.; Zhang, J.; Zhou, H.; Chen, L.; Tang, Y.H.; Zheng, Y. Protective effect of hydrogen sulfide on hypoxic respiratory suppression in medullary slice of neonatal rats. Respir. Physiol. Neurobiol. 2010, 171, 181–186. [Google Scholar] [CrossRef]
- Pan, J.G.; Zhang, J.; Zhou, H.; Chen, L.; Tang, Y.H.; Zheng, Y. Protective action of endogenously generated H2S on hypoxia-induced respiratory suppression and its relation to antioxidation and down-regulation of c-fos mRNA in medullary slices of neonatal rats. Respir. Physiol. Neurobiol. 2011, 178, 230–234. [Google Scholar] [CrossRef]
- Li, M.; Nie, L.; Hu, Y.; Yan, X.; Xue, L.; Chen, L.; Zhou, H.; Zheng, Y. Chronic intermittent hypoxia promotes expression of 3-mercaptopyruvate sulfurtransferase in adult rat medulla oblongata. Auton. Neurosci. 2013, 179, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Donatti, A.F.; Soriano, R.N.; Sabino, J.P.; Branco, L.G. Involvement of endogenous hydrogen sulfide (H2S) in the rostral ventrolateral medulla (RVLM) in hypoxia-induced hypothermia. Brain Res. Bull. 2014, 108, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Donatti, A.F.; Soriano, R.N.; Sabino, J.P.; Branco, L.G. Endogenous hydrogen sulfide in the rostral ventrolateral medulla/Botzinger complex downregulates ventilatory responses to hypoxia. Respir. Physiol. Neurobiol. 2014, 200, 97–104. [Google Scholar] [CrossRef]
- Buckler, K.J. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012, 463, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, B.; Wang, X.; Jin, Z.; Zhou, Y.; Dong, L.; Jiang, L.H.; Rong, W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid. Redox. Signal. 2010, 12, 1179–1189. [Google Scholar] [CrossRef]
- Makarenko, V.V.; Nanduri, J.; Raghuraman, G.; Fox, A.P.; Gadalla, M.M.; Kumar, G.K.; Snyder, S.H.; Prabhakar, N.R. Endogenous H2S is required for hypoxic sensing by carotid body glomus cells. Am. J. Physiol. Cell Physiol. 2012, 303, C916–C923. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.D.; Del, R.R.; Ding, Y.; Marcus, N.J. Role of neurotransmitter gases in the control of the carotid body in heart failure. Respir. Physiol. Neurobiol. 2012, 184, 197–203. [Google Scholar] [CrossRef]
- Fitzgerald, R.S.; Shirahata, M.; Chang, I.; Kostuk, E.; Kiihl, S. The impact of hydrogen sulfide (H2S) on neurotransmitter release from the cat carotid body, Respir. Physiol. Neurobiol. 2011, 176, 80–89. [Google Scholar] [CrossRef]
- Peng, Y.J.; Zhang, X.; Gridina, A.; Chupikova, I.; McCormick, D.L.; Thomas, R.J.; Scammell, T.E.; Kim, G.; Vasavda, C.; Nanduri, J.; et al. Complementary roles of gasotransmitters CO and H2S in sleep apnea. Proc. Natl. Acad. Sci. USA 2017, 114, 1413–1418. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Recent advances in understanding the physiology of hypoxic sensing by the carotid body. F1000Research 2018, 7, 1900. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J. Oxygen Sensing by the Carotid Body: Past and Present. Adv. Exp. Med. Biol. 2017, 977, 3–8. [Google Scholar] [PubMed]
- Wu, B.; Teng, H.; Zhang, L.; Li, H.; Li, J.; Wang, L.; Li, H. Interaction of Hydrogen Sulfide with Oxygen Sensing under Hypoxia. Oxid. Med. Cell Longev. 2015, 2015, 758678. [Google Scholar] [CrossRef] [PubMed]
- Telezhkin, V.; Brazier, S.P.; Cayzac, S.; Muller, C.T.; Riccardi, D.; Kemp, P.J. Hydrogen sulfide inhibits human BK(Ca) channels. Adv. Exp. Med. Biol. 2009, 648, 65–72. [Google Scholar] [PubMed]
