Siegesbeckiae Herba Extract and Chlorogenic Acid Ameliorate the Death of HaCaT Keratinocytes Exposed to Airborne Particulate Matter by Mitigating Oxidative Stress
Abstract
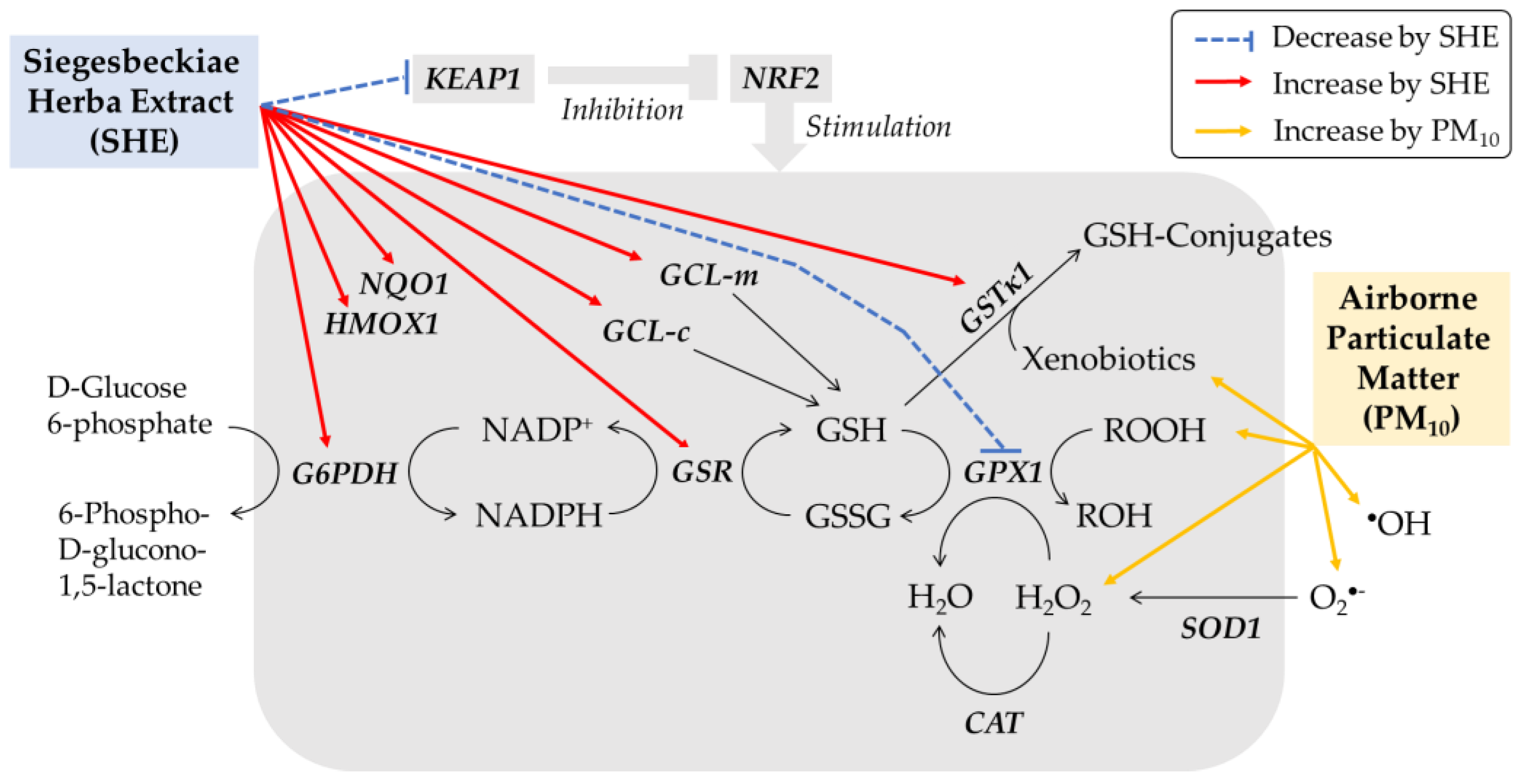
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Extracts of Medicinal Plants
2.3. Siegesbeckiae Herba Extract and Its Solvent Fractions
2.4. High-Performance Liquid Chromatography with Photodiode Array Detection (HPLC-DAD)
2.5. Cell Culture and PM10 Treatment
2.6. Cell Viability and Lactate Dehydrogenase (LDH) Release Assays
2.7. Cellular Lipid Peroxidation Assay
2.8. Cellular ROS Production Assay
2.9. Glutathione (GSH) and Glutathione Disulfide (GSSG) Assay
2.10. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
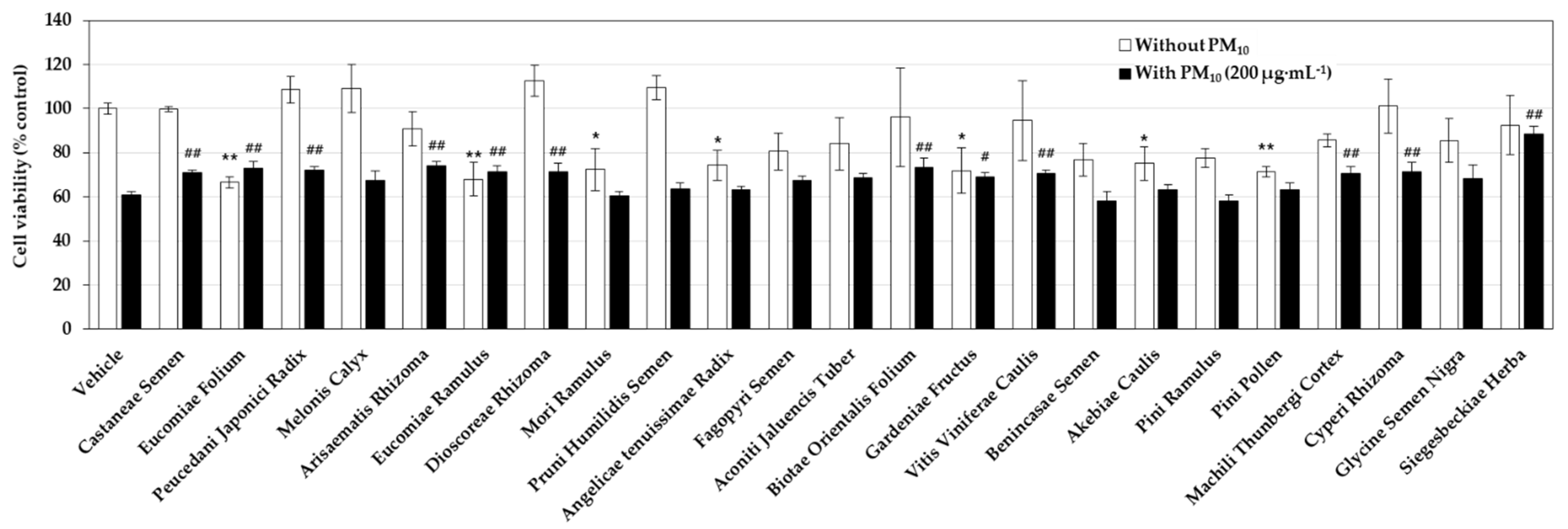
3.1. Effects of Medicinal Plant Extracts on the PM10-Induced Toxicity in HaCaT Keratinocytes
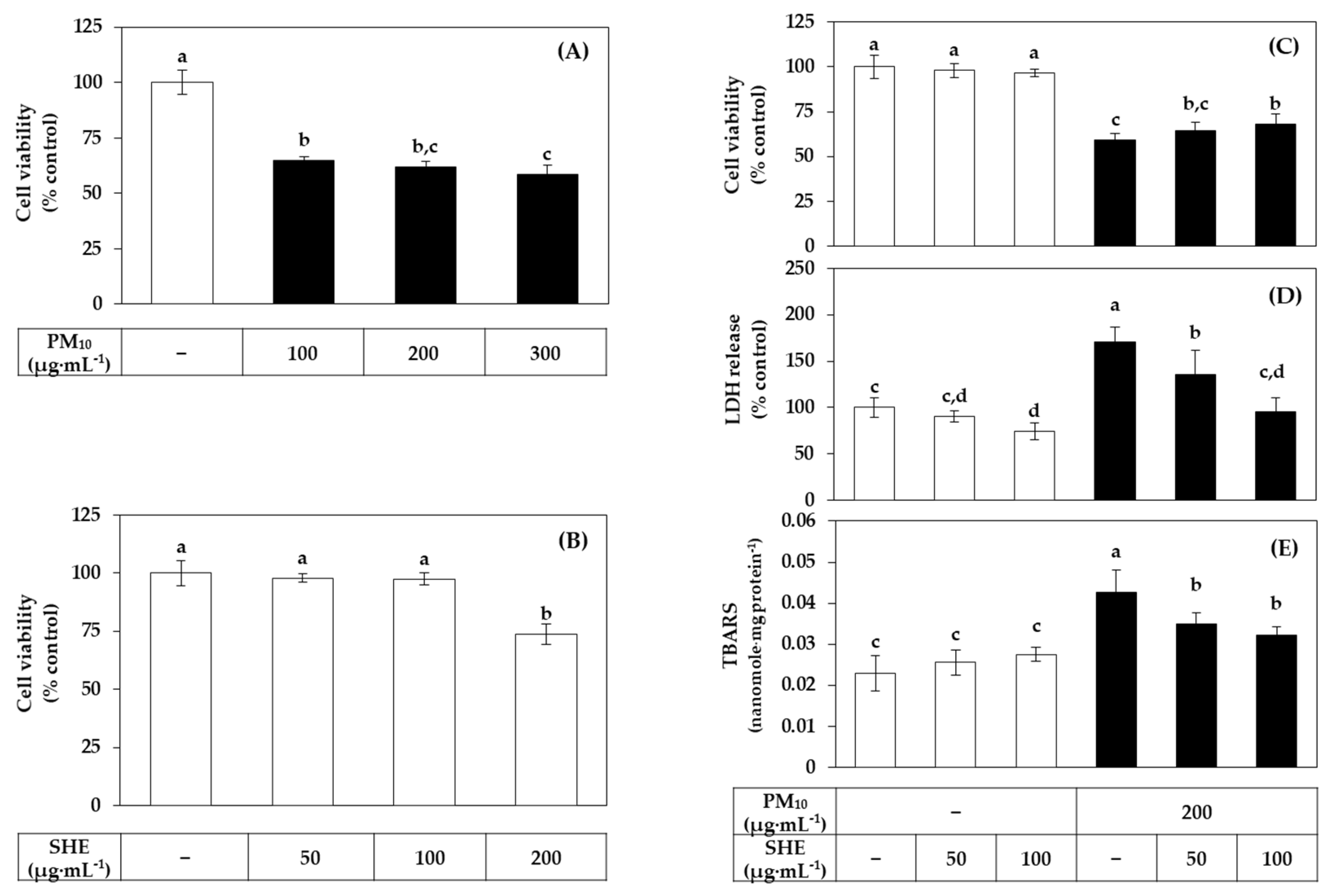
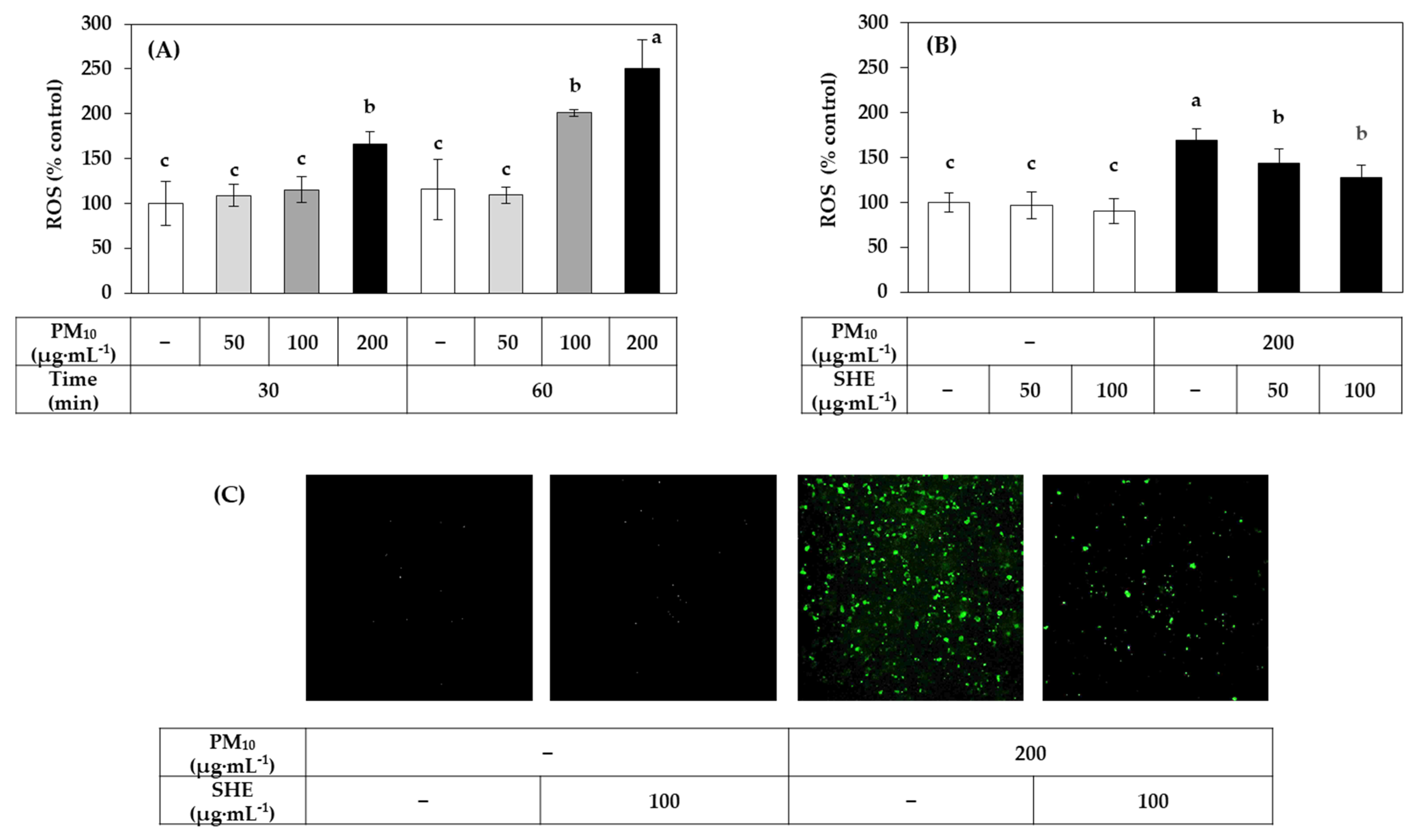
3.2. Effects of SHE on the Viability, LDH Release, Lipid Peroxidation, and ROS Production in HaCaT Cells Exposed to PM10
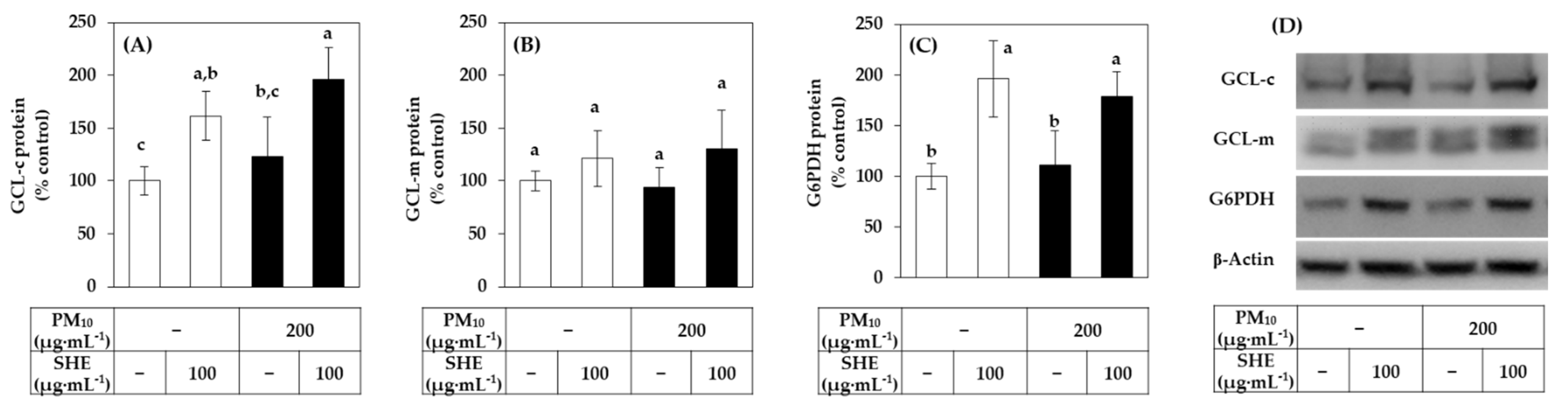
3.3. Effects of SHE on the Expression of the Defense Genes in HaCaT Cells under Basal and PM10-Exposed Conditions
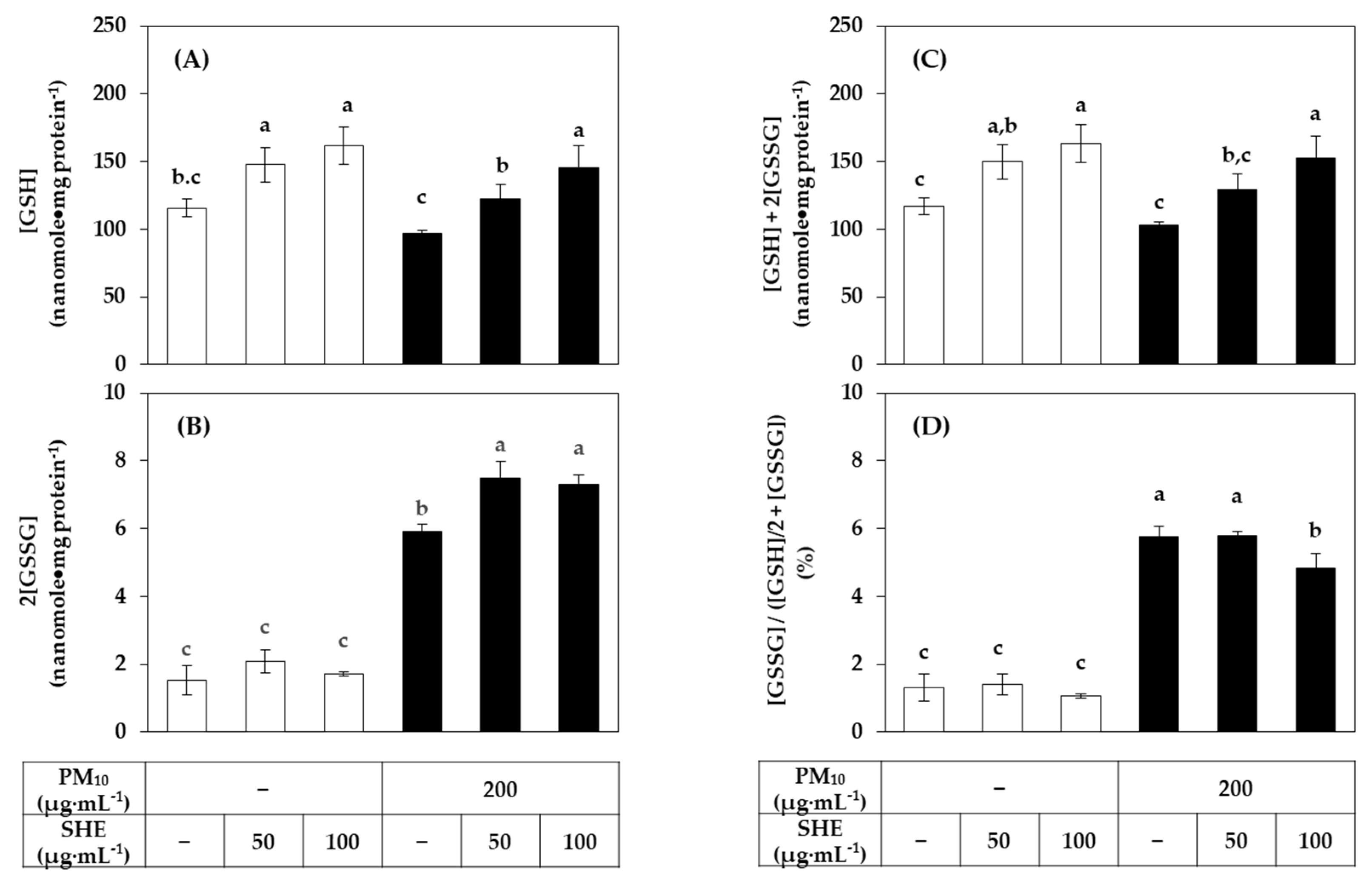
3.4. Effects of SHE on the GSH and GSSG Levels in HaCaT Cells Exposed to PM10
3.5. Effects of Solvent Fractions of SHE on the Viability of HaCaT Cells Exposed to PM10
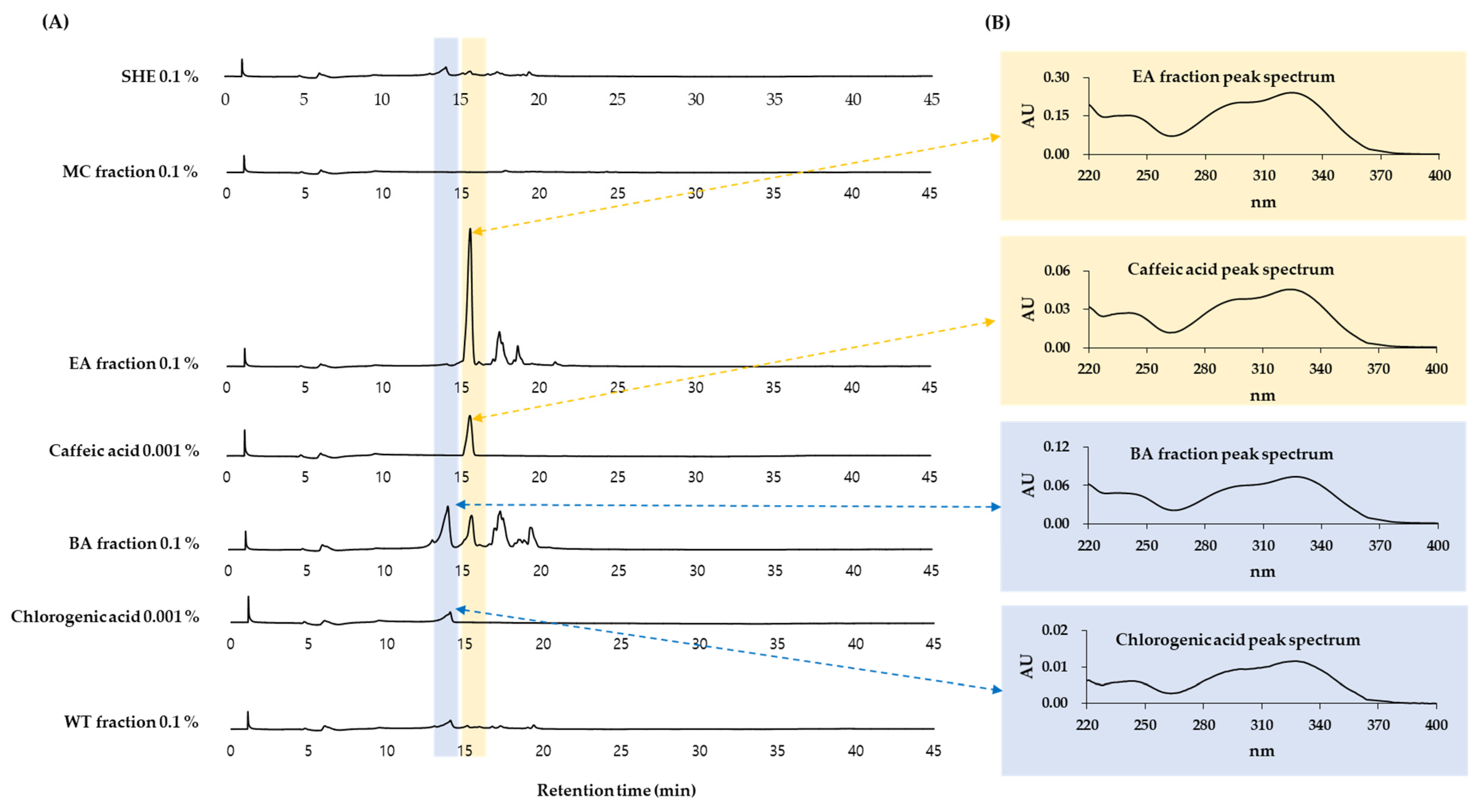
3.6. HPLC-DAD Analysis of Solvent Fractions of SHE
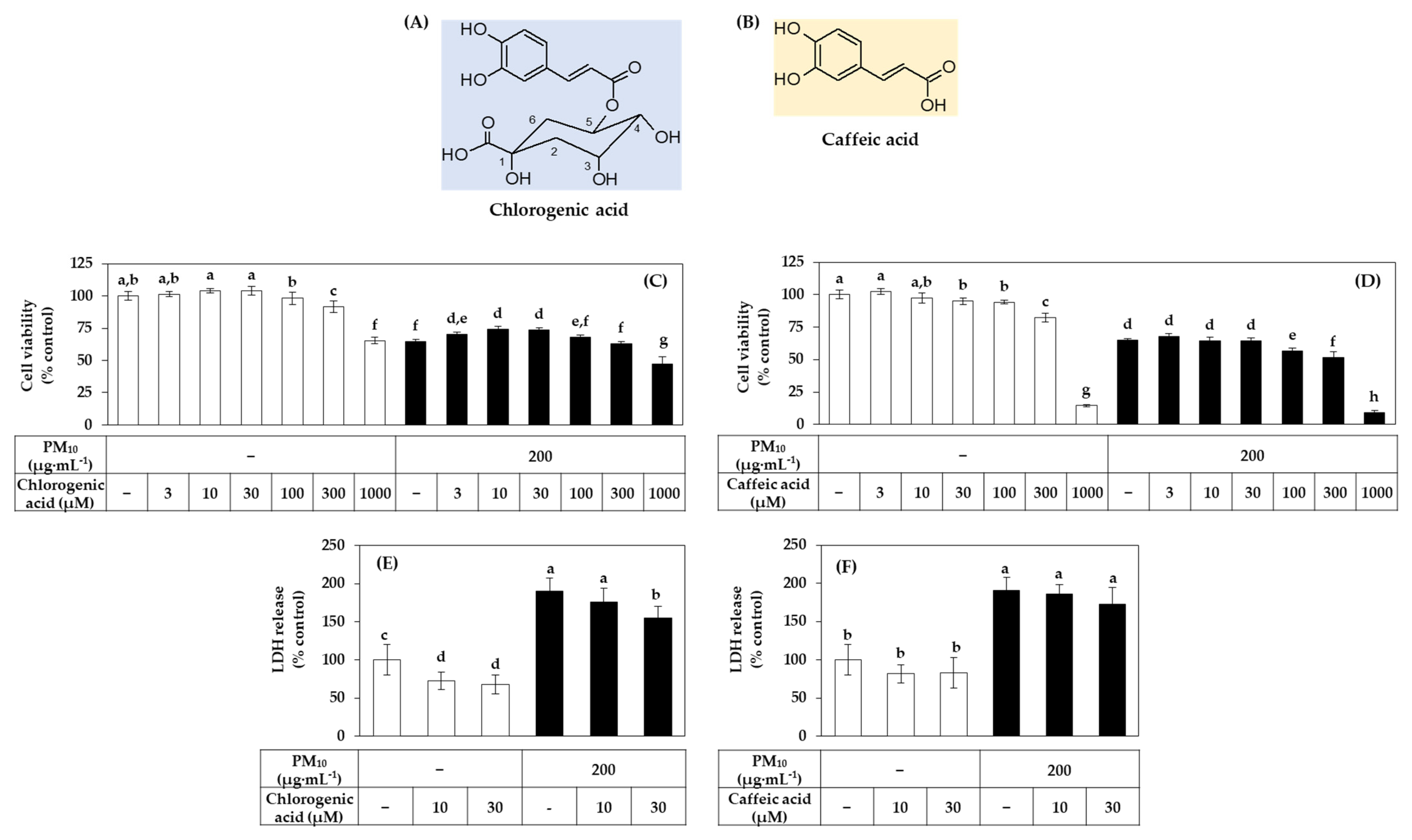
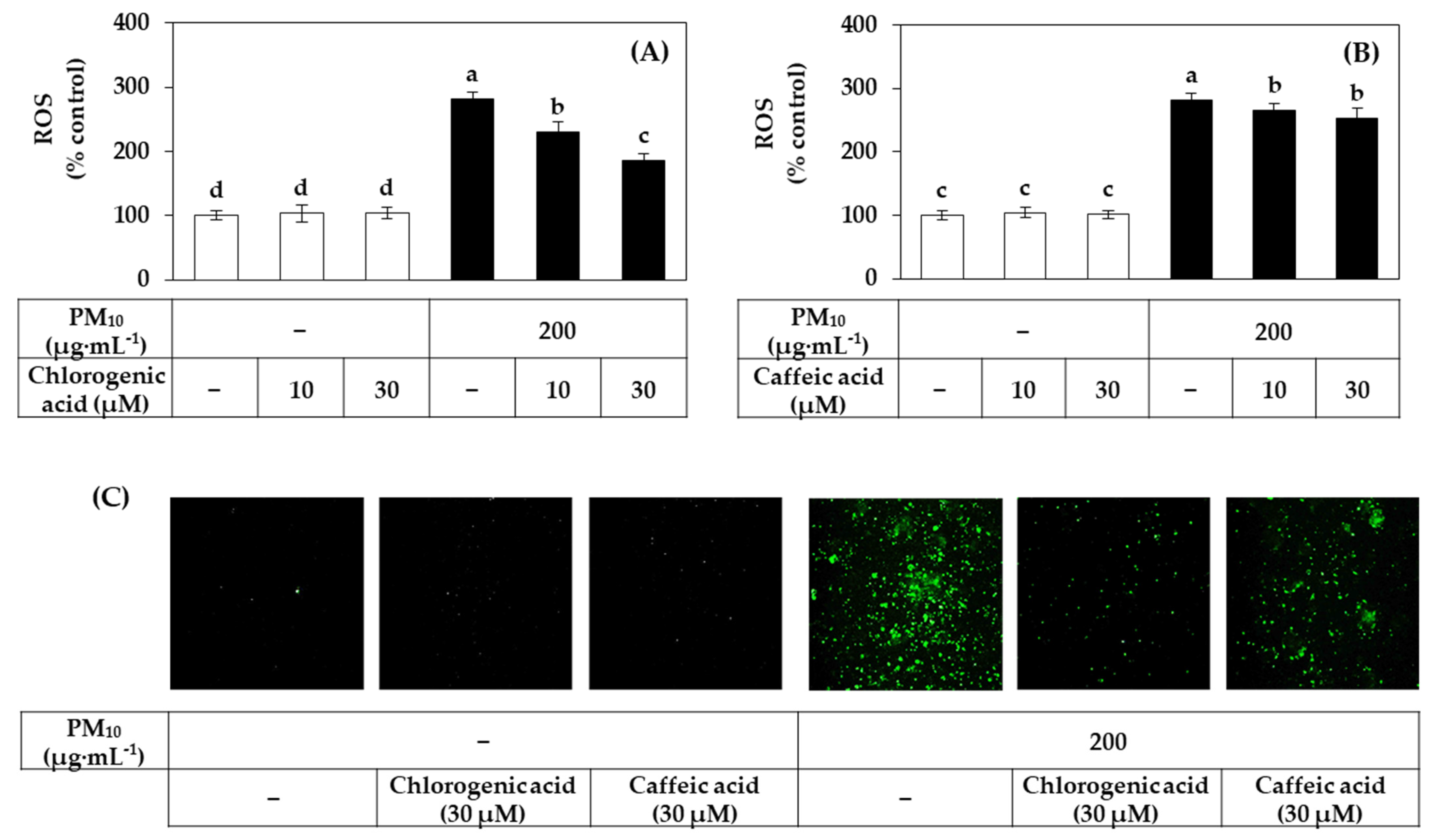
3.7. Effects of Chlorogenic Acid vs. Caffeic Acid on the Viability, LDH Release, and ROS Production of HaCaT Cells Exposed to PM10
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARE | antioxidant response element |
| BA | n-butyl alcohol |
| CAT | catalase |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DTNB | 5,5-dithio-bis-2-nitrobenzoic acid |
| EA | ethyl acetate |
| G6PDH | glucose 6-phosphate dehydrogenase |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GCL-c | glutamate-cysteine ligase catalytic subunit |
| GCL-m | glutamate-cysteine ligase modifier subunit |
| GPX | glutathione peroxidase |
| GSH | glutathione |
| GSR | glutathione disulfide reductase |
| GSSG | glutathione disulfide |
| GST | glutathione S-transferase |
| HMOX | heme oxygenase |
| HPLC-DAD | high-performance liquid chromatography-photodiode array detection |
