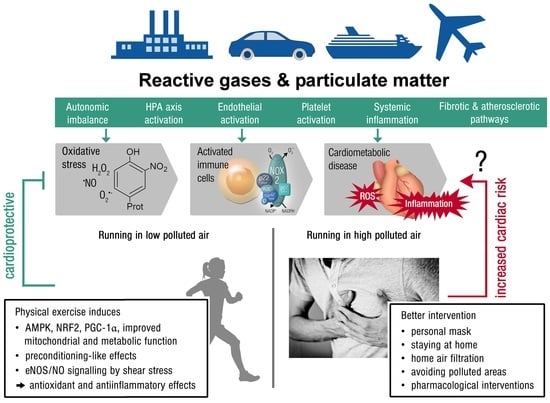
Physical Activity in Polluted Air—Net Benefit or Harm to Cardiovascular Health? A Comprehensive Review
Abstract
:1. Introduction
2. Pathomechanisms of Air Pollution with Focus on Oxidative Stress and Inflammation
| First Author/Year | Population/Cohort | Air Pollutants | Major Outcomes | Ref. |
|---|---|---|---|---|
| Liu, 2021 | 40 chronic obstructive pulmonary disease patients and 75 controls | PAHs | A one fold increase in hydroxylated PAHs was associated with a 4.1–15.1% elevation of malondialdehyde, which was stronger in subjects with impaired lung function. | [18] |
| Abohashem, 2021 | 503 subjects without cardiovascular disease | PM2.5 | Higher PM2.5 was associated with increased risk for major adverse cardiovascular events, mediated by an increase in leucopoietic activity and arterial inflammation. | [21] |
| Ni, 2021 | 740 subjects | PM2.5 | Acute increases in PM2.5 were associated with increased soluble lectin like oxidized LDL receptor-1, but not with nitrite. | [46] |
| Nassan, 2021 | 456 men | PM2.5 species | Acute increases in PM2.5 species were associated with metabolic pathways involved in inflammation, oxidative stress, immunity, and nucleic acid damage and repair. | [19] |
| Mann, 2021 | 299 children | Traffic related air pollutants (sum of PAH456, NO2, elemental carbon, PM2.5) | Acute increases in traffic related air pollutants were associated with 8-isoprostane. | [22] |
| Prunicki, 2020 | 100 subjects | PM2.5, NO, NO2, CO, PAHs | Air pollutants were associated with oxidative stress, acute inflammation, altered hemostasis, endothelial dysfunction, monocyte enrichment, and diastolic blood pressure. | [23] |
| Riggs, 2020 | 100 subjects | PM2.5 | A 10 μg/m3 increase in PM2.5 was associated with a 12.4% decrease in reactive hyperemia index (95% CI −21.0–−2.7). Increased PM2.5 was associated with elevated F-2 isoprostane metabolite, angiopoietin 1, vascular endothelial growth factor, placental growth factor, intracellular adhesion molecule-1, and matrix metalloproteinase-9 as well as reduced vascular adhesion molecule-1. | [47] |
| Li, 2019 | 73 subjects | PM2.5, BC, NO2, CO | Increases in air pollutants were associated with reductions in circulating high density lipoprotein cholesterol and apolipoprotein A-I, as well as elevations in HDL oxidation index, oxidized LDL, malondialdehyde, and C-reactive protein. | [48] |
| Lin, 2019 | 26 subjects | PAHs | Increases in 5-, 12-, and 15-hydroxyeicosatetraenoic acid, as well as 9- and 13-hydroxyoctadecadienoic acid, were observed. Decreases in paraoxonase and arylesterase, as well increases in C-reactive protein and fibrinogen, were observed. | [49] |
| Balmes, 2019 | 87 subjects | O3 | Acute O3 exposure did not alter C-reactive protein, monocyte–platelet conjugates, and microparticle associated tissue factor activity, whereas increases in endothelin-1 and decreases in nitrotyrosine were observed. | [50] |
| Han, 2019 | 60 subjects with prediabetes and 60 healthy subjects | PM2.5 | Acute exposure to PM2.5 resulted in increased exhaled NO, white blood cells, neutrophils, interleukin-1α, and glycated hemoglobin. Compared to healthy subjects, prediabetic subjects displayed pronounced PM2.5 associated systemic inflammation, elevated systolic and diastolic blood pressure, impaired endothelial function, and elevated fasting glucose. | [51] |
| Xia, 2019 | 215 pregnant women | PM2.5 | Acute increases in PM2.5 and lead constituent were associated with endothelial dysfunction (increased endothelin-1, E-selectin, and intracellular adhesion molecule-1) and inflammation (increased interleukin-1β, interleukin-6, tumor necrosis factor-α). Endothelial dysfunction and elevated inflammation were partially mediated by the effect of PM2.5 and lead constituent on blood pressure. | [52] |
| Li, 2019 | 3820 subjects | PM2.5, BC, O3, sulfate, NOX | Negative associations of acute PM2.5 and BC with P-selectin, of O3 with monocyte chemoattractant protein 1, and of sulfate and NOx with osteoprotegerin were found. | [53] |
| Li, 2017 | 3996 subjects | PM2.5, sulfate, NOx, BC, O3 | Acute increases in PM2.5 and sulfate were associated with increased C-reactive protein, which was also true for NOx in case of interleukin-6 and for BC, sulfate, and O3 in case of tumor necrosis factor receptor 2. Conversely, BC, sulfate, and NOx were negatively associated with fibrinogen, and sulfate was negatively associated with tumor necrosis factor α. | [24] |
| Mirowsky, 2017 | 13 subjects with coronary artery disease | O3 | Per acute IQR increase in O3, changes in tissue plasminogen factor (6.6%, 95% CI 0.4–13.2), plasminogen activator inhibitor-1 (40.5%, 95% CI 8.7–81.6), neutrophils (8.7%, 95% CI 1.5–16.4), monocytes (10.2%, 95% CI 1.0–20.1), interleukin-6 (15.9%, 95% CI 3.6–29.6), large artery elasticity index (−19.5%, 95% CI −34.0–−1.7), and the baseline diameter of the brachial artery (−2.5%, 95% CI −5.0–0.1) were observed. | [54] |
| Pope 3rd, 2016 | 24 subjects | PM2.5 | Episodic increases in PM2.5 were associated with increased endothelial cell apoptosis, an anti-angiogenic plasma profile, and elevated circulating monocytes, and T, but not B, lymphocytes. | [55] |
| Wu, 2016 | 89 subjects | PM2.5, NO2 | Acute increases in PM2.5 were associated with brachial–ankle pulse wave velocity, whereas no association was found for NO2. NO2 was associated with increased C-reactive protein. | [56] |
3. Key Mechanisms of Antioxidant and Anti-Inflammatory Effects of Physical Activity
4. Recent Epidemiological Evidence on the Association between Physical Activity, Long Term Exposure to Air Pollution, and (Cardiovascular) Health
5. Evidence on the (Cardiovascular) Health Effects of Physical Activity and Air Pollution from Animal Studies
6. Mitigation Strategies and Practical Recommendations

7. Gaps in Current Knowledge and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ApoE−/− | Apolipoprotein E knockout |
| BC | Black carbon |
| BAL | Bronchoalveolar lavage |
| Ca2+ | Tetrahydrobiopterin |
| Cat | Catalase |
| CD36 | Cluster of differentiation 36 |