- Telezhkin, V.; Brazier, S.P.; Cayzac, S.H.; Wilkinson, W.J.; Riccardi, D.; Kemp, P.J. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir. Physiol. Neurobiol. 2010, 172, 169–178. [Google Scholar] [CrossRef]
- Kim, D.; Kim, I.; Wang, J.; White, C.; Carroll, J.L. Hydrogen sulfide and hypoxia-induced changes in TASK (K2P3/9) activity and intracellular Ca(2+) concentration in rat carotid body glomus cells. Respir. Physiol. Neurobiol. 2015, 215, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hogan, J.O.; Wang, R.; White, C.; Kim, D. Role of cystathionine-gamma-lyase in hypoxia-induced changes in TASK activity, intracellular [Ca(2+)] and ventilation in mice. Respir. Physiol. Neurobiol. 2017, 246, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Porteus, C.S.; Abdallah, S.J.; Pollack, J.; Kumai, Y.; Kwong, R.W.; Yew, H.M.; Milsom, W.K.; Perry, S.F. The role of hydrogen sulphide in the control of breathing in hypoxic zebrafish (Danio rerio). J. Physiol. 2014, 592, 3075–3088. [Google Scholar] [CrossRef]
- Zhu, D.; Yu, X.; Sun, J.; Li, J.; Ma, X.; Yao, W. H2S induces catecholamine secretion in rat adrenal chromaffin cells. Toxicology 2012, 302, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F.; McNeill, B.; Elia, E.; Nagpal, A.; Vulesevic, B. Hydrogen sulfide stimulates catecholamine secretion in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol Regul. Integr. Comp. Physiol. 2009, 296, R133–R140. [Google Scholar] [CrossRef][Green Version]
- Kemp, P.J.; Searle, G.J.; Hartness, M.E.; Lewis, A.; Miller, P.; Williams, S.; Wootton, P.; Adriaensen, D.; Peers, C. Acute oxygen sensing in cellular models: Relevance to the physiology of pulmonary neuroepithelial and carotid bodies. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 270, 41–50. [Google Scholar] [CrossRef]
- Chung, C.L.; Lin, Y.S.; Chan, N.J.; Chen, Y.Y.; Hsu, C.C. Hypersensitivity of Airway Reflexes Induced by Hydrogen Sulfide: Role of TRPA1 Receptors. Int. J. Mol. Sci. 2020, 21, 3929. [Google Scholar] [CrossRef]
- Huang, J.; Luo, Y.L.; Hao, Y.; Zhang, Y.L.; Chen, P.X.; Xu, J.W.; Chen, M.H.; Luo, Y.F.; Zhong, N.S.; Xu, J.; et al. Cellular mechanism underlying hydrogen sulfide induced mouse tracheal smooth muscle relaxation: Role of BKCa. Eur. J. Pharmacol. 2014, 741, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Doe, I.; Kurokawa, Y.; Kawabata, A. Hydrogen sulfide causes relaxation in mouse bronchial smooth muscle. J. Pharmacol. Sci. 2007, 104, 392–396. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, P.P.; Wang, X.M.; He, Y.J.; Yao, W.Z.; Qi, Y.F.; Tang, C.S. Involvement of endogenous hydrogen sulfide in cigarette smoke-induced changes in airway responsiveness and inflammation of rat lung. Cytokine 2011, 53, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Castro-Piedras, I.; Perez-Zoghbi, J.F. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J. Physiol. 2013, 591, 5999–6015. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, M.; Patacchini, R.; Nicoletti, P.; Gatti, R.; Gazzieri, D.; Lissi, N.; Zagli, G.; Creminon, C.; Geppetti, P.; Harrison, S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br. J. Pharmacol. 2005, 145, 1123–1131. [Google Scholar] [CrossRef]
- Cao, X.; Bian, J.S. The Role of Hydrogen Sulfide in Renal System. Front. Pharmacol. 2016, 7, 385. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J. The smell of renal protection against chronic kidney disease: Hydrogen sulfide offers a potential stinky remedy. Pharmacol. Rep. 2018, 70, 196–205. [Google Scholar] [CrossRef]
- Beltowski, J. Hypoxia in the renal medulla: Implications for hydrogen sulfide signaling. J. Pharmacol. Exp. Ther. 2010, 334, 358–363. [Google Scholar] [CrossRef]
