Differences in Cadmium Accumulation, Detoxification and Antioxidant Defenses between Contrasting Maize Cultivars Implicate a Role of Superoxide Dismutase in Cd Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Stress Treatment
2.2. Cadmium Content, Bioconcentration Factor (BCF) and Translocation Factor (TF)
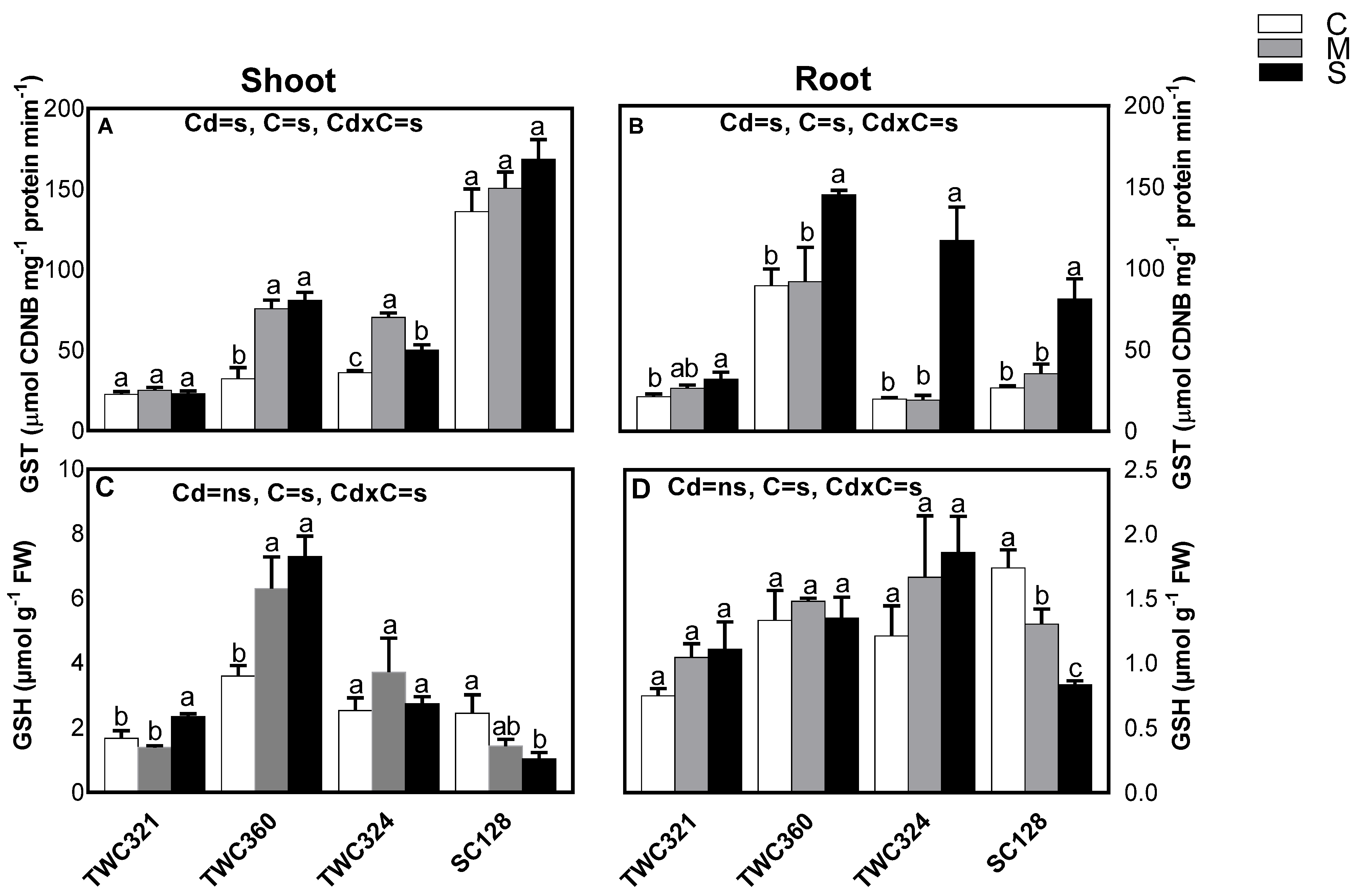
2.3. Detoxification
2.4. Photosynthesis
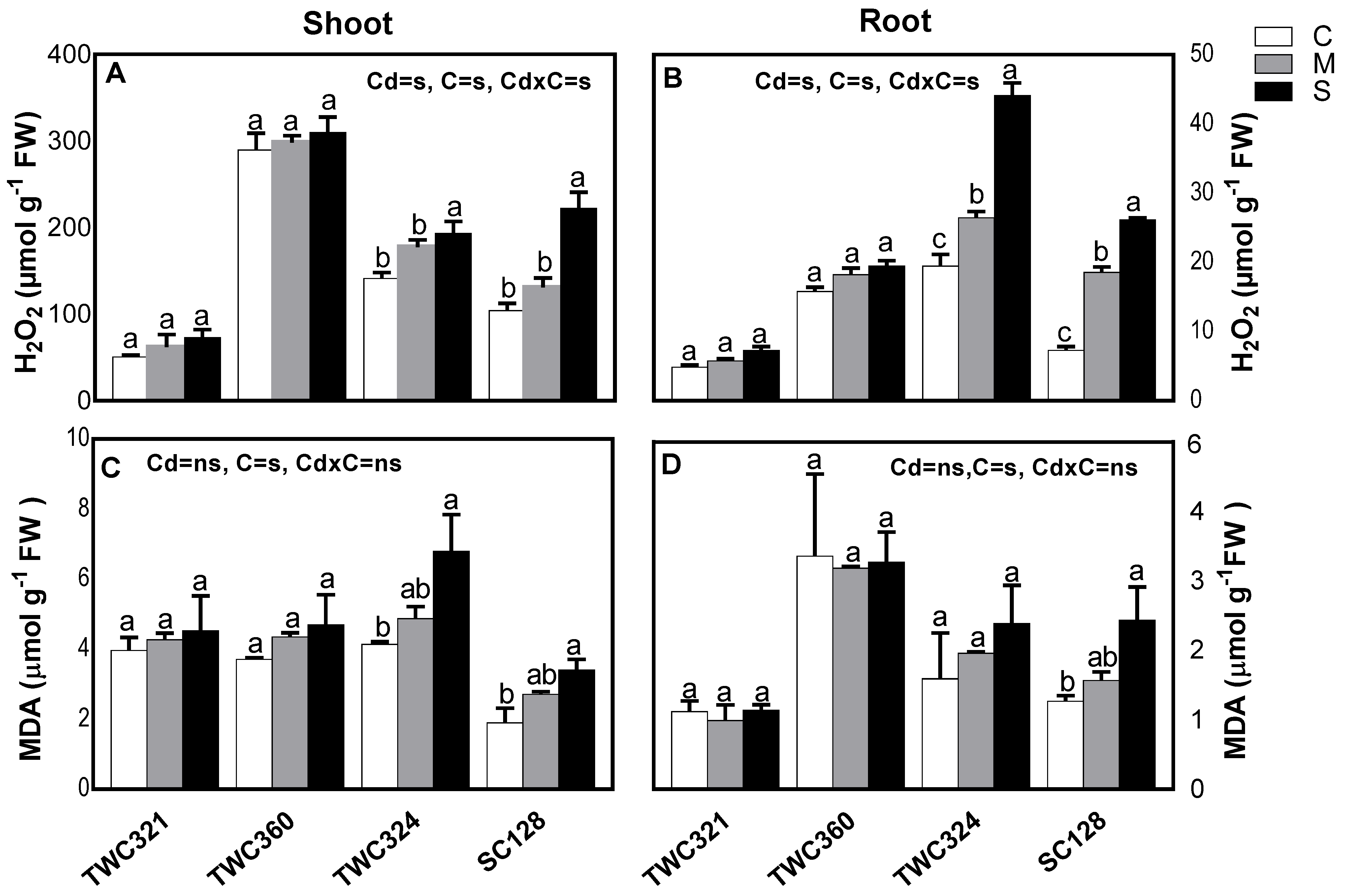
2.5. Oxidative Stress
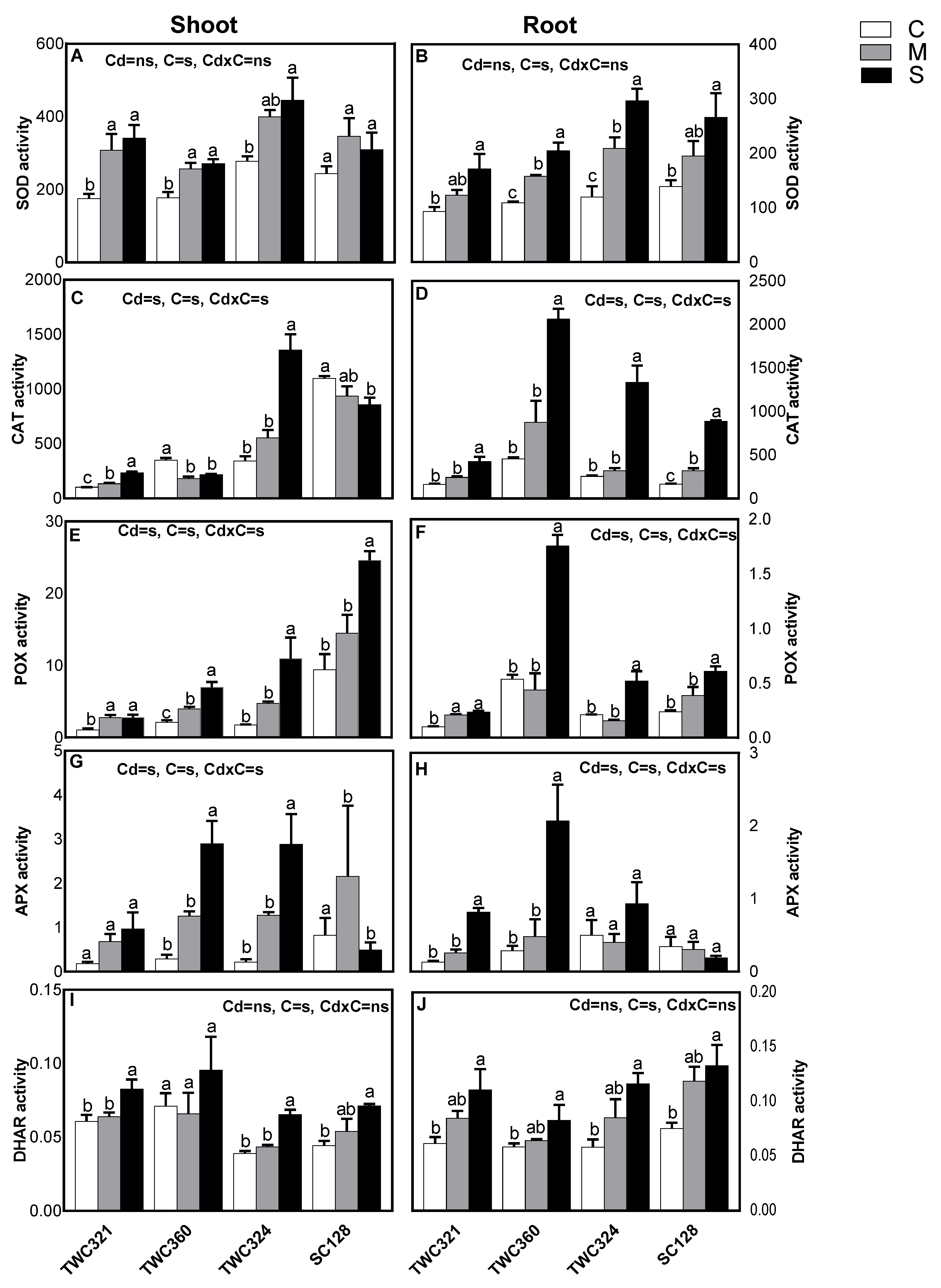