| CHD | Coronary heart disease |
| CI | Confidence interval |
| CO | Carbon monoxide |
| Cox2/Cox4 | Cytochrome C oxidase 2/4 |
| CVD | Cardiovascular disease |
| CXCL1/KC | Chemokine (C-X-C motif) ligand 1 |
| eNOS | Endothelial nitric oxide synthase |
| ER | Endoplasmic reticulum |
| GEMM | Global Exposure-Mortality Model |
| GPx | Glutathione peroxidase |
| HDL | High-density lipoprotein |
| eHSP70/iHSP70 | Extracellular/intracellular heat shock protein 70 |
| HR | Hazard ratio |
| IFNγ | Interferon gamma |
| iNOS | Inducible nitric oxide synthase |
| IHD | Ischemic heart disease |
| IL-6/IL-1β | Interleukin 6/1β |
| IQR | Interquartile range |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehyde |
| MET-min/week | Minutes of metabolic equivalent tasks per week |
| MI | Myocardial infarction |
| Mn-SOD | Manganese superoxide dismutase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOX | NADPH oxidase |
| NO | Nitric oxide/nitrogen monoxide |
| NO2 | Nitrogen dioxide |
| NOx | Nitrogen oxides |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| O2− | Superoxide anion |
| O3 | Ozone |
| ONOO− | Peroxynitrite |
| OR | Odds ratio |
| PA | Physical activity |
| PAHs | Polycyclic aromatic hydrocarbons |
| PGC-1α | Peroxisome proliferator activated receptor gamma coactivator 1-alpha |
| PM(diameter size) | Particulate matter |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TiO2 | Titanium dioxide |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| VCAM-1 | Vascular cell adhesion protein 1 |
| VOCs | Volatile organic compounds |
| WHO | World Health Organization |
References
- Hallal, P.C.; Victora, C.G.; Azevedo, M.R.; Wells, J.C. Adolescent physical activity and health: A systematic review. Sports Med. 2006, 36, 1019–1030. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Liu, X.; Tu, R.; Dong, X.; Zhai, Z.; Mao, Z.; Huo, W.; Chen, G.; Xiang, H.; Guo, Y.; et al. Long-term exposure to ambient air pollution attenuated the association of physical activity with metabolic syndrome in rural Chinese adults: A cross-sectional study. Environ. Int. 2020, 136, 105459. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Paul, K.; Arah, O.A.; Mayeda, E.R.; Wu, J.; Lee, E.; Shih, I.F.; Su, J.; Jerrett, M.; Haan, M.; et al. Air pollution, noise exposure, and metabolic syndrome—A cohort study in elderly Mexican-Americans in Sacramento area. Environ. Int. 2020, 134, 105269. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Fenichel, P. Endocrine disruptors: New players in the pathophysiology of type 2 diabetes? Diabetes Metab. 2015, 41, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chan, T.C.; Huang, Y.J.; Pan, W.C. The Association between Noise Exposure and Metabolic Syndrome: A Longitudinal Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 4236. [Google Scholar] [CrossRef]
- World Health Organization. 9 out of 10 People Worldwide Breathe Polluted Air, but More Countries Are Taking Action. Available online: https://www.who.int/news-room/detail/02-05-2018-9-out-of-10-peopleworldwide-breathe-polluted-air-but-more-countries-are-taking-action (accessed on 6 August 2021).
- Tainio, M.; Jovanovic Andersen, Z.; Nieuwenhuijsen, M.J.; Hu, L.; de Nazelle, A.; An, R.; Garcia, L.M.T.; Goenka, S.; Zapata-Diomedi, B.; Bull, F.; et al. Air pollution, physical activity and health: A mapping review of the evidence. Environ. Int. 2021, 147, 105954. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Sbihi, H.; Tamburic, L.; Brauer, M.; Frank, L.D.; Davies, H.W. Association of Long-Term Exposure to Transportation Noise and Traffic-Related Air Pollution with the Incidence of Diabetes: A Prospective Cohort Study. Environ. Health Perspect. 2017, 125, 087025. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ying, Z.; Harkema, J.; Sun, Q.; Rajagopalan, S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol. Pathol. 2013, 41, 361–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopalan, S.; Brook, R.D. Air pollution and type 2 diabetes: Mechanistic insights. Diabetes 2012, 61, 3037–3045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 6 August 2021).
- World Health Organization. Physical Activity. Available online: https://www.who.int/westernpacific/health-topics/physical-activity (accessed on 6 August 2021).
- Lelieveld, J.; Klingmuller, K.; Pozzer, A.; Poschl, U.; Fnais, M.; Daiber, A.; Munzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019, 40, 1590–1596. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Personal Interventions and Risk Communication on air Pollution. Available online: https://www.who.int/publications/i/item/9789240000278 (accessed on 6 August 2021).
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Munzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef] [PubMed]
- Hahad, O.; Frenis, K.; Kuntic, M.; Daiber, A.; Munzel, T. Accelerated Aging and Age-Related Diseases (CVD and Neurological) Due to Air Pollution and Traffic Noise Exposure. Int. J. Mol. Sci. 2021, 22, 2419. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Qiu, X.; Zhang, H.; Lu, X.; Li, H.; Chen, W.; Zhang, L.; Que, C.; Zhu, T. Association between exposure to polycyclic aromatic hydrocarbons and lipid peroxidation in patients with chronic obstructive pulmonary disease. Sci. Total Environ. 2021, 780, 146660. [Google Scholar] [CrossRef]
- Nassan, F.L.; Wang, C.; Kelly, R.S.; Lasky-Su, J.A.; Vokonas, P.S.; Koutrakis, P.; Schwartz, J.D. Ambient PM2.5 species and ultrafine particle exposure and their differential metabolomic signatures. Environ. Int. 2021, 151, 106447. [Google Scholar] [CrossRef]
- Daiber, A.; Kuntic, M.; Hahad, O.; Delogu, L.G.; Rohrbach, S.; Di Lisa, F.; Schulz, R.; Munzel, T. Effects of air pollution particles (ultrafine and fine particulate matter) on mitochondrial function and oxidative stress—Implications for cardiovascular and neurodegenerative diseases. Arch. Biochem. Biophys. 2020, 696, 108662. [Google Scholar] [CrossRef] [PubMed]