- Bianca, R.D.D.; Fusco, F.; Mirone, V.; Cirino, G.; Sorrentino, R. The Role of the Hydrogen Sulfide Pathway in Male and Female Urogenital System in Health and Disease. Antioxid. Redox Signal. 2017, 27, 654–668. [Google Scholar] [CrossRef]
- Shen, F.; Zhao, C.S.; Shen, M.F.; Wang, Z.; Chen, G. The role of hydrogen sulfide in gastric mucosal damage. Med. Gas. Res. 2019, 9, 88–92. [Google Scholar] [CrossRef]
- Verbeure, W.; van Goor, H.; Mori, H.; van Beek, A.P.; Tack, J.; van Dijk, P.R. The Role of Gasotransmitters in Gut Peptide Actions. Front Pharmacol. 2021, 12, 720703. [Google Scholar] [CrossRef]
- Dombkowski, R.A.; Doellman, M.M.; Head, S.K.; Olson, K.R. Hydrogen sulfide mediates hypoxia-induced relaxation of trout urinary bladder smooth muscle. J. Exp. Biol. 2006, 209, 3234–3240. [Google Scholar] [CrossRef]
- Battino, M.; Bompadre, S.; Politi, A.; Fioroni, M.; Rubini, C.; Bullon, P. Antioxidant status (CoQ10 and Vit. E levels) and immunohistochemical analysis of soft tissues in periodontal diseases. Biofactors 2005, 25, 213–217. [Google Scholar] [CrossRef]
- Fusco, F.; d’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Cirino, G.; Sorrentino, R.; Mirone, V. Sildenafil Effect on the Human Bladder Involves the L-cysteine/Hydrogen Sulfide Pathway: A Novel Mechanism of Action of Phosphodiesterase Type 5 Inhibitors. Eur. Urol. 2012, 62, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Wang, J.; Li, D.; Li, Z.; Liu, H.; Ding, M.; Cai, Z.; Liang, X.; Yang, Q.; Long, Z.; et al. Hydrogen sulfide inhibits cigarette smoke-induced inflammation and injury in alveolar epithelial cells by suppressing PHD2/HIF-1alpha/MAPK signaling pathway. Int. Immunopharmacol. 2020, 81, 105979. [Google Scholar] [CrossRef] [PubMed]
- Kai, S.; Tanaka, T.; Daijo, H.; Harada, H.; Kishimoto, S.; Suzuki, K.; Takabuchi, S.; Takenaga, K.; Fukuda, K.; Hirota, K. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindau- and mitochondria-dependent manner. Antioxid. Redox. Signal. 2012, 16, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.F.; Wang, J.; Guan, J.; Zhou, L.; Sheng, Y.; Zhao, J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br. J. Pharmacol. 2013, 169, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Teng, H.; Yang, G.; Wu, L.; Wang, R. Hydrogen sulfide inhibits the translational expression of hypoxia-inducible factor-1alpha. Br. J. Pharmacol. 2012, 167, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Budde, M.W.; Roth, M.B. Hydrogen sulfide increases hypoxia-inducible factor-1 activity independently of von Hippel-Lindau tumor suppressor-1 in C. elegans. Mol. Biol. Cell 2010, 21, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, K.L.; Agbor, T.A.; Motta, J.P.; Ferraz, J.G.; Wang, R.; Buret, A.G.; Wallace, J.L. Proresolution effects of hydrogen sulfide during colitis are mediated through hypoxia-inducible factor-1alpha. FASEB J. 2015, 29, 1591–1602. [Google Scholar] [CrossRef]
- Ling, K.; Xu, A.; Chen, Y.; Chen, X.; Li, Y.; Wang, W. Protective effect of a hydrogen sulfide donor on balloon injury-induced restenosis via the Nrf2/HIF-1alpha signaling pathway. Int. J. Mol. Med. 2019, 43, 1299–1310. [Google Scholar]
- Liu, X.; Pan, L.; Zhuo, Y.; Gong, Q.; Rose, P.; Zhu, Y. Hypoxia-inducible factor-1alpha is involved in the pro-angiogenic effect of hydrogen sulfide under hypoxic stress. Biol. Pharm. Bull. 2010, 33, 1550–1554. [Google Scholar] [CrossRef]