2.6. Enzymatic Antioxidants
2.7. Non-Enzymatic Antioxidants
2.8. Statistical Analysis
3. Results
3.1. Screening of Maize Cultivars for Cd-Stress Tolerance
3.2. Differential Cd Accumulation and Detoxification in Tolerant and Sensitive Cultivars
3.3. Cd Differentially Induced Oxidative Stress and Antioxidant Defense System in Tolerant and Sensitive Cultivars
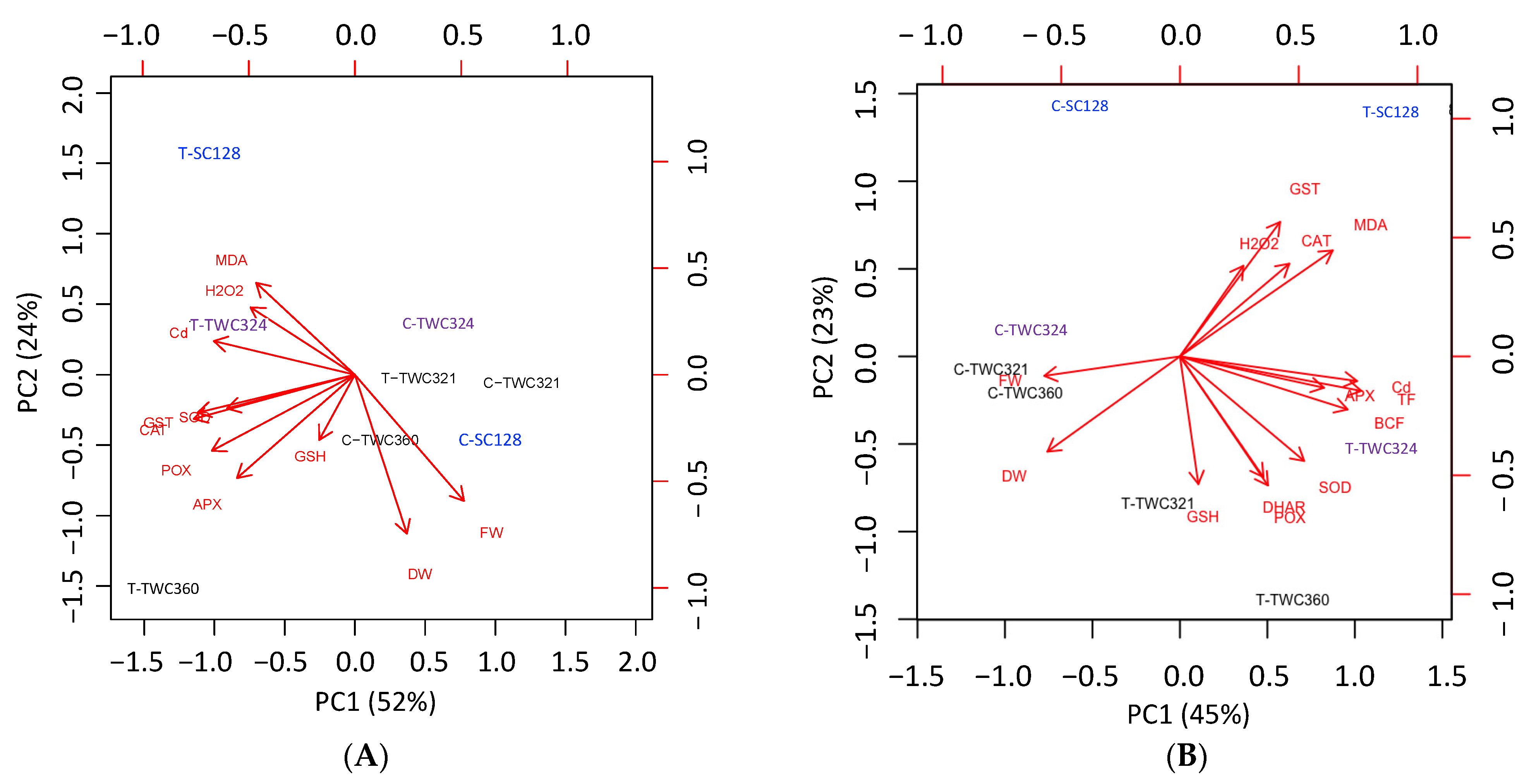
3.4. Principal Component Analysis (PCA) Confirmed Cultivar Specific Responses