| IL | interleukin |
| KEAP | kelch-like ECH-associated protein |
| LDH | lactate dehydrogenase |
| MC | methylene chloride |
| MMP | matrix metalloproteinase |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide |
| NQO | NAD(P)H quinone oxidoreductase |
| NRF | nuclear factor erythroid 2-related factor |
| PBS | phosphate-buffered saline |
| PM | particulate matter |
| qRT-PCR | quantitative reverse transcriptase-polymerase chain reaction |
| ROS | reactive oxygen species |
| SHE | Siegesbeckiae Herba extract |
| SOD | superoxide dismutase |
| TBA | 2-thiobarbituric acid |
| TBARS | 2-thiobarbituric acid-reactive substance |
| WT | water |
References
- Mukherjee, A.; Agrawal, M. World air particulate matter: Sources, distribution and health effects. Environ. Chem. Lett. 2017, 15, 283–309. [Google Scholar] [CrossRef]
- Fuzzi, S.; Baltensperger, U.; Carslaw, K.; Decesari, S.; Van Der Gon, H.D.; Facchini, M.C.; Fowler, D.; Koren, I.; Langford, B.; Lohmann, U.; et al. Particulate matter, air quality and climate: Lessons learned and future needs. Atmos. Chem. Phys. Discuss. 2015, 15, 8217–8299. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Park, D.; Lee, Y.; Lee, Y.-C. Systematic Review and Meta-Analysis of Human Skin Diseases Due to Particulate Matter. Int. J. Environ. Res. Public Health 2017, 14, 1458. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qiu, H.; Wang, L.; Duan, Z.; Yu, H.; Deng, R.; Zhang, Y.; Zhou, L. Risks of hospital admissions from a spectrum of causes associated with particulate matter pollution. Sci. Total Environ. 2019, 656, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Schaefer, H.; Otberg, N.; Teichmann, A.; Blume-Peytavi, U.; Sterry, W. Penetration von Mikropartikeln in die menschliche Haut. Der Hautarzt 2004, 55, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lee, K.; Lee, Y.-M.; Lee, J.-H.; Lee, S.I.; Yu, S.-D.; Paek, D. Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis. Environ. Res. 2011, 111, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999. [Google Scholar] [CrossRef]