- Lohninger, L.; Tomasova, L.; Praschberger, M.; Hintersteininger, M.; Erker, T.; Gmeiner, B.M.; Laggner, H. Hydrogen sulphide induces HIF-1alpha and Nrf2 in THP-1 macrophages. Biochimie 2015, 112, 187–195. [Google Scholar] [CrossRef]
- Ma, D.K.; Vozdek, R.; Bhatla, N.; Horvitz, H.R. CYSL-1 interacts with the O2-sensing hydroxylase EGL-9 to promote H2S-modulated hypoxia-induced behavioral plasticity in C. elegans. Neuron 2012, 73, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yan, J.; Cao, X.; Hua, P.; Li, Z. Hydrogen sulfide modulates epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via HIF-1alpha activation. Biochem. Pharmacol. 2020, 172, 113775. [Google Scholar] [CrossRef] [PubMed]
- Uba, T.; Matsuo, Y.; Sumi, C.; Shoji, T.; Nishi, K.; Kusunoki, M.; Harada, H.; Kimura, H.; Bono, H.; Hirota, K. Polysulfide inhibits hypoxia-elicited hypoxia-inducible factor activation in a mitochondria-dependent manner. Mitochondrion 2021, 59, 255–266. [Google Scholar] [CrossRef]
- Biermann, J.; Lagreze, W.A.; Schallner, N.; Schwer, C.I.; Goebel, U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol. Vis. 2011, 17, 1275–1286. [Google Scholar]
- Hu, Y.; Li, R.; Yang, H.; Luo, H.; Chen, Z. Sirtuin 6 Is Essential for Sodium Sulfide-mediated Cytoprotective Effect in Ischemia/Reperfusion-Stimulated Brain Endothelial Cells. J. Stroke Cerebrovasc. Dis. 2015, 24, 601–609. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Zheng, Q.; Wan, X.; Ouyang, S.; Yin, Y.; Sui, X.; Liu, J.; Yang, X. Hydrogen sulfide prevents hypoxia-induced apoptosis via inhibition of an H2O2-activated calcium signaling pathway in mouse hippocampal neurons. Biochem. Biophys. Res. Commun. 2012, 425, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xie, X.; Chen, D.; Zhang, J.; Zhou, Y.; Yang, G. Protective and biogenesis effects of sodium hydrosulfide on brain mitochondria after cardiac arrest and resuscitation. Eur. J. Pharmacol. 2014, 741, 74–82. [Google Scholar] [CrossRef]
- Qin, H.; Gu, L.Z.; Gao, L.; Guo, J. Protective effect of H2S pretreatment on cerebral ischemia-reperfusion injury and its mechanisms in rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2013, 35, 249–253. [Google Scholar] [PubMed]
- Shen, Y.; Shen, Z.; Guo, L.; Zhang, Q.; Wang, Z.; Miao, L.; Wang, M.; Wu, J.; Guo, W.; Zhu, Y. MiR-125b-5p is involved in oxygen and glucose deprivation injury in PC-12 cells via CBS/H2S pathway. Nitric. Oxide. 2018, 78, 11–21. [Google Scholar] [CrossRef]
- Wen, X.; Qi, D.; Sun, Y.; Huang, X.; Zhang, F.; Wu, J.; Fu, Y.; Ma, K.; Du, Y.; Dong, H.; et al. H(2)S attenuates cognitive deficits through Akt1/JNK3 signaling pathway in ischemic stroke. Behav. Brain Res. 2014, 269, 6–14. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, Y.Q.; Wang, W.S.; Wang, X.Q. Excessive nNOS/NO/AMPK signaling activation mediated by the blockage of the CBS/H2S system contributes to oxygenglucose deprivationinduced endoplasmic reticulum stress in PC12 cells. Int. J. Mol. Med. 2017, 40, 549–557. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Wu, X.; Xu, Y.; He, M.; Yang, J.; Li, J.; Li, Y.; Ao, G.; Cheng, J.; Jia, J. The cystathionine beta-synthase/hydrogen sulfide pathway contributes to microglia-mediated neuroinflammation following cerebral ischemia. Brain Behav. Immun. 2017, 66, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, K.; Wang, X.; Ding, Y.; Ren, Z.; Fang, J.; Sun, T.; Guo, Y.; Chen, Z.; Wen, J. CSE-Derived H2S Inhibits Reactive Astrocytes Proliferation and Promotes Neural Functional Recovery after Cerebral Ischemia/Reperfusion Injury in Mice Via Inhibition of RhoA/ROCK2 Pathway. ACS Chem. Neurosci. 2021, 12, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Chen, C.P.; Halliwell, B.; Moore, P.K.; Wong, P.T. Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke 2006, 37, 889–893. [Google Scholar] [CrossRef]
