A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Rutin Glycoside
2.3. Analysis of Rutin and Rutin Glycoside
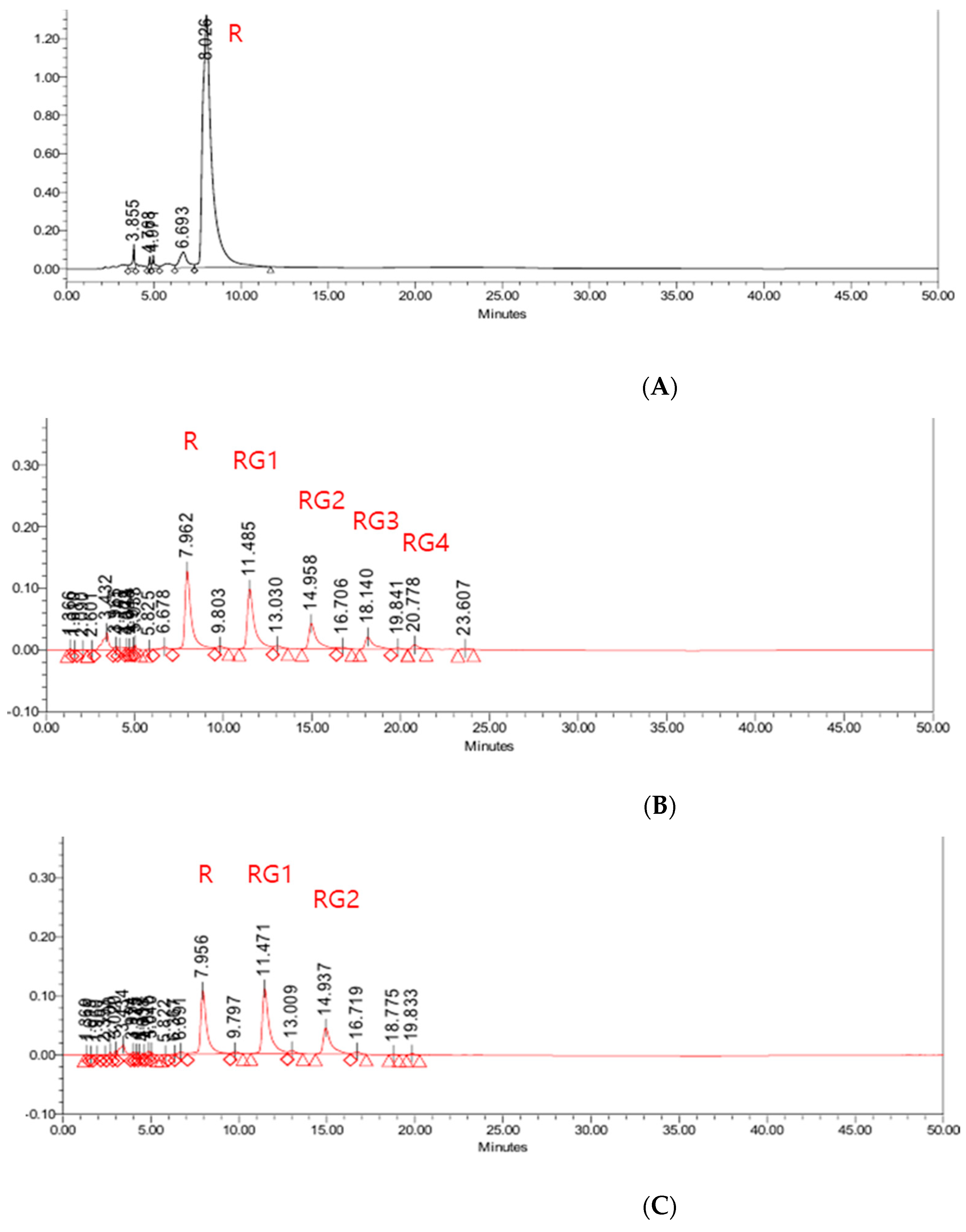
2.3.1. Determination of Rutin and Rutin Glycoside
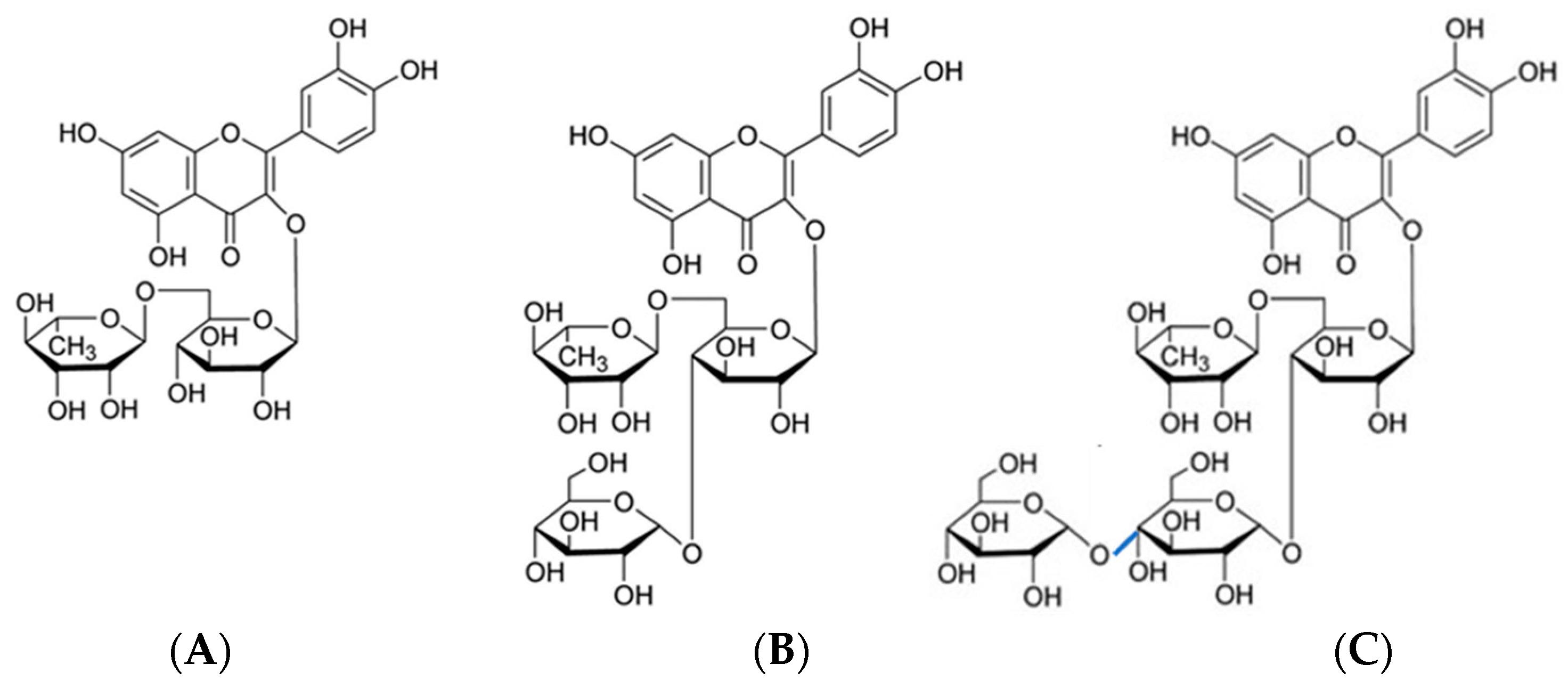
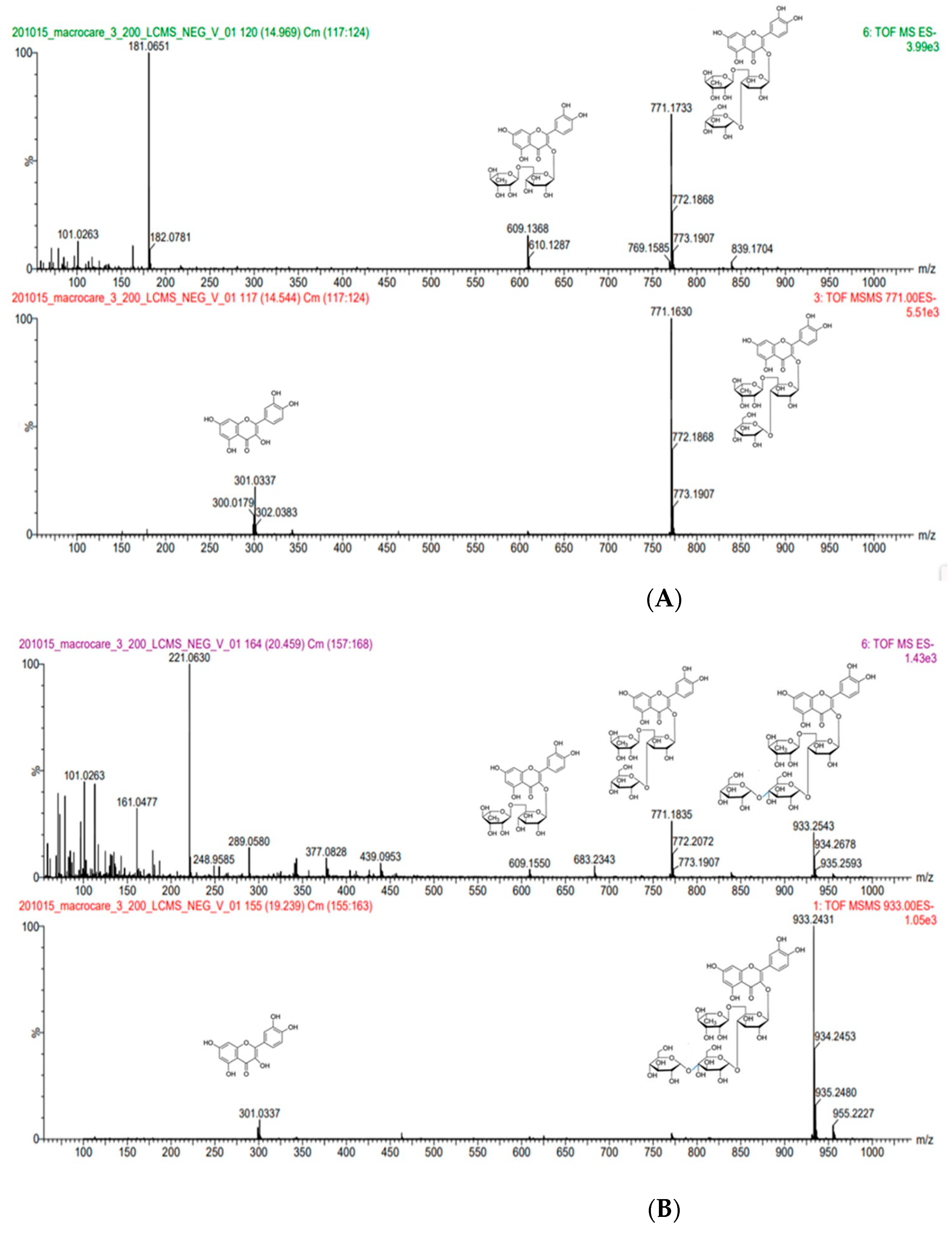
2.3.2. Identification of Rutin and Rutin Glycoside
2.4. Determination of Partition Coefficient
2.5. Determination of Antioxidant Activity
2.5.1. DPPH Assay
2.5.2. ABTS Assay
2.6. Determination of Effects on NO, PGE2 and Pro-Inflammatory Cytokine
2.6.1. Cell Culture and Cytotoxicity Assay
2.6.2. Measurement of NO and PGE2 Levels
2.6.3. Measurement of Pro-Inflammatory Cytokine Levels
2.7. Platelet Aggregation and Blood Coagulation In Vitro and In Vivo