- Jin, S.-P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018, 91, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Ji, M.J.; Kang, J.Y.; Kyung, S.Y.; Hong, J.H. Dust particles-induced intracellular Ca2+ signaling and reactive oxygen species in lung fibroblast cell line MRC5. Korean J. Physiol. Pharmacol. 2017, 21, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Contribution of transition metals in the reactive oxygen species activity of PM emissions from retrofitted heavy-duty vehicles. Atmos. Environ. 2010, 44, 5165–5173. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Hyun, Y.-M.; Kim, S.H.; Ko, M.K.; Park, C.O.; Hyun, J.W. Particulate matter induces inflammatory cytokine production via activation of NFκB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019, 21, 101080. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Cho, M.A.; Ha, J.W.; Boo, Y.C. Role of Dual Oxidase 2 in Reactive Oxygen Species Production Induced by Airborne Particulate Matter PM10 in Human Epidermal Keratinocytes. J. Soc. Cosmet. Sci. Korea 2018, 45, 57–67. [Google Scholar] [CrossRef]
- Ko, E.; Choi, H.; Park, K.-N.; Park, J.-Y.; Lee, T.R.; Shin, N.W.; Bae, Y.S. Dual oxidase 2 is essential for house dust mite-induced pro-inflammatory cytokine production in human keratinocytes. Exp. Dermatol. 2015, 24, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Lin, Z.-C.; Hsu, L.-F.; Fang, J.-Y.; Chiang, Y.-C.; Tsai, M.-H.; Lee, M.-H.; Li, S.-Y.; Hu, S.C.-S.; Lee, I.-T.; et al. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants-exposed human keratinocytes through ROS/MAPKs pathways. J. Ethnopharmacol. 2016, 189, 300–309. [Google Scholar] [CrossRef]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine Alga Ecklonia cava Extract and Dieckol Attenuate Prostaglandin E2 Production in HaCaT Keratinocytes Exposed to Airborne Particulate Matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Lee, J.-W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (–)-Epigallocatechin-3-Gallate Rescue Cell Viability and Attenuate Inflammatory Responses of Human Epidermal Keratinocytes Exposed to Airborne Particulate Matter PM10. Ski. Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, J.H.; Jeong, G.J.; Park, K.Y.; Lee, M.; Seo, S.J. Particulate matter induces pro-inflammatory cytokines via phosphorylation of p38 MAPK possibly leading to dermal inflammaging. Exp. Dermatol. 2019, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From In Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Pasciu, V.; Posadino, A.M.; Cossu, A.; Sanna, B.; Tadolini, B.; Gaspa, L.; Marchisio, A.; Dessole, S.; Capobianco, G.; Pintus, G. Akt Downregulation by Flavin Oxidase–Induced ROS Generation Mediates Dose-Dependent Endothelial Cell Damage Elicited by Natural Antioxidants. Toxicol. Sci. 2010, 114, 101–112. [Google Scholar] [CrossRef]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Deiana, L.; Carru, C.; Pintus, G. Coumaric Acid Induces Mitochondrial Damage and Oxidative-Mediated Cell Death of Human Endothelial Cells. Cardiovasc. Toxicol. 2013, 13, 301–306. [Google Scholar] [CrossRef]