- Abohashem, S.; Osborne, M.T.; Dar, T.; Naddaf, N.; Abbasi, T.; Ghoneem, A.; Radfar, A.; Patrich, T.; Oberfeld, B.; Tung, B.; et al. A leucopoietic-arterial axis underlying the link between ambient air pollution and cardiovascular disease in humans. Eur. Heart J. 2021, 42, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.K.; Lutzker, L.; Holm, S.M.; Margolis, H.G.; Neophytou, A.M.; Eisen, E.A.; Costello, S.; Tyner, T.; Holland, N.; Tindula, G.; et al. Traffic-related air pollution is associated with glucose dysregulation, blood pressure, and oxidative stress in children. Environ. Res. 2021, 195, 110870. [Google Scholar] [CrossRef] [PubMed]
- Prunicki, M.; Cauwenberghs, N.; Ataam, J.A.; Movassagh, H.; Kim, J.B.; Kuznetsova, T.; Wu, J.C.; Maecker, H.; Haddad, F.; Nadeau, K. Immune biomarkers link air pollution exposure to blood pressure in adolescents. Environ. Health 2020, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dorans, K.S.; Wilker, E.H.; Rice, M.B.; Ljungman, P.L.; Schwartz, J.D.; Coull, B.A.; Koutrakis, P.; Gold, D.R.; Keaney, J.F., Jr.; et al. Short-Term Exposure to Ambient Air Pollution and Biomarkers of Systemic Inflammation: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1793–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prows, D.R.; Shertzer, H.G.; Daly, M.J.; Sidman, C.L.; Leikauf, G.D. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat. Genet. 1997, 17, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Marchini, T.; Zirlik, A.; Wolf, D. Pathogenic Role of Air Pollution Particulate Matter in Cardiometabolic Disease: Evidence from Mice and Humans. Antioxid. Redox Signal. 2020, 33, 263–279. [Google Scholar] [CrossRef]
- Sunyer, J.; Ballester, F.; Tertre, A.L.; Atkinson, R.; Ayres, J.G.; Forastiere, F.; Forsberg, B.; Vonk, J.M.; Bisanti, L.; Tenias, J.M.; et al. The association of daily sulfur dioxide air pollution levels with hospital admissions for cardiovascular diseases in Europe (The Aphea-II study). Eur. Heart J. 2003, 24, 752–760. [Google Scholar] [CrossRef]
- Chuang, G.C.; Yang, Z.; Westbrook, D.G.; Pompilius, M.; Ballinger, C.A.; White, C.R.; Krzywanski, D.M.; Postlethwait, E.M.; Ballinger, S.W. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L209–L216. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Colombo, E.S.; Lucas, S.N.; Hall, P.R.; Febbraio, M.; Paffett, M.L.; Campen, M.J. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol. Sci. 2013, 134, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Allen, K.; Rao, X.; Ying, Z.; Braunstein, Z.; Kankanala, S.R.; Xia, C.; Wang, X.; Bramble, L.A.; Wagner, J.G.; et al. Repeated ozone exposure exacerbates insulin resistance and activates innate immune response in genetically susceptible mice. Inhal. Toxicol. 2016, 28, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Hamade, A.K.; Rabold, R.; Tankersley, C.G. Adverse cardiovascular effects with acute particulate matter and ozone exposures: Interstrain variation in mice. Environ. Health Perspect. 2008, 116, 1033–1039. [Google Scholar] [CrossRef]
- Munzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018, 39, 3543–3550. [Google Scholar] [CrossRef] [Green Version]
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 2011, 108, 716–726. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Yue, P.; Ying, Z.; Cardounel, A.J.; Brook, R.D.; Devlin, R.; Hwang, J.S.; Zweier, J.L.; Chen, L.C.; Rajagopalan, S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1760–1766. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Arora, R.C.; Hiebert, B.M.; Lerner, B.; Szwajcer, A.; McDonald, K.; Rigatto, C.; Komenda, P.; Sood, M.M.; Tangri, N. Non-invasive endothelial function testing and the risk of adverse outcomes: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 736–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Munzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Kwon, T.G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef] [Green Version]
- Crow, J.P.; Beckman, J.S. Reaction between Nitric Oxide, Superoxide, and Peroxynitrite: Footprints of Peroxynitrite in Vivo. Adv. Pharmacol. 1995, 35, 17–43. [Google Scholar]
- Sun, Q.; Wang, A.; Jin, X.; Natanzon, A.; Duquaine, D.; Brook, R.D.; Aguinaldo, J.G.; Fayad, Z.A.; Fuster, V.; Lippmann, M.; et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005, 294, 3003–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherng, T.W.; Paffett, M.L.; Jackson-Weaver, O.; Campen, M.J.; Walker, B.R.; Kanagy, N.L. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ. Health Perspect. 2011, 119, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Davel, A.P.; Lemos, M.; Pastro, L.M.; Pedro, S.C.; de Andre, P.A.; Hebeda, C.; Farsky, S.H.; Saldiva, P.H.; Rossoni, L.V. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 2012, 295, 39–46. [Google Scholar] [CrossRef]
- Ying, Z.; Xu, X.; Chen, M.; Liu, D.; Zhong, M.; Chen, L.C.; Sun, Q.; Rajagopalan, S. A synergistic vascular effect of airborne particulate matter and nickel in a mouse model. Toxicol. Sci. 2013, 135, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Rao, X.; Zhong, J.; Maiseyeu, A.; Gopalakrishnan, B.; Villamena, F.A.; Chen, L.C.; Harkema, J.R.; Sun, Q.; Rajagopalan, S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ. Res. 2014, 115, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Courtois, A.; Andujar, P.; Ladeiro, Y.; Baudrimont, I.; Delannoy, E.; Leblais, V.; Begueret, H.; Galland, M.A.; Brochard, P.; Marano, F.; et al. Impairment of NO-dependent relaxation in intralobar pulmonary arteries: Comparison of urban particulate matter and manufactured nanoparticles. Environ. Health Perspect. 2008, 116, 1294–1299. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Tracy, R.P.; Cornell, E.; Kaufman, J.D.; Szpiro, A.A.; Campen, M.J.; Vedal, S. Short-term exposure to air pollution and biomarkers of cardiovascular effect: A repeated measures study. Environ. Pollut. 2021, 279, 116893. [Google Scholar] [CrossRef]