2.7.1. Condition for In Vitro Test
2.7.2. Condition for In Vivo Test
2.7.3. Preparation of Platelet Rich Plasma (PRP) and Platelet Poor Plasma (PPP)
2.7.4. Determination of Platelet Aggregation Rate
2.7.5. Determination of Blood Coagulation Time
2.8. Statistical Analysis
3. Results
3.1. Preparation of Rutin Glycoside
3.1.1. Enzymatic Conversion of Rutin to Rutin Glycoside
3.1.2. Mass Spectral Fragmentation of Rutin Glycosides
3.2. Partition Coefficient of Rutin and Rutin Glycosides
3.3. Antioxidant Activity of Rutin and Rutin Glycoside
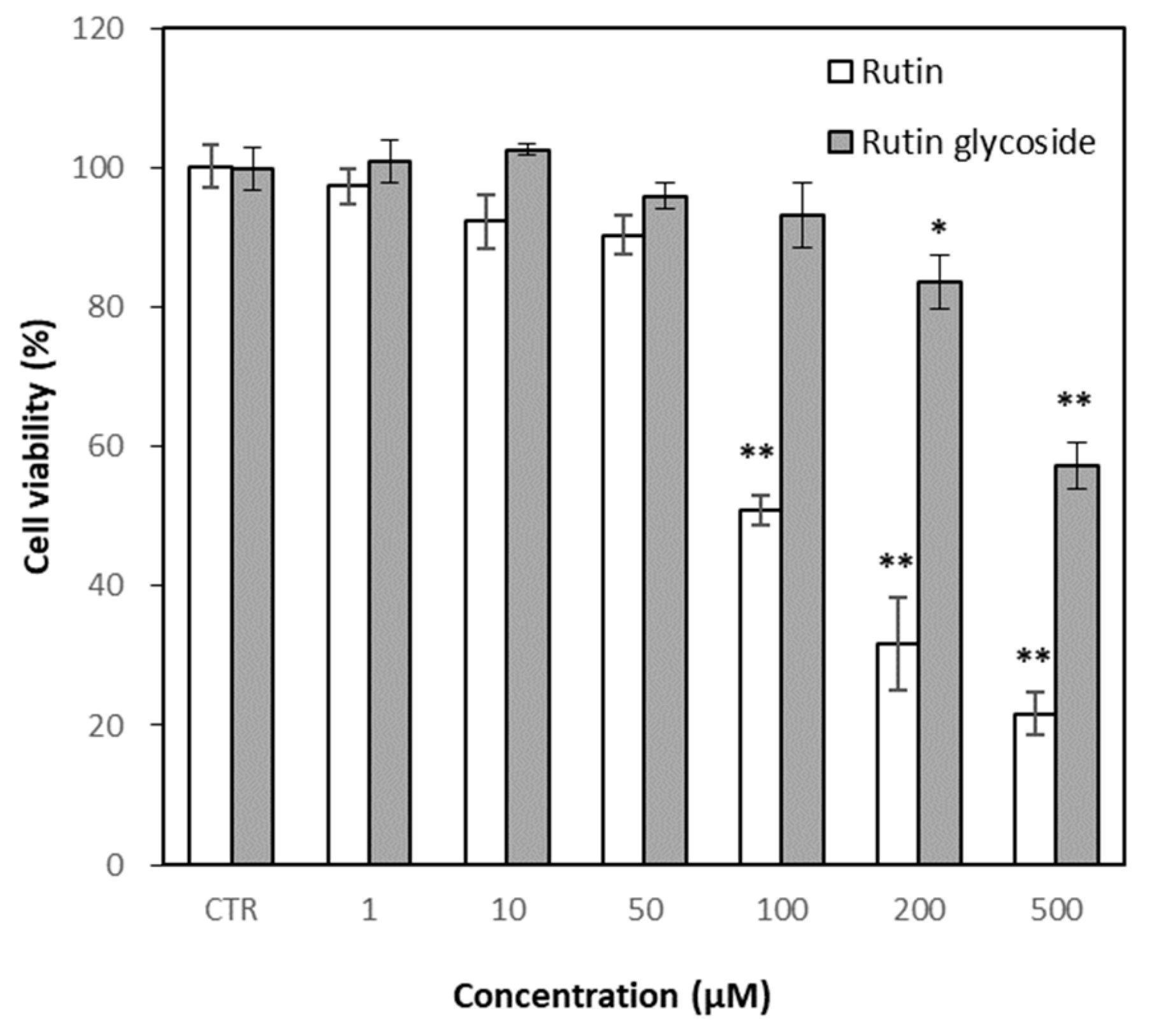
3.4. Effects of Rutin and Rutin Glycoside on Cell Viability
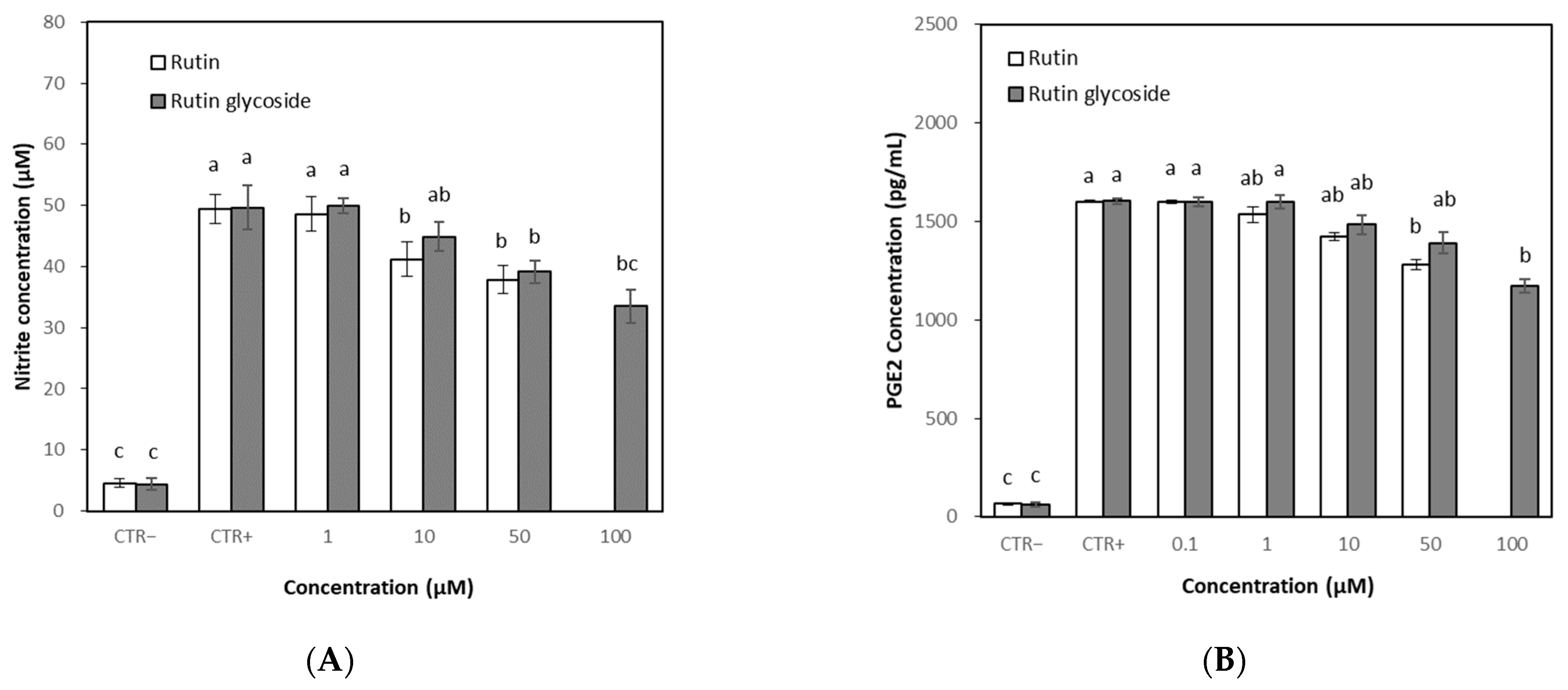