- Giordo, R.; Cossu, A.; Pasciu, V.; Hoa, P.T.; Posadino, A.M.; Pintus, G. Different Redox Response Elicited by Naturally Occurring Antioxidants in Human Endothelial Cells. Open Biochem. J. 2013, 7, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Edged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Natural Nrf2 Modulators for Skin Protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, Y.-Y.; Li, K.-W.; Li, Y.; Niu, F.-J.; Zhou, S.-J.; Wei, H.-C.; Zhou, C.-Z. Herba Siegesbeckiae: A review on its traditional uses, chemical constituents, pharmacological activities and clinical studies. J. Ethnopharmacol. 2021, 275, 114117. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.-X.; Xiong, W.; Zhao, G.-D.; Peng, Y.; Zhong, Z.-F.; Xu, L.; Duan, R.; Tsim, K.W.; Yu, H.; Wang, Y.-T. Discrimination of three Siegesbeckiae Herba species using UPLC-QTOF/MS-based metabolomics approach. Food Chem. Toxicol. 2018, 119, 400–406. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Cheng, B.C.-Y.; Lau, M.-Y.; Fu, X.-Q.; Li, T.; Su, T.; Zhu, P.-L.; Chan, Y.-C.; Tse, A.K.-W.; et al. Comparison of the chemical profiles and inflammatory mediator-inhibitory effects of three Siegesbeckia herbs used as Herba Siegesbeckiae (Xixiancao). BMC Complement. Altern. Med. 2018, 18, 141. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Lee, J.-W.; Seok, J.K.; Boo, Y.C. Ecklonia cava Extract and Dieckol Attenuate Cellular Lipid Peroxidation in Keratinocytes Exposed to PM10. Evid.-Based Complement. Altern. Med. 2018, 2018, 8248323. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS Using Oxidized DCFDA and Flow-Cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Sun, X.; Li, B.; Li, X.; Wang, Y.; Xu, Y.; Jin, Y.; Piao, F.; Sun, G. Effects of sodium arsenite on catalase activity, gene and protein expression in HaCaT cells. Toxicol. Vitr. 2006, 20, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Rolfs, F.; Huber, M.; Kuehne, A.; Kramer, S.; Haertel, E.; Muzumdar, S.; Wagner, J.; Tanner, Y.; Böhm, F.; Smola, S.; et al. Nrf2 Activation Promotes Keratinocyte Survival during Early Skin Carcinogenesis via Metabolic Alterations. Cancer Res. 2015, 75, 4817–4829. [Google Scholar] [CrossRef]