- Riggs, D.W.; Zafar, N.; Krishnasamy, S.; Yeager, R.; Rai, S.N.; Bhatnagar, A.; O’Toole, T.E. Exposure to airborne fine particulate matter is associated with impaired endothelial function and biomarkers of oxidative stress and inflammation. Environ. Res. 2020, 180, 108890. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Xu, H.; Brook, R.D.; Liu, S.; Yi, T.; Wang, Y.; Feng, B.; Zhao, M.; Wang, X.; et al. Ambient Air Pollution Is Associated With HDL (High-Density Lipoprotein) Dysfunction in Healthy Adults. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Ramanathan, G.; Zhu, Y.; Yin, F.; Rea, N.D.; Lu, X.; Tseng, C.H.; Faull, K.F.; Yoon, A.J.; Jerrett, M.; et al. Pro-Oxidative and Proinflammatory Effects After Traveling from Los Angeles to Beijing: A Biomarker-Based Natural Experiment. Circulation 2019, 140, 1995–2004. [Google Scholar] [CrossRef]
- Balmes, J.R.; Arjomandi, M.; Bromberg, P.A.; Costantini, M.G.; Dagincourt, N.; Hazucha, M.J.; Hollenbeck-Pringle, D.; Rich, D.Q.; Stark, P.; Frampton, M.W. Ozone effects on blood biomarkers of systemic inflammation, oxidative stress, endothelial function, and thrombosis: The Multicenter Ozone Study in oldEr Subjects (MOSES). PLoS ONE 2019, 14, e0222601. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.; Li, W.; Chen, X.; Xue, T.; Chen, W.; Fan, Y.; Qiu, X.; Zhu, T. Susceptibility of prediabetes to the health effect of air pollution: A community-based panel study with a nested case-control design. Environ. Health 2019, 18, 65. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Zhou, Y.; Zhu, Q.; Zhao, Y.; Wang, Y.; Ge, W.; Yang, Q.; Zhao, Y.; Wang, P.; Si, J.; et al. Personal exposure to PM2.5 constituents associated with gestational blood pressure and endothelial dysfunction. Environ. Pollut. 2019, 250, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dorans, K.S.; Wilker, E.H.; Rice, M.B.; Ljungman, P.L.; Schwartz, J.D.; Coull, B.A.; Koutrakis, P.; Gold, D.R.; Keaney, J.F., Jr.; et al. Short-term exposure to ambient air pollution and circulating biomarkers of endothelial cell activation: The Framingham Heart Study. Environ. Res. 2019, 171, 36–43. [Google Scholar] [CrossRef]
- Mirowsky, J.E.; Carraway, M.S.; Dhingra, R.; Tong, H.; Neas, L.; Diaz-Sanchez, D.; Cascio, W.; Case, M.; Crooks, J.; Hauser, E.R.; et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ. Health 2017, 16, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.F.; Shen, F.H.; Li, Y.R.; Tsao, T.M.; Tsai, M.J.; Chen, C.C.; Hwang, J.S.; Hsu, S.H.; Chao, H.; Chuang, K.J.; et al. Association of short-term exposure to fine particulate matter and nitrogen dioxide with acute cardiovascular effects. Sci. Total Environ. 2016, 569–570, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.C.E.; Tittlbach, S.; Bos, K.; Woll, A. Different Types of Physical Activity and Fitness and Health in Adults: An 18-Year Longitudinal Study. Biomed. Res. Int. 2017, 2017, 1785217. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.; Deiseroth, A.; Hahad, O.; Diestelmeier, S.; Schulz, A.; Daubenbuchel, A.; Gori, T.; Binder, H.; Pfeiffer, N.; Prochaska, J.; et al. Domains of Physical Activity in Relation to Stiffness Index in the General Population. J. Am. Heart Assoc. 2021, 10, e020930. [Google Scholar] [CrossRef] [PubMed]
- Mury, P.; Chirico, E.N.; Mura, M.; Millon, A.; Canet-Soulas, E.; Pialoux, V. Oxidative Stress and Inflammation, Key Targets of Atherosclerotic Plaque Progression and Vulnerability: Potential Impact of Physical Activity. Sports Med. 2018, 48, 2725–2741. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Montgomery, P.S.; Casanegra, A.I.; Silva-Palacios, F.; Ungvari, Z.; Csiszar, A. Association between gait characteristics and endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. Age 2016, 38, 64. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.W.; Montgomery, P.S.; Zhao, Y.D.; Silva-Palacios, F.; Ungvari, Z.; Csiszar, A.; Sonntag, W.E. Association between daily walking and antioxidant capacity in patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2017, 65, 1762–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, E.S. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology 2002, 13, 561–568. [Google Scholar] [CrossRef]
- Mora, S.; Lee, I.M.; Buring, J.E.; Ridker, P.M. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA 2006, 295, 1412–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Ye, X.; Wang, J.; Qi, Q.; Franco, O.H.; Rennie, K.L.; Pan, A.; Li, H.; Liu, Y.; Hu, F.B.; et al. Associations of physical activity with inflammatory factors, adipocytokines, and metabolic syndrome in middle-aged and older chinese people. Circulation 2009, 119, 2969–2977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmefors, H.; DuttaRoy, S.; Rundqvist, B.; Borjesson, M. The effect of physical activity or exercise on key biomarkers in atherosclerosis—A systematic review. Atherosclerosis 2014, 235, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Glenn, J.M.; Bott, N.; Masmoudi, L.; Hakim, A.; Chtourou, H.; Driss, T.; Hoekelmann, A.; et al. Effects of Aerobic-, Anaerobic- and Combined-Based Exercises on Plasma Oxidative Stress Biomarkers in Healthy Untrained Young Adults. Int. J. Environ. Res. Public Health 2020, 17, 2601. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Andreadou, I.; Efentakis, P.; Frenis, K.; Daiber, A.; Schulz, R. Thiol-based redox-active proteins as cardioprotective therapeutic agents in cardiovascular diseases. Basic Res. Cardiol. 2021, 116, 44. [Google Scholar] [CrossRef]
- Dillard, C.J.; Litov, R.E.; Savin, W.M.; Dumelin, E.E.; Tappel, A.L. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 45, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Brady, P.S.; Brady, L.J.; Ullrey, D.E. Selenium, vitamin E and the response to swimming stress in the rat. J. Nutr. 1979, 109, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Jones, D.A.; Edwards, R.H. Vitamin E and skeletal muscle. In Biology of Vitamin E; Pitman Books: London, UK, 1983; pp. 224–239. [Google Scholar] [CrossRef]