3.5. Effects of Rutin and Rutin Glycoside on NO and PGE2 Levels
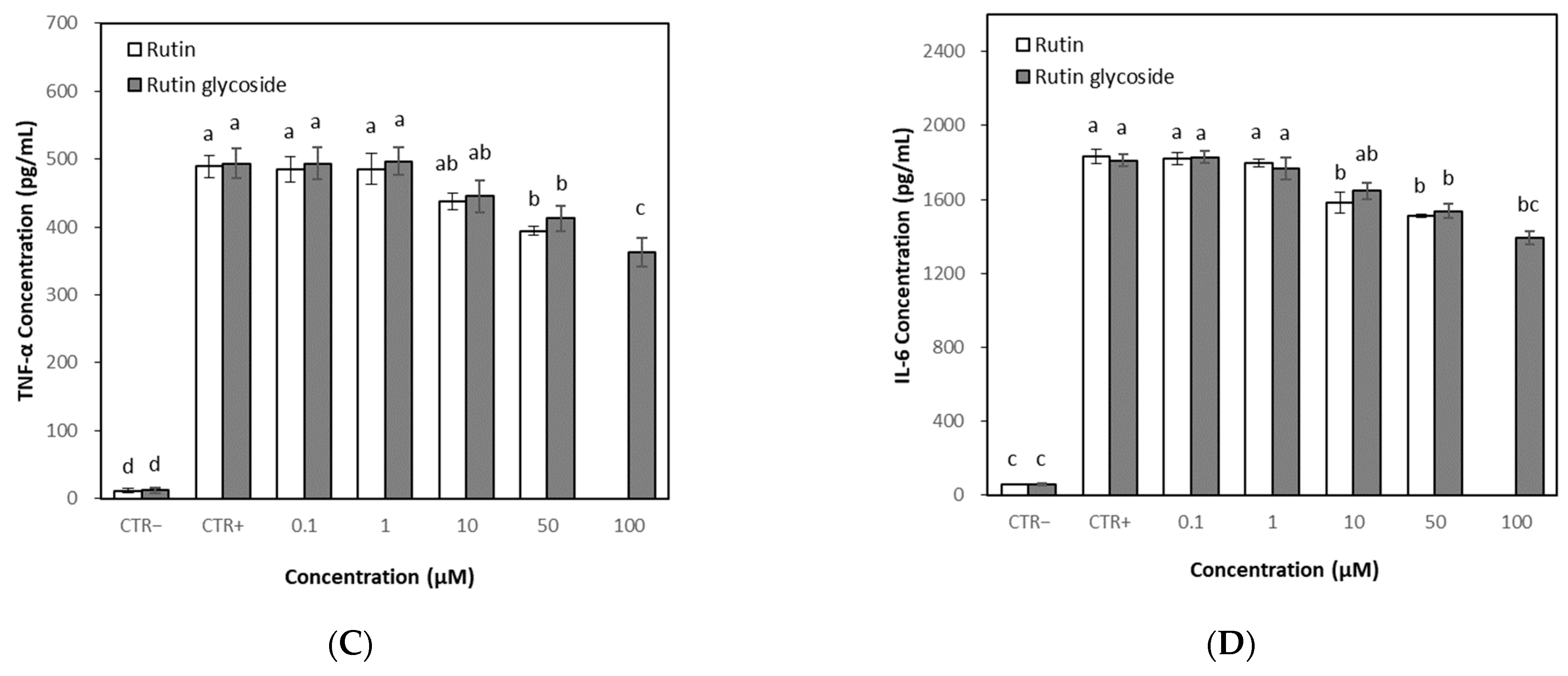
3.6. Effects of Rutin and Rutin Glycoside on TNF-α and IL-6
3.7. Platelet Aggregation and Blood Coagulation In Vitro and In Vivo
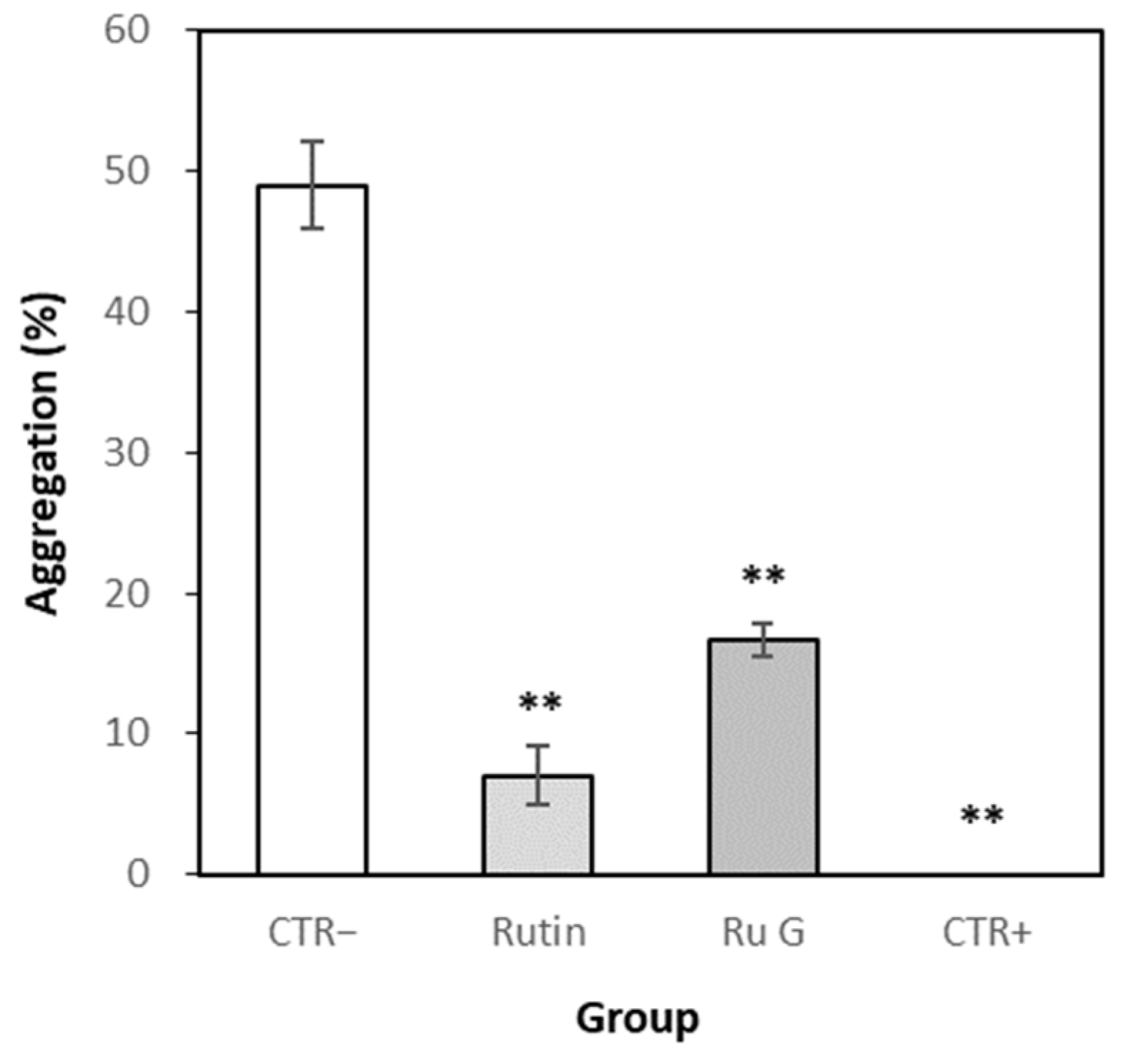
3.7.1. Effects of Rutin and Rutin Glycoside on Platelet Aggregation In Vitro
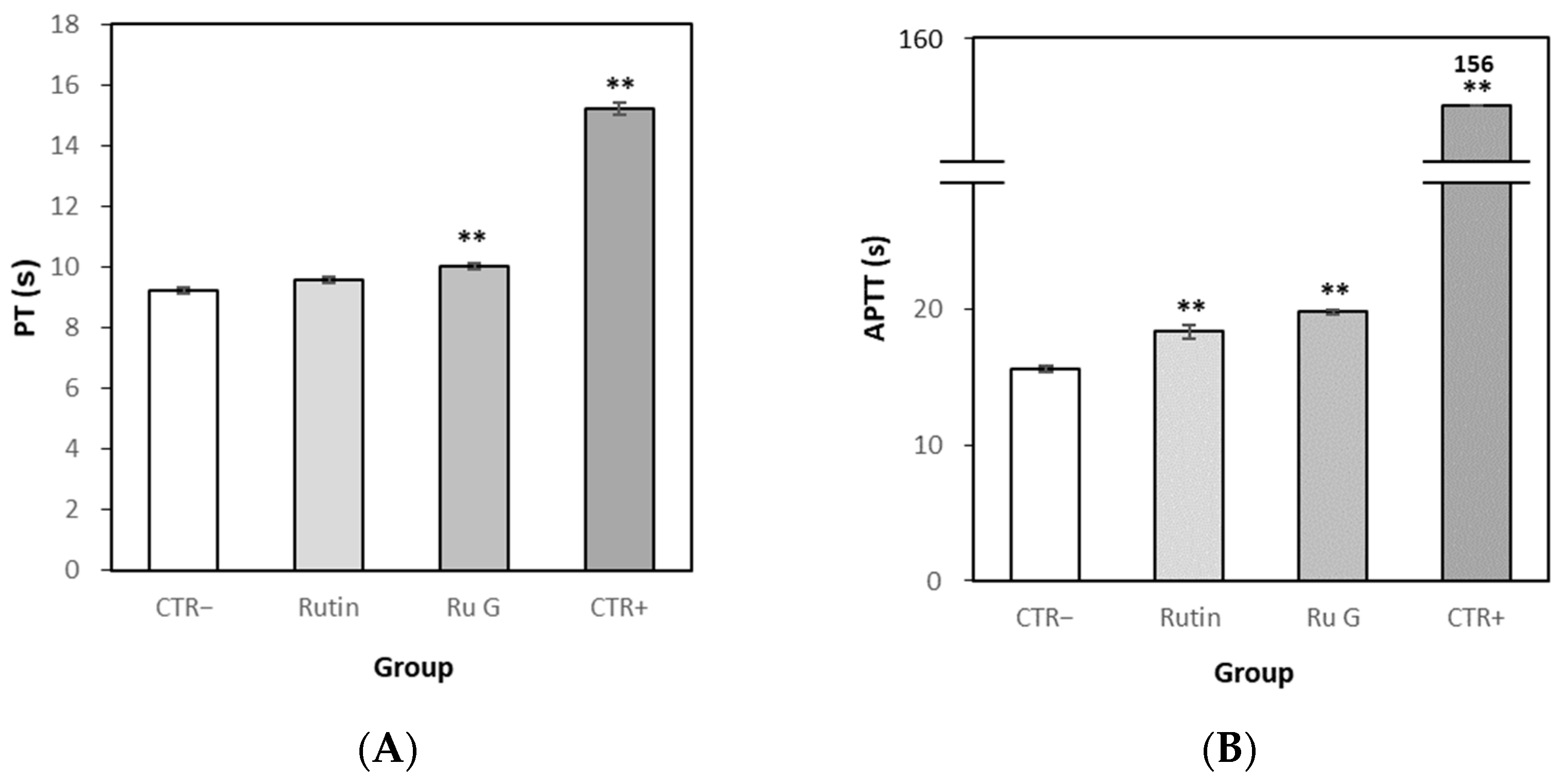
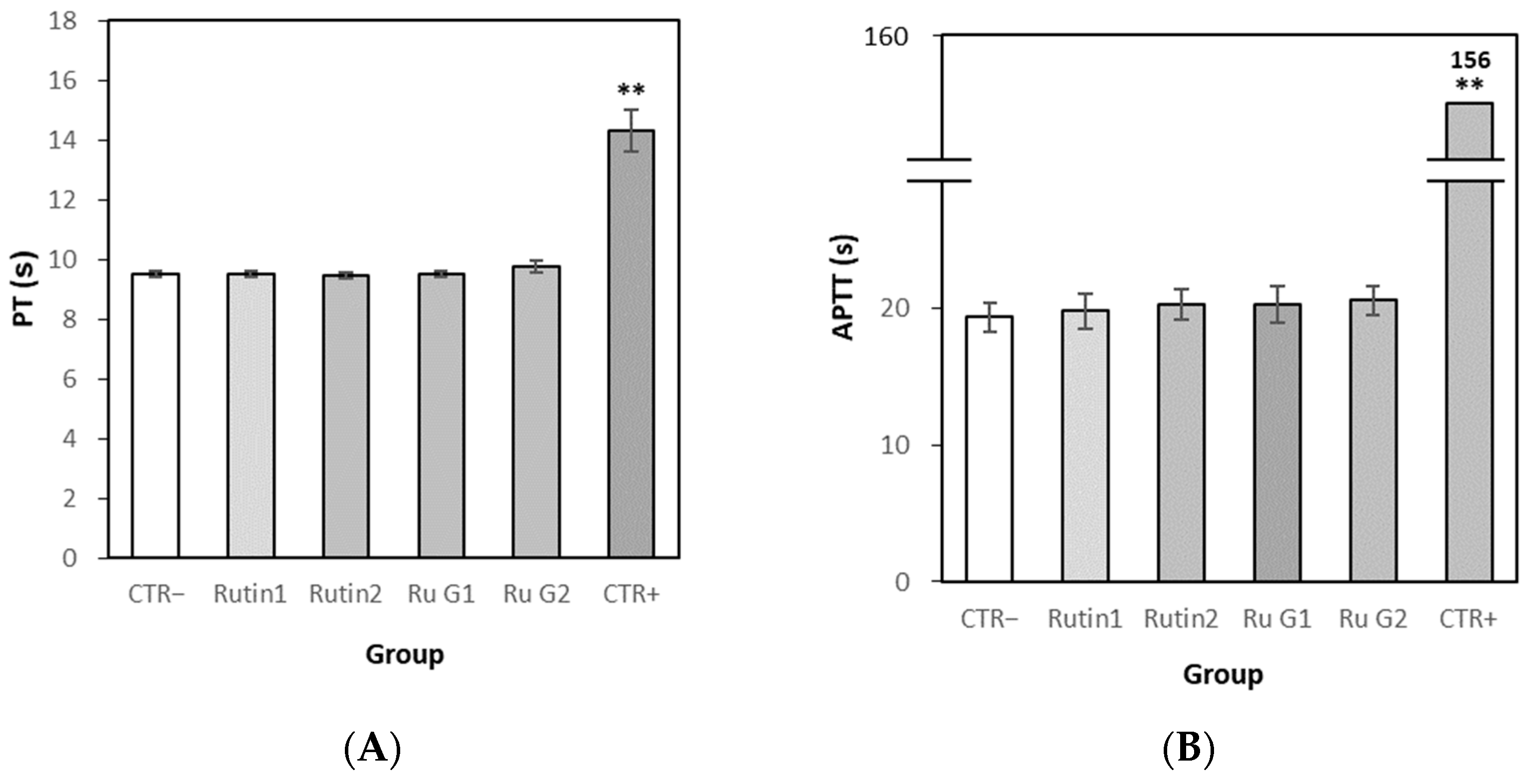
3.7.2. Effects of Rutin and Rutin Glycoside on Blood Coagulation In Vitro
3.7.3. Effects of Rutin and Rutin Glycoside on Platelet Aggregation In Vivo
3.7.4. Effects of Rutin and Rutin Glycoside on Blood Coagulation In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018, 241017. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Rutin: A potential antiviral for repurposing as a SARS-CoV-2 main protease (Mpro) inhibitor. Nat. Prod. Commun. 2021, 16, 1934578X21991723. [Google Scholar]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, M.L.; Tommasini, S.; Donato, P.; Stancanelli, R.; Raneri, D.; Catania, S.; Costa, C.; Villari, V.; Ficarra, P.; Ficarra, R. The rutin/β-cyclodextrin interactions in fully aqueous solution: Spectroscopic studies and biological assays. J. Pharm. Biomed. Anal. 2005, 35, 1019–1027. [Google Scholar] [CrossRef]
- Danciu, C.; Pinzaru, I.A.; Dehelan, C.A. Antiproliferative and antimicrobial properties of pure and encapsulated rutin. Farmacia 2018, 66, 302–308. [Google Scholar]
- Pivec, T.; Kargl, R.; Maver, U.; Bračič, M.; Elschner, T.; Žagar, E.; Gradišnik, L.; Kleinschek, K.S. Chemical structure—antioxidant activity relationship of water—based enzymatic polymerized rutin and its wound healing potential. Polymers 2019, 11, 1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedriali, C.A.; Fernandes, A.U.; Bernusso, L.C.; Polakiewicz, B. The synthesis of a water-soluble derivative of rutin as an antiradical agent. Quim. Nova 2008, 31, 2147–2151. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Huh, J.-Y.; Nam, S.-H.; Kim, D.; Lee, S.-B. Synthesis of quercetin-3-O-glucoside from rutin by Penicillium decumbens naringinase. J. Food Sci. 2013, 78, C411–C415. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet flavonoids”: Glycosidase-catalyzed modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef] [Green Version]
- Chaisin, T.; Rudeekulthamrong, P.; Kaulpiboon, J. Enzymatic synthesis, structural analysis, and evaluation of antibacterial activity and α-glucosidase inhibition of hesperidin glycosides. Catalysts 2021, 11, 532. [Google Scholar] [CrossRef]
- Ioannou, I.; Barboza, E.; Willig, G.; Marié, T.; Texeira, A.; Darme, P.; Renault, J.-H.; Allais, F. Implementation of an enzyme membrane reactor to intensify the α-O-glycosylation of resveratrol using cyclodextrins. Pharmaceuticals 2021, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, H.; Wada, Y.; Kadota, K.; Tozuka, Y. Improved solubility of quercetin by preparing amorphous solid with transglycosylated rutin and isoquercitrin. Environ. Control. Biol. 2018, 56, 161–165. [Google Scholar] [CrossRef]
- Maeda, J.; Roybal, E.; Brents, C.; Uesaka, M.; Aizawa, Y.; Kato, T. Natural and glucosyl flavonoids inhibit poly(ADP-ribose) polymerase activity and induce synthetic lethality in BRCA mutant cells. Oncol. Rep. 2014, 31, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Jiang, B.; Pan, B. Microwave accelerated transglycosylation of rutin by cyclodextrin glucanotransferase from Bacillus sp. SK13.002. Int. J. Mol. Sci. 2011, 12, 3786–3796. [Google Scholar] [CrossRef] [Green Version]
- KFDA. Enzymatically modified rutin. Food Addit. Code #2020-59, 336.