- Kanno, T.; Tanaka, K.; Yanagisawa, Y.; Yasutake, K.; Hadano, S.; Yoshii, F.; Hirayama, N.; Ikeda, J.-E. A novel small molecule, N-(4-(2-pyridyl)(1,3-thiazol-2-yl))-2-(2,4,6-trimethylphenoxy) acetamide, selectively protects against oxidative stress-induced cell death by activating the Nrf2–ARE pathway: Therapeutic implications for ALS. Free. Radic. Biol. Med. 2012, 53, 2028–2042. [Google Scholar] [CrossRef]
- Priftis, A.; Angeli-Terzidou, A.; Veskoukis, A.S.; Spandidos, D.; Kouretas, D. Cell-specific and roasting-dependent regulation of the Keap1/Nrf2 pathway by coffee extracts. Mol. Med. Rep. 2018, 17, 8325–8331. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Ku, J.-E.; Rhie, S.-J.; Ryu, J.Y.; Bae, S.; Kim, Y.-S. Anti-oxidant and Anti-inflammatory Effects of Sinapic Acid in UVB Irradiation-Damaged HaCaT Keratinocytes. Asian J. Beauty Cosmetol. 2017, 15, 513–522. [Google Scholar] [CrossRef]
- Lou, D.; Wei, X.; Xiao, P.; Huo, Q.; Hong, X.; Sun, J.; Shuai, Y.; Tao, G. Demethylation of the NRF2 Promoter Protects against Carcinogenesis Induced by Nano-SiO2. Front. Genet. 2020, 11, 818. [Google Scholar] [CrossRef]
- Warabi, E.; Wada, Y.; Kajiwara, H.; Kobayashi, M.; Koshiba, N.; Hisada, T.; Shibata, M.; Ando, J.; Tsuchiya, M.; Kodama, T.; et al. Effect on Endothelial Cell Gene Expression of Shear Stress, Oxygen Concentration, and Low-Density Lipoprotein as Studied by a Novel Flow Cell Culture System. Free. Radic. Biol. Med. 2004, 37, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hou, Y.; Zhang, Q.; Woods, C.G.; Xue, P.; Fu, J.; Yarborough, K.; Guan, D.; Andersen, M.; Pi, J. Cross-Regulations among NRFs and KEAP1 and Effects of their Silencing on Arsenic-Induced Antioxidant Response and Cytotoxicity in Human Keratinocytes. Environ. Health Perspect. 2012, 120, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-H. Anti-oxidant Activities of Phytol on Keratinocytes. Asian J. Beauty Cosmetol. 2017, 15, 457–465. [Google Scholar] [CrossRef]
- An, S.-M.; Koh, J.-S.; Boo, Y.-C. Inhibition of melanogenesis by tyrosinase siRNA in human melanocytes. BMB Rep. 2009, 42, 178–183. [Google Scholar] [CrossRef]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Chernov, N.N.; Novichkova, M.D. Role of glutathione, glutathione transferase, and glutaredoxin in regulation of redox-dependent processes. Biochemistry 2014, 79, 1562–1583. [Google Scholar] [CrossRef]