- Quintanilha, A.T.; Packer, L. Vitamin E, physical exercise and tissue oxidative damage. In Biology of Vitamin E; Pitman Books: London, UK, 1983; pp. 56–69. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, M.; Hafstad, A.D.; Nabeebaccus, A.A.; Catibog, N.; Logan, A.; Smyrnias, I.; Hansen, S.S.; Lanner, J.; Schroder, K.; Murphy, M.P.; et al. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. eLife 2018, 7, e41044. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio Alves, J.; Matta Pereira, L.; Cabral Coutinho do Rego Monteiro, I.; Pontes Dos Santos, L.H.; Soares Marreiros Ferraz, A.; Carneiro Loureiro, A.C.; Calado Lima, C.; Leal-Cardoso, J.H.; Pires Carvalho, D.; Soares Fortunato, R.; et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants 2020, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vina, J.; Gimeno, A.; Sastre, J.; Desco, C.; Asensi, M.; Pallardo, F.V.; Cuesta, A.; Ferrero, J.A.; Terada, L.S.; Repine, J.E. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life 2000, 49, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Daiber, A.; Di Lisa, F.; Oelze, M.; Kroller-Schon, S.; Steven, S.; Schulz, E.; Munzel, T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017, 174, 1670–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta 2010, 1797, 897–906. [Google Scholar] [CrossRef] [Green Version]
- Schulz, E.; Wenzel, P.; Munzel, T.; Daiber, A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014, 20, 308–324. [Google Scholar] [CrossRef]
- Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.J.; Vasilaki, A.; McArdle, A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic. Biol. Med. 2016, 98, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Cartier, L.J.; Chen, M.; Holloszy, J.O. Superoxide dismutase and catalase in skeletal muscle: Adaptive response to exercise. J. Gerontol. 1985, 40, 281–286. [Google Scholar] [CrossRef]
- Kanter, M.M.; Hamlin, R.L.; Unverferth, D.V.; Davis, H.W.; Merola, A.J. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J. Appl. Physiol. 1985, 59, 1298–1303. [Google Scholar] [CrossRef]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med. Sci. Sports Exerc. 1993, 25, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Fu, R.; Mitchell, E.W. Glutathione and antioxidant enzymes in skeletal muscle: Effects of fiber type and exercise intensity. J. Appl. Physiol. 1992, 73, 1854–1859. [Google Scholar] [CrossRef]
- Ji, L.L.; Stratman, F.W.; Lardy, H.A. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch. Biochem. Biophys. 1988, 263, 150–160. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Simpson, T.; Sexton, W.L.; Brown, O.R.; Smith, J.K.; Korthuis, R.J. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J. Appl. Physiol. 1990, 68, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Kvandova, M.; Daiber, A.; Stamm, P.; Frenis, K.; Schulz, E.; Munzel, T.; Kroller-Schon, S. The AMP-Activated Protein Kinase Plays a Role in Antioxidant Defense and Regulation of Vascular Inflammation. Antioxidants 2020, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Kroller-Schon, S.; Jansen, T.; Hauptmann, F.; Schuler, A.; Heeren, T.; Hausding, M.; Oelze, M.; Viollet, B.; Keaney, J.F., Jr.; Wenzel, P.; et al. alpha1AMP-activated protein kinase mediates vascular protective effects of exercise. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1632–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, P.; Kossmann, S.; Munzel, T.; Daiber, A. Redox regulation of cardiovascular inflammation—Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2017, 109, 48–60. [Google Scholar] [CrossRef]
- Sen, C.K. Glutathione homeostasis in response to exercise training and nutritional supplements. Mol. Cell Biochem. 1999, 196, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Li, H.; Laher, I. Oxidative Stress: A Unifying Mechanism for Cell Damage Induced by Noise, (Water-Pipe) Smoking, and Emotional Stress-Therapeutic Strategies Targeting Redox Imbalance. Antioxid. Redox Signal. 2018, 28, 741–759. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbidi, S.; Daiber, A.; Korac, B.; Li, H.; Essop, M.F.; Laher, I. Health Benefits of Fasting and Caloric Restriction. Curr. Diab. Rep. 2017, 17, 123. [Google Scholar] [CrossRef] [PubMed]
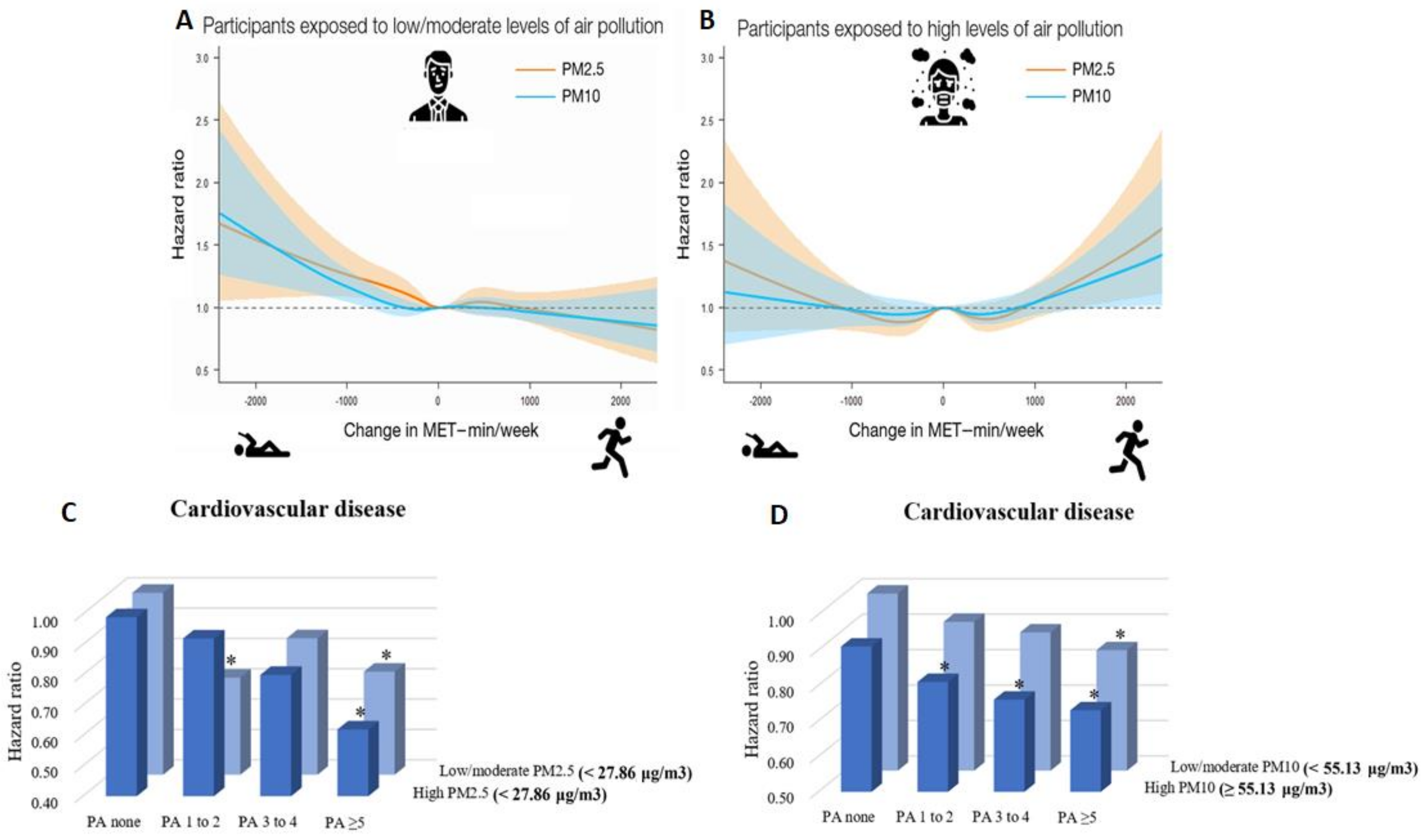
- Kim, S.R.; Choi, S.; Kim, K.; Chang, J.; Kim, S.M.; Cho, Y.; Oh, Y.H.; Lee, G.; Son, J.S.; Kim, K.H.; et al. Association of the combined effects of air pollution and changes in physical activity with cardiovascular disease in young adults. Eur. Heart J. 2021, 42, 2487–2497. [Google Scholar] [CrossRef]
- Munzel, T.; Hahad, O.; Daiber, A. Running in polluted air is a two-edged sword—Physical exercise in low air pollution areas is cardioprotective but detrimental for the heart in high air pollution areas. Eur. Heart J. 2021, 42, 2498–2500. [Google Scholar] [CrossRef]
- Kim, S.R.; Choi, S.; Keum, N.; Park, S.M. Combined Effects of Physical Activity and Air Pollution on Cardiovascular Disease: A Population-Based Study. J. Am. Heart Assoc. 2020, 9, e013611. [Google Scholar] [CrossRef]
- Raza, W.; Krachler, B.; Forsberg, B.; Sommar, J.N. Does Physical Activity Modify the Association between Air Pollution and Recurrence of Cardiovascular Disease? Int. J. Environ. Res. Public Health 2021, 18, 2631. [Google Scholar] [CrossRef]
- Raza, W.; Krachler, B.; Forsberg, B.; Sommar, J.N. Air pollution, physical activity and ischaemic heart disease: A prospective cohort study of interaction effects. BMJ Open 2021, 11, e040912. [Google Scholar] [CrossRef]
- Tu, R.; Hou, J.; Liu, X.; Li, R.; Dong, X.; Pan, M.; Mao, Z.; Huo, W.; Chen, G.; Guo, Y.; et al. Physical activity attenuated association of air pollution with estimated 10-year atherosclerotic cardiovascular disease risk in a large rural Chinese adult population: A cross-sectional study. Environ. Int. 2020, 140, 105819. [Google Scholar] [CrossRef]
- Sun, S.; Cao, W.; Qiu, H.; Ran, J.; Lin, H.; Shen, C.; Siu-Yin Lee, R.; Tian, L. Benefits of physical activity not affected by air pollution: A prospective cohort study. Int. J. Epidemiol. 2020, 49, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.G.; Laden, F.; James, P.; Rimm, E.B.; Rexrode, K.M.; Hart, J.E. Interaction between Long-Term Exposure to Fine Particulate Matter and Physical Activity, and Risk of Cardiovascular Disease and Overall Mortality in U.S. Women. Environ. Health Perspect. 2020, 128, 127012. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Choi, D.; Choi, S.; Kim, K.; Lee, G.; Son, J.S.; Kim, K.H.; Park, S.M. Association of combined effects of physical activity and air pollution with diabetes in older adults. Environ. Int. 2020, 145, 106161. [Google Scholar] [CrossRef]