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, L.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Expr. 2020, 10, 187. [Google Scholar] [CrossRef]
- Schroeder, P.; Klotz, L.O.; Sies, H. Amphiphilic properties of (-)-epicatechin and their significance for protection of cells against peroxynitrite. Biochem. Biophys. Res. Commun. 2003, 307, 69–73. [Google Scholar] [CrossRef]
- Ratha, P.; Jhon, D.Y. Increase of rutin, quercetin, and antioxidant activity during germinated buckwheat (Fagopyrum esculentum Moench) fermentation. Ferment. Technol. 2017, 6, 147. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, M.S. Antiplatelet and antithrombotic activities of methanol extract of Usnea longissima. Phyto. Res. 2005, 19, 1061–1064. [Google Scholar] [CrossRef]
- Laskin, D.L.; Pendino, K.J. Macrophages and inflammatory mediators in tissue injury. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 655–677. [Google Scholar] [CrossRef]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Armstrong, P.C.J.; Truss, N.J.; Ali, F.Y.; Dhanji, A.A.; Vojnovic, I.; Zain, Z.N.M.; Bishop-Bailey, D.; Paul-Clark, M.J.; Tucker, A.T.; Mitchell, J.A.; et al. Aspirin and the in vitro linear relationship between thromboxane A 2 -mediated platelet aggregation and platelet production of thromboxane A. J. Thromb. Haemost. 2008, 6, 1933–1943. [Google Scholar] [CrossRef]
- Schrör, K. Aspirin and platelets: The antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin. Thromb. Hemost. 1997, 23, 349–356. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, K.A. Antithrombotic activity of methanolic extract of Umbilicaria esculenta. J. Ethnopharmacol. 2006, 105, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.H.; Koo, B.K.; Bang, S.H.; Kim, H.J.; Cho, Y.K. Analysis of coagulation factor activity of normal adults with APTT limit range. Korean J. Clin. Lab. Sci. 2015, 47, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Wang, L.; Walzem, R.L.; Miller, E.G.; Pike, L.M.; Patil, B.S. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J. Agric. Food Chem. 2005, 53, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Luthar, Z.; Germ, M.; Likar, M.; Golob, A.; Vogel-Mikuš, K.; Pongrac, P.; Kušar, A.; Pravst, I.; Kreft, I. Breeding buckwheat for increased levels of rutin, quercetin and other bioactive compounds with potential antiviral effects. Plants 2020, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure—Activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.E. Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 2008, 28, s11–s16. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Mitsuzumi, H.; Tsuzaki, Y.; Miwa, Y.; Chaen, H.; Yamamoto, I. Antioxidant activity of glycosylated vitamin P and its suppressive effect on oxidative stress in hyperlipidemic mice. Jpn. Soc. Nutr. Food Sci. 2003, 56, 355–363. [Google Scholar] [CrossRef]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of in vitro and in vivo antioxidant activities of six flavonoids with similar structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.H.; Beak, E.J.; Han, C.H.; Kang, N.J. Anti-oxidant and anti-inflammatory effects of rutin and its metabolites. Curr. Res. Agric. Life Sci. 2013, 31, 165–169. [Google Scholar]
- Rakshit, S.; Shukla, P.; Verma, A.; Nirala, S.K.; Bhadauria, M. Protective role of rutin against combined exposure to lipopolysaccharide and D-galactosamine-induced dysfunctions in liver, kidney, and brain: Hematological, biochemical, and histological evidences. J. Food Biochem. 2021, 45, e13605. [Google Scholar] [CrossRef]
- De Caterina, R.; D’Ugo, E.; Libby, P. Inflammation and thrombosis—Testing the hypothesis with anti-inflammatory drug trials. J. Thromb. Haemost. 2016, 116, 1012–1021. [Google Scholar]
- Branchford, B.R.; Carpenter, S.L. The role of inflammation in venous thromboembolism. Front. Pediatrs. 2018, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Zhang, J.; Zou, L.; Deng, G.; Xu, X.; Wang, F.; Ma, Z.; Zhang, J.; Zhao, T.; et al. C5aR, TNF-α, and FGL2 contribute to coagulation and complement activation in virus-induced fulminant hepatitis. J. Hepatol. 2015, 62, 354–362. [Google Scholar] [CrossRef]
- Page, M.J.; Bester, J.; Pretorius, E. The inflammatory effects of TNF-α and complement component 3 on coagulation. Sci. Rep. 2018, 8, 1812. [Google Scholar] [CrossRef] [Green Version]
- Grigniani, G.; Maiolo, A. Cytokines and hemostasis. Haematologica 2000, 85, 967–972. [Google Scholar]
- Day, S.M.; Reeve, J.L.; Myers, D.D.; Fay, W.P. Murine thrombosis models. Thromb. Haemost. 2004, 92, 486–494. [Google Scholar] [CrossRef]
- Liu, Y.; Jennings, N.L.; Dart, A.M.; Du, X.J. Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J. Exp. Med. 2012, 20, 30–36. [Google Scholar] [CrossRef]
- Gullon, B.; Lu-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, D.-W.; Park, S.-E.; Lee, H.-J.; Kim, K.-M.; Kim, K.-J.; Kim, M.-K.; Kim, S.-J.; Kim, S. Anti-thrombotic effect of rutin isolated from Dendropanax morbifera Leveille. J. Biosci. Bioeng. 2015, 120, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotito, S.B.; Frei, B. Relevance of apple polyphenols as antioxidants in human plasma: Contrasting in vitro and in vivo effects. Free. Radic. Biol. Med. 2004, 36, 201–211. [Google Scholar]
- Dong, D.; Quan, E.; Yuan, X.; Xie, Q.; Li, Z.; Wu, B. Sodium oleate-based nanoemulsion enhances oral absorption of chrysin through inhibition of UGT-mediated metabolism. Mol. Pharmaceut. 2017, 14, 2864–2874. [Google Scholar] [CrossRef] [PubMed]


| Compound | Log Po/w |
|---|---|
| Rutin | 0.61 ± 0.02 a |
| Rutin mono-glucoside Rutin mono-/di-glucoside | −1.40 ± 0.01 b −1.45 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-S.; Park, H.-R.; Lee, K.-A. A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation. Antioxidants 2021, 10, 1696. https://doi.org/10.3390/antiox10111696
Choi S-S, Park H-R, Lee K-A. A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation. Antioxidants. 2021; 10(11):1696. https://doi.org/10.3390/antiox10111696
Chicago/Turabian StyleChoi, Sung-Sook, Hye-Ryung Park, and Kyung-Ae Lee. 2021. "A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation" Antioxidants 10, no. 11: 1696. https://doi.org/10.3390/antiox10111696