- Gong, Z.-H.; Tian, G.-L.; Huang, Q.-W.; Wang, Y.-M.; Xu, H.-P. Reduced glutathione and glutathione disulfide in the blood of glucose-6-phosphate dehydrogenase-deficient newborns. BMC Pediatr. 2017, 17, 172. [Google Scholar] [CrossRef]
- Hoffmann, C.; Dietrich, M.; Herrmann, A.-K.; Schacht, T.; Albrecht, P.; Methner, A. Dimethyl Fumarate Induces Glutathione Recycling by Upregulation of Glutathione Reductase. Oxidative Med. Cell. Longev. 2017, 2017, 6093903. [Google Scholar] [CrossRef]
- Föller, M.; Harris, I.S.; Elia, A.; John, R.; Lang, F.; Kavanagh, T.J.; Mak, T.W. Functional significance of glutamate–cysteine ligase modifier for erythrocyte survival in vitro and in vivo. Cell Death Differ. 2013, 20, 1350–1358. [Google Scholar] [CrossRef]
- Beyer, T.A.; Keller, U.A.D.; Braun, S.; Schäfer, M.; Werner, S. Roles and mechanisms of action of the Nrf2 transcription factor in skin morphogenesis, wound repair and skin cancer. Cell Death Differ. 2007, 14, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Wang, D.; Hou, J.; Wan, J.; Yang, Y.; Liu, S.; Li, X.; Li, W.; Dai, X.; Zhou, P.; Liu, W.; et al. Dietary chlorogenic acid ameliorates oxidative stress and improves endothelial function in diabetic mice via Nrf2 activation. J. Int. Med. Res. 2021, 49, 300060520985363. [Google Scholar] [CrossRef]
- Han, D.; Chen, W.; Gu, X.; Shan, R.; Zou, J.; Liu, G.; Shahid, M.; Gao, J.; Han, B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget 2017, 8, 14680–14692. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Wang, J.-P.; Ruan, J.-L.; Cai, Y.-L.; Luo, Q.; Xu, H.-X.; Wu, Y.-X. In vitro and in vivo evaluation of the wound healing properties of Siegesbeckia pubescens. J. Ethnopharmacol. 2011, 134, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, Y.; Wu, Y. Antiinflammatory and analgesic activity of topical administration of Siegesbeckia pubescens. Pak. J. Pharm. Sci. 2008, 21, 89–91. [Google Scholar] [PubMed]
- Sang, W.; Zhong, Z.; Linghu, K.; Xiong, W.; Tse, A.K.W.; Cheang, W.S.; Yu, H.; Wang, Y. Siegesbeckia pubescens Makino inhibits Pam3CSK4-induced inflammation in RAW 264.7 macrophages through suppressing TLR1/TLR2-mediated NF-κB activation. Chin. Med. 2018, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Zhang, J.; Tian, G.-H.; Shang, H.-C.; Tang, H.-B. Kirenol, darutoside and hesperidin contribute to the anti-inflammatory and analgesic activities of Siegesbeckia pubescens makino by inhibiting COX-2 expression and inflammatory cell infiltration. J. Ethnopharmacol. 