- Guo, C.; Yang, H.T.; Chang, L.Y.; Bo, Y.; Lin, C.; Zeng, Y.; Tam, T.; Lau, A.K.H.; Hoek, G.; Lao, X.Q. Habitual exercise is associated with reduced risk of diabetes regardless of air pollution: A longitudinal cohort study. Diabetologia 2021, 64, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Petrowski, K.; Buhrer, S.; Strauss, B.; Decker, O.; Brahler, E. Examining air pollution (PM10), mental health and well-being in a representative German sample. Sci. Rep. 2021, 11, 18436. [Google Scholar] [CrossRef]
- Goeminne, P.C.; Bijnens, E.; Nemery, B.; Nawrot, T.S.; Dupont, L.J. Impact of traffic related air pollution indicators on non-cystic fibrosis bronchiectasis mortality: A cohort analysis. Respir. Res. 2014, 15, 108. [Google Scholar] [CrossRef] [Green Version]
- Majewski, S.; Piotrowski, W.J. Air Pollution-An Overlooked Risk Factor for Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2020, 10, 77. [Google Scholar] [CrossRef]
- Pereira, P.R.; Oliveira-Junior, M.C.; Mackenzie, B.; Chiovatto, J.E.; Matos, Y.; Greiffo, F.R.; Rigonato-Oliveira, N.C.; Brugemman, T.R.; Delle, H.; Idzko, M.; et al. Exercise Reduces Lung Fibrosis Involving Serotonin/Akt Signaling. Med. Sci. Sports Exerc. 2016, 48, 1276–1284. [Google Scholar] [CrossRef] [Green Version]
- Silva-Renno, A.; Baldivia, G.C.; Oliveira-Junior, M.C.; Brandao-Rangel, M.A.R.; El-Mafarjeh, E.; Dolhnikoff, M.; Mauad, T.; Britto, J.M.; Saldiva, P.H.N.; Oliveira, L.V.F.; et al. Exercise Performed Concomitantly with Particulate Matter Exposure Inhibits Lung Injury. Int. J. Sports Med. 2018, 39, 133–140. [Google Scholar] [CrossRef]
- Millerick-May, M.L.; Karmaus, W.; Derksen, F.J.; Berthold, B.; Robinson, N.E. Airborne particulates (PM10) and tracheal mucus: A case-control study at an American Thoroughbred racetrack. Equine Vet. J. 2015, 47, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.P.; Toledo, A.C.; Silva, L.B.; Almeida, F.M.; Damaceno-Rodrigues, N.R.; Caldini, E.G.; Santos, A.B.; Rivero, D.H.; Hizume, D.C.; Lopes, F.D.; et al. Anti-inflammatory effects of aerobic exercise in mice exposed to air pollution. Med. Sci. Sports Exerc. 2012, 44, 1227–1234. [Google Scholar] [CrossRef]
- Long, N.C.; Suh, J.; Morrow, J.D.; Schiestl, R.H.; Murthy, G.G.; Brain, J.D.; Frei, B. Ozone causes lipid peroxidation but little antioxidant depletion in exercising and nonexercising hamsters. J. Appl. Physiol. 2001, 91, 1694–1700. [Google Scholar] [CrossRef]
- Bhalla, D.K.; Mannix, R.C.; Lavan, S.M.; Phalen, R.F.; Kleinman, M.T.; Crocker, T.T. Tracheal and bronchoalveolar permeability changes in rats inhaling oxidant atmospheres during rest or exercise. J. Toxicol. Environ. Health 1987, 22, 417–437. [Google Scholar] [CrossRef] [PubMed]
- Decaesteker, T.; Vanhoffelen, E.; Trekels, K.; Jonckheere, A.C.; Cremer, J.; Vanstapel, A.; Dilissen, E.; Bullens, D.; Dupont, L.J.; Vanoirbeek, J.A. Differential effects of intense exercise and pollution on the airways in a murine model. Part. Fibre Toxicol. 2021, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [Green Version]
- Willerson, J.T.; Ridker, P.M. Inflammation as a cardiovascular risk factor. Circulation 2004, 109, II2-10. [Google Scholar] [CrossRef] [Green Version]
- Farah, C.; Meyer, G.; Andre, L.; Boissiere, J.; Gayrard, S.; Cazorla, O.; Richard, S.; Boucher, F.; Tanguy, S.; Obert, P.; et al. Moderate exercise prevents impaired Ca2+ handling in heart of CO-exposed rat: Implication for sensitivity to ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H2076–H2081. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.S.; Dos Santos, A.B.; Beber, L.C.C.; Basso, R.D.B.; Sulzbacher, L.M.; Goettems-Fiorin, P.B.; Frizzo, M.N.; Rhoden, C.R.; Ludwig, M.S.; Heck, T.G. Exercise Training under Exposure to Low Levels of Fine Particulate Matter: Effects on Heart Oxidative Stress and Extra-to-Intracellular HSP70 Ratio. Oxid. Med. Cell Longev. 2017, 2017, 9067875. [Google Scholar] [CrossRef]
- Kostrycki, I.M.; Wildner, G.; Donato, Y.H.; Dos Santos, A.B.; Beber, L.C.C.; Frizzo, M.N.; Ludwig, M.S.; Keane, K.N.; Cruzat, V.; Rhoden, C.R.; et al. Effects of High-Fat Diet on eHSP72 and Extra-to-Intracellular HSP70 Levels in Mice Submitted to Exercise under Exposure to Fine Particulate Matter. J. Diabetes Res. 2019, 2019, 4858740. [Google Scholar] [CrossRef]
- Marmett, B.; Nunes, R.B.; de Souza, K.S.; Lago, P.D.; Rhoden, C.R. Aerobic training reduces oxidative stress in skeletal muscle of rats exposed to air pollution and supplemented with chromium picolinate. Redox Rep. 2018, 23, 146–152. [Google Scholar] [CrossRef]
- Feng, B.; Qi, R.; Gao, J.; Wang, T.; Xu, H.; Zhao, Q.; Wu, R.; Song, X.; Guo, J.; Zheng, L.; et al. Exercise training prevented endothelium dysfunction from particulate matter instillation in Wistar rats. Sci. Total Environ. 2019, 694, 133674. [Google Scholar] [CrossRef]
- Olivo, C.R.; Castro, T.B.P.; Riane, A.; Regonha, T.; Rivero, D.; Vieira, R.P.; Saraiva-Romanholo, B.M.; Lopes, F.; Tiberio, I.; Martins, M.A.; et al. The effects of exercise training on the lungs and cardiovascular function of animals exposed to diesel exhaust particles and gases. Environ. Res. 2021, 203, 111768. [Google Scholar] [CrossRef] [PubMed]
- Uysal, N.; Kiray, M.; Sisman, A.R.; Baykara, B.; Aksu, I.; Dayi, A.; Gencoglu, C.; Evren, M.; Buyuk, E.; Cetin, F.; et al. Effects of exercise and poor indoor air quality on learning, memory and blood IGF-1 in adolescent mice. Biotech. Histochem. 2014, 89, 126–135. [Google Scholar] [CrossRef]