- Marutani, E.; Morita, M.; Hirai, S.; Kai, S.; Grange, R.M.H.; Miyazaki, Y.; Nagashima, F.; Traeger, L.; Magliocca, A.; Ida, T.; et al. Sulfide catabolism ameliorates hypoxic brain injury. Nat. Commun. 2021, 12, 3108. [Google Scholar] [CrossRef]
- Abou-Hamdan, A.; Guedouari-Bounihi, H.; Lenoir, V.; Andriamihaja, M.; Blachier, F.; Bouillaud, F. Oxidation of H2S in mammalian cells and mitochondria. Methods Enzym. 2015, 554, 201–228. [Google Scholar]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Kandel, R.S.; Mishra, R.; Gautam, J.; Alaref, A.; Hassan, A.; Jahan, N. Patchy Vasoconstriction Versus Inflammation: A Debate in the Pathogenesis of High Altitude Pulmonary Edema. Cureus 2020, 12, e10371. [Google Scholar]
- Swenson, E.R. Early hours in the development of high-altitude pulmonary edema: Time course and mechanisms. J. Appl. Physiol. 2020, 128, 1539–1546. [Google Scholar] [CrossRef]
- Agne, A.M.; Baldin, J.P.; Benjamin, A.R.; Orogo-Wenn, M.C.; Wichmann, L.; Olson, K.R.; Walters, D.V.; Althaus, M. Hydrogen sulfide decreases beta-adrenergic agonist stimulated lung liquid clearance by inhibiting ENaC-mediated transepithelial sodium absorption. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R636–R649. [Google Scholar] [CrossRef][Green Version]
- Althaus, M.; Urness, K.D.; Clauss, W.G.; Baines, D.L.; Fronius, M. The gasotransmitter hydrogen sulphide decreases Na(+) transport across pulmonary epithelial cells. Br. J. Pharmacol. 2012, 166, 1946–1963. [Google Scholar] [CrossRef]
- Krause, N.C.; Kutsche, H.S.; Santangelo, F.; DeLeon, E.R.; Dittrich, N.P.; Olson, K.R.; Althaus, M. Hydrogen sulfide contributes to hypoxic inhibition of airway transepithelial sodium absorption. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R607–R617. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. The re-emerging pathophysiological role of the cystathionine-beta-synthase—Hydrogen sulfide system in Down syndrome. FEBS J. 2020, 287, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Pecze, L.; Randi, E.B.; Szabo, C. Meta-analysis of metabolites involved in bioenergetic pathways reveals a pseudohypoxic state in Down syndrome. Mol. Med. 2020, 26, 102–128. [Google Scholar] [CrossRef]
- Durmowicz, A.G. Pulmonary edema in 6 children with Down syndrome during travel to moderate altitudes. Pediatrics 2001, 108, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.P.; Chenivesse, C.; Larmignat, P.; Meille, L. High altitude pulmonary edema, down syndrome, and obstructive sleep apneas. High Alt. Med. Biol. 2008, 9, 179–181. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic pulmonary vasoconstriction. Physiol. Rev. 2012, 92, 367–520. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Lloret, J.; Snetkov, V.A.; Shaifta, Y.; Docio, I.; Connolly, M.J.; MacKay, C.E.; Knock, G.A.; Ward, J.P.T.; Aaronson, P.I. Role of reactive oxygen species and sulfide-quinone oxoreductase in hydrogen sulfide-induced contraction of rat pulmonary arteries. Am. J. Physiol. Lung. Cell Mol. Physiol. 2018, 314, L670–L685. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Tielens, A.G.M.; Mentel, M. Mitochondria and Anaerobic Energy Metabolism in Eukaryotes; Walter de Gruyter: Berlin, Germany, 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).