2021, 268, 113547. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Kim, M.; Kim, C.E.; Kang, J.; Yoo, H.; Sung, S.H.; Lee, M. Quercetin 3,7-O-dimethyl ether from Siegesbeckia pubescens suppress the production of inflammatory mediators in lipopolysaccharide-induced macrophages and colon epithelial cells. Biosci. Biotechnol. Biochem. 2016, 80, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, S.H.; Lee, H.K.; Cho, Y.; Kang, J.; Sung, S.H. ent-Kaurane and ent-Pimarane Diterpenes from Siegesbeckia pubescens Inhibit Lipopolysaccharide-Induced Nitric Oxide Production in BV2 Microglia. Biol. Pharm. Bull. 2014, 37, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Lee, M.; Sung, S.H.; Jeong, G.S. Involvement of heme oxygenase-1 induction in the cytoprotective and neuroinflammatory activities of Siegesbeckia pubescens isolated from 5,3′-dihydroxy-3,7,4′-trimethoxyflavone in HT22 cells and BV2 cells. Int. Immunopharmacol. 2016, 40, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Jung, D.-H.; Sung, J.; Min, I.; Lee, S.-J. Tart Cherry Extract Containing Chlorogenic Acid, Quercetin, and Kaempferol Inhibits the Mitochondrial Apoptotic Cell Death Elicited by Airborne PM10 in Human Epidermal Keratinocytes. Antioxidants 2021, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, B.; Ren, P. Reduction of MTT by flavonoids in the absence of cells. Colloids Surf. B Biointerfaces 2005, 45, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Holder, A.; Goth-Goldstein, R.; Lucas, D.; Koshland, C.P. Particle-Induced Artifacts in the MTT and LDH Viability Assays. Chem. Res. Toxicol. 2012, 25, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Catechols and quercetin reduce MTT through iron ions: A possible artefact in cell viability assays. Phytother. Res. 1995, 9, 603–605. [Google Scholar] [CrossRef]



| Gene Name | GenBank Accession # | Forward (F) and Reverse (R) Primer Sequences | Reference |
|---|---|---|---|
| Catalase (CAT) | NM_001752.4 | F: 5′-CATCGCCACATGAATGGATA-3′ | [38] |
| R: 5′-CCAACTGGGATGAGAGGGTA-3′ | |||
| Glucose 6-phosphate dehydrogenase (G6PDH) | NM_001042351.3 | F: 5′-GACATCCGCAAACAGAGTGA-3′ | [39] |
| R: 5′-GGAGGCTGCATCATCGTACT-3′ | |||
| Glutamate-cysteine ligase-catalytic subunit (GCL-c) | NM_001197115.2 | F: 5′-CTGGGAGTGATTTCTGCAT-3′ | [40] |
| R: 5′-AGGAGGGGGCTTAAATCTCA-3′ | |||
| Glutamate-cysteine ligase-modifier subunit (GCL-m) | NM_002061.4 | F: 5′-TTTGGTCAGGGAGTTTCCAG-3′ | [40] |