- Toledo, A.C.; Magalhaes, R.M.; Hizume, D.C.; Vieira, R.P.; Biselli, P.J.; Moriya, H.T.; Mauad, T.; Lopes, F.D.; Martins, M.A. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur. Respir. J. 2012, 39, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Nesi, R.T.; de Souza, P.S.; Dos Santos, G.P.; Thirupathi, A.; Menegali, B.T.; Silveira, P.C.; da Silva, L.A.; Valenca, S.S.; Pinho, R.A. Physical exercise is effective in preventing cigarette smoke-induced pulmonary oxidative response in mice. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.B.; Liao, Y.W.; Su, K.H.; Chang, T.M.; Shyue, S.K.; Kou, Y.R.; Lee, T.S. Prior exercise training alleviates the lung inflammation induced by subsequent exposure to environmental cigarette smoke. Acta Physiol. 2012, 205, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.C.R.; Nai, G.A.; Laurindo, C.P.; Gregorio, K.C.R.; Olean-Oliveira, T.; Teixeira, M.F.S.; Seraphim, P.M. Resistance training prevents right ventricle hypertrophy in rats exposed to secondhand cigarette smoke. PLoS ONE 2020, 15, e0236988. [Google Scholar] [CrossRef]
- Bevan, G.H.; Al-Kindi, S.G.; Brook, R.D.; Munzel, T.; Rajagopalan, S. Ambient Air Pollution and Atherosclerosis: Insights Into Dose, Time, and Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 628–637. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.N.; Balde, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Available online: https://apps.who.int/iris/handle/10665/345329 (accessed on 27 September 2021).
- Lelieveld, J.; Poschl, U. Chemists can help to solve the air-pollution health crisis. Nature 2017, 551, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Daiber, A. The air pollution constituent particulate matter (PM2.5) destabilizes coronary artery plaques. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1365–1367. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lee, S.P.; Park, J.B.; Lee, H.; Kang, S.H.; Lee, S.E.; Kim, J.B.; Choi, S.Y.; Kim, Y.J.; Chang, H.J. PM2.5 concentration in the ambient air is a risk factor for the development of high-risk coronary plaques. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1355–1364. [Google Scholar] [CrossRef] [Green Version]
- Rich, D.Q.; Kipen, H.M.; Huang, W.; Wang, G.; Wang, Y.; Zhu, P.; Ohman-Strickland, P.; Hu, M.; Philipp, C.; Diehl, S.R.; et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA 2012, 307, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Kashima, S.; Doi, H. Fine-particulate Air Pollution from Diesel Emission Control and Mortality Rates in Tokyo: A Quasi-experimental Study. Epidemiology 2016, 27, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Giani, P.; Castruccio, S.; Anav, A.; Howard, D.; Hu, W.; Crippa, P. Short-term and long-term health impacts of air pollution reductions from COVID-19 lockdowns in China and Europe: A modelling study. Lancet Planet. Health 2020, 4, e474–e482. [Google Scholar] [CrossRef]
- Munzel, T.; Sorensen, M.; Lelieveld, J.; Hahad, O.; Al-Kindi, S.; Nieuwenhuijsen, M.; Giles-Corti, B.; Daiber, A.; Rajagopalan, S. Heart healthy cities: Genetics loads the gun but the environment pulls the trigger. Eur. Heart J. 2021, 42, 2422–2438. [Google Scholar] [CrossRef]
- Nieuwenhuijsen, M.J. Influence of urban and transport planning and the city environment on cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rueda, D.; Nieuwenhuijsen, M.J.; Gascon, M.; Perez-Leon, D.; Mudu, P. Green spaces and mortality: A systematic review and meta-analysis of cohort studies. Lancet Planet. Health 2019, 3, e469–e477. [Google Scholar] [CrossRef] [Green Version]
- Nieuwenhuijsen, M.J. New urban models for more sustainable, liveable and healthier cities post covid19; reducing air pollution, noise and heat island effects and increasing green space and physical activity. Environ. Int. 2021, 157, 106850. [Google Scholar] [CrossRef] [PubMed]
- Sallis, J.F.; Cerin, E.; Conway, T.L.; Adams, M.A.; Frank, L.D.; Pratt, M.; Salvo, D.; Schipperijn, J.; Smith, G.; Cain, K.L.; et al. Physical activity in relation to urban environments in 14 cities worldwide: A cross-sectional study. Lancet 2016, 387, 2207–2217. [Google Scholar] [CrossRef] [Green Version]
- James, P.; Banay, R.F.; Hart, J.E.; Laden, F. A Review of the Health Benefits of Greenness. Curr. Epidemiol. Rep. 2015, 2, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.C.; Maheswaran, R. The health benefits of urban green spaces: A review of the evidence. J. Public Health 2011, 33, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Cerin, E.; Owen, N.; Oyeyemi, A.L.; Conway, T.L.; Van Dyck, D.; Schipperijn, J.; Macfarlane, D.J.; Salvo, D.; Reis, R.S.; et al. Perceived neighbourhood environmental attributes associated with adults recreational walking: IPEN Adult study in 12 countries. Health Place 2014, 28, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, D.J.; Hirabayashi, S.; Bodine, A.; Greenfield, E. Tree and forest effects on air quality and human health in the United States. Environ. Pollut. 2014, 193, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niinemets, U.; Fares, S.; Harley, P.; Jardine, K.J. Bidirectional exchange of biogenic volatiles with vegetation: Emission sources, reactions, breakdown and deposition. Plant Cell Environ. 2014, 37, 1790–1809. [Google Scholar] [CrossRef] [PubMed]
- Vohra, K.; Vodonos, A.; Schwartz, J.; Marais, E.A.; Sulprizio, M.P.; Mickley, L.J. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: Results from GEOS-Chem. Environ. Res. 2021, 195, 110754. [Google Scholar] [CrossRef]
- Lelieveld, J.; Klingmuller, K.; Pozzer, A.; Burnett, R.T.; Haines, A.; Ramanathan, V. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. USA 2019, 116, 7192–7197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marselle, M.R.; Hartig, T.; Cox, D.T.C.; de Bell, S.; Knapp, S.; Lindley, S.; Triguero-Mas, M.; Bohning-Gaese, K.; Braubach, M.; Cook, P.A.; et al. Pathways linking biodiversity to human health: A conceptual framework. Environ. Int. 2021, 150, 106420. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.T.C.; Shanahan, D.F.; Hudson, H.L.; Plummer, K.E.; Siriwardena, G.M.; Fuller, R.A.; Anderson, K.; Hancock, S.; Gaston, K.J. Doses of Neighborhood Nature: The Benefits for Mental Health of Living with Nature. BioScience 2017, 67, 147–155. [Google Scholar] [CrossRef]