| R: 5′-TGGTTTTACCTGTGCCCACT-3′ | |||
| Glutathione disulfide reductase (GSR) | NM_000637.5 | F: 5′-CCAGCTTAGGAATAACCAGCGATGG-3′ | [41] |
| R: 5′-GTCTTTTTAACCTCCTTGACCTGGGAGAAC-3′ | |||
| Glutathione peroxidase (GPX) 1 | NM_001329503.2 | F: 5′-TTCCCGTGCAACCAGTTTG-3′ | [42] |
| R: 5′-GGACGTACTTGAGGGAATTCAGA-3′ | |||
| Glutathione S-transferase (GST) κ1 | NM_001143679.2 | F: 5′-TCTCCAGATTCCCATCCACTTCCC-3′ | [43] |
| R: 5′-CTGCGGCTCGGTGATGTCTTC-3′ | |||
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | NM_001357943.2 | F: 5′-ATGGGGAAGGTGAAGGTCG-3′ | [17] |
| R: 5′-GGGGTCATTGATGGCAACAA-3′ | |||
| Heme oxygenase (HMOX) 1 | NM_002133.3 | F: 5′-CGGGCCAGCAACAAAGTG-3′ | [44] |
| R: 5′-ACTGTCGCCACCAGAAAGCT-3′ | |||
| Kelch-like ECH-associated protein (KEAP) 1 | NM_012289.4 | F: 5′-CAGAGGTGGTGGTGTTGCTTAT-3′ | [45] |
| R: 5′-AGCTCGTTCATGATGCCAAAG-3′ | |||
| NAD(P)H quinone oxidoreductase (NQO) 1 | NM_001025434.2 | F: 5′-GCACTGATCGTACTGGCTCACT-3′ | This study |
| R: 5′-CCACCACCTCCCATCCTTT-3′ | |||
| Nuclear factor erythroid 2-related factor (NRF) 2 | NM_006164.5 | F: 5′-GAGAGCCCAGTCTTCATTGC-3′ | This study |
| R: 5′-ACTGGTTGGGGTCTTGTGTG-3′ | |||
| Superoxide dismutase (SOD) 1 | NM_000454.5 | F: 5′-AGGGCATCATCAATTTCGAG-3′ | [46] |
| R: 5′-ACATTGCCCAAGTCTCCAAC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, J.W.; Boo, Y.C. Siegesbeckiae Herba Extract and Chlorogenic Acid Ameliorate the Death of HaCaT Keratinocytes Exposed to Airborne Particulate Matter by Mitigating Oxidative Stress. Antioxidants 2021, 10, 1762. https://doi.org/10.3390/antiox10111762
Ha JW, Boo YC. Siegesbeckiae Herba Extract and Chlorogenic Acid Ameliorate the Death of HaCaT Keratinocytes Exposed to Airborne Particulate Matter by Mitigating Oxidative Stress. Antioxidants. 2021; 10(11):1762. https://doi.org/10.3390/antiox10111762
Chicago/Turabian StyleHa, Jae Won, and Yong Chool Boo. 2021. "Siegesbeckiae Herba Extract and Chlorogenic Acid Ameliorate the Death of HaCaT Keratinocytes Exposed to Airborne Particulate Matter by Mitigating Oxidative Stress" Antioxidants 10, no. 11: 1762. https://doi.org/10.3390/antiox10111762
APA StyleHa, J. W., & Boo, Y. C. (2021). Siegesbeckiae Herba Extract and Chlorogenic Acid Ameliorate the Death of HaCaT Keratinocytes Exposed to Airborne Particulate Matter by Mitigating Oxidative Stress. Antioxidants, 10(11), 1762. https://doi.org/10.3390/antiox10111762