- Methorst, J.; Rehdanz, K.; Mueller, T.; Hansjürgens, B.; Bonn, A.; Böhning-Gaese, K. The importance of species diversity for human well-being in Europe. Ecol. Econ. 2021, 181, 106917. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Brook, R.D.; Biswal, S.; Rajagopalan, S. Environmental determinants of cardiovascular disease: Lessons learned from air pollution. Nat. Rev. Cardiol. 2020, 17, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef]
- Shaw, K.; Butcher, S.; Ko, J.; Zello, G.A.; Chilibeck, P.D. Wearing of Cloth or Disposable Surgical Face Masks has no Effect on Vigorous Exercise Performance in Healthy Individuals. Int. J. Environ. Res. Public Health 2020, 17, 8110. [Google Scholar] [CrossRef]
- Ahmadian, M.; Ghasemi, M.; Nasrollahi Borujeni, N.; Afshan, S.; Fallah, M.; Ayaseh, H.; Pahlavan, M.; Nabavi Chashmi, S.M.; Haeri, T.; Imani, F.; et al. Does wearing a mask while exercising amid COVID-19 pandemic affect hemodynamic and hematologic function among healthy individuals? Implications of mask modality, sex, and exercise intensity. Phys. Sportsmed. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, M.; Salvioni, E.; De Martino, F.; Mattavelli, I.; Gugliandolo, P.; Vignati, C.; Farina, S.; Palermo, P.; Campodonico, J.; Maragna, R.; et al. “You can leave your mask on”: Effects on cardiopulmonary parameters of different airway protection masks at rest and during maximal exercise. Eur. Respir. J. 2021, 58, 2004473. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, B.; Fernandes, S. “Exercise with facemask; Are we handling a devil’s sword?”—A physiological hypothesis. Med. Hypotheses 2020, 144, 110002. [Google Scholar] [CrossRef] [PubMed]
- Tainio, M.; de Nazelle, A.J.; Gotschi, T.; Kahlmeier, S.; Rojas-Rueda, D.; Nieuwenhuijsen, M.J.; de Sa, T.H.; Kelly, P.; Woodcock, J. Can air pollution negate the health benefits of cycling and walking? Prev. Med. 2016, 87, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Giallouros, G.; Kouis, P.; Papatheodorou, S.I.; Woodcock, J.; Tainio, M. The long-term impact of restricting cycling and walking during high air pollution days on all-cause mortality: Health impact Assessment study. Environ. Int. 2020, 140, 105679. [Google Scholar] [CrossRef]
- Hahad, O.; Daiber, A.; Michal, M.; Kuntic, M.; Lieb, K.; Beutel, M.; Munzel, T. Smoking and Neuropsychiatric Disease-Associations and Underlying Mechanisms. Int. J. Mol. Sci. 2021, 22, 7272. [Google Scholar] [CrossRef] [PubMed]
- Olchowski, A.E.; Graham, J.W.; Beverly, E.A.; Dupkanick, C.W. Cigarette Smoking, Physical Activity, and the Health Status of College Students. J. Appl. Soc. Psychol. 2009, 39, 683–706. [Google Scholar] [CrossRef]
- Ussher, M.H.; Taylor, A.H.; Faulkner, G.E. Exercise interventions for smoking cessation. Cochrane Database Syst. Rev. 2014, CD002295. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, R.; Jiang, Y.; Xia, Y.; Niu, Y.; Wang, C.; Liu, C.; Chen, C.; Ge, Y.; Wang, W.; et al. Cardiovascular Benefits of Fish-Oil Supplementation Against Fine Particulate Air Pollution in China. J. Am. Coll. Cardiol. 2019, 73, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, X.; Tian, G.; Shi, X.; Huang, W.; Wu, Y.; Sun, L.; Peng, C.; Liu, S.; Huang, Y.; et al. AMPKalpha2 deficiency exacerbates long-term PM2.5 exposure-induced lung injury and cardiac dysfunction. Free Radic. Biol. Med. 2018, 121, 202–214. [Google Scholar] [CrossRef]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.H.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2015, 36, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopalan, S.; Brauer, M.; Bhatnagar, A.; Bhatt, D.L.; Brook, J.R.; Huang, W.; Munzel, T.; Newby, D.; Siegel, J.; Brook, R.D.; et al. Personal-Level Protective Actions Against Particulate Matter Air Pollution Exposure: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e411–e431. [Google Scholar] [CrossRef] [PubMed]
- Poschl, U.; Shiraiwa, M. Multiphase chemistry at the atmosphere-biosphere interface influencing climate and public health in the anthropocene. Chem. Rev. 2015, 115, 4440–4475. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Kroller-Schon, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Vujacic-Mirski, K.; Kuntic, M.; Bayo Jimenez, M.T.; Helmstadter, J.; Steven, S.; et al. Environmental noise induces the release of stress hormones and inflammatory signaling molecules leading to oxidative stress and vascular dysfunction—Signatures of the internal exposome. Biofactors 2019, 45, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijsen, M.J. Urban and transport planning, environmental exposures and health-new concepts, methods and tools to improve health in cities. Environ. Health 2016, 15 (Suppl. S1), 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munzel, T.; Hahad, O.; Sorensen, M.; Lelieveld, J.; Duerr, G.D.; Nieuwenhuijsen, M.; Daiber, A. Environmental risk factors and cardiovascular diseases: A comprehensive review. Cardiovasc. Res. 2021, cvab316. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahad, O.; Kuntic, M.; Frenis, K.; Chowdhury, S.; Lelieveld, J.; Lieb, K.; Daiber, A.; Münzel, T. Physical Activity in Polluted Air—Net Benefit or Harm to Cardiovascular Health? A Comprehensive Review. Antioxidants 2021, 10, 1787. https://doi.org/10.3390/antiox10111787
Hahad O, Kuntic M, Frenis K, Chowdhury S, Lelieveld J, Lieb K, Daiber A, Münzel T. Physical Activity in Polluted Air—Net Benefit or Harm to Cardiovascular Health? A Comprehensive Review. Antioxidants. 2021; 10(11):1787. https://doi.org/10.3390/antiox10111787
Chicago/Turabian StyleHahad, Omar, Marin Kuntic, Katie Frenis, Sourangsu Chowdhury, Jos Lelieveld, Klaus Lieb, Andreas Daiber, and Thomas Münzel. 2021. "Physical Activity in Polluted Air—Net Benefit or Harm to Cardiovascular Health? A Comprehensive Review" Antioxidants 10, no. 11: 1787. https://doi.org/10.3390/antiox10111787
APA StyleHahad, O., Kuntic, M., Frenis, K., Chowdhury, S., Lelieveld, J., Lieb, K., Daiber, A., & Münzel, T. (2021). Physical Activity in Polluted Air—Net Benefit or Harm to Cardiovascular Health? A Comprehensive Review. Antioxidants, 10(11), 1787. https://doi.org/10.3390/antiox10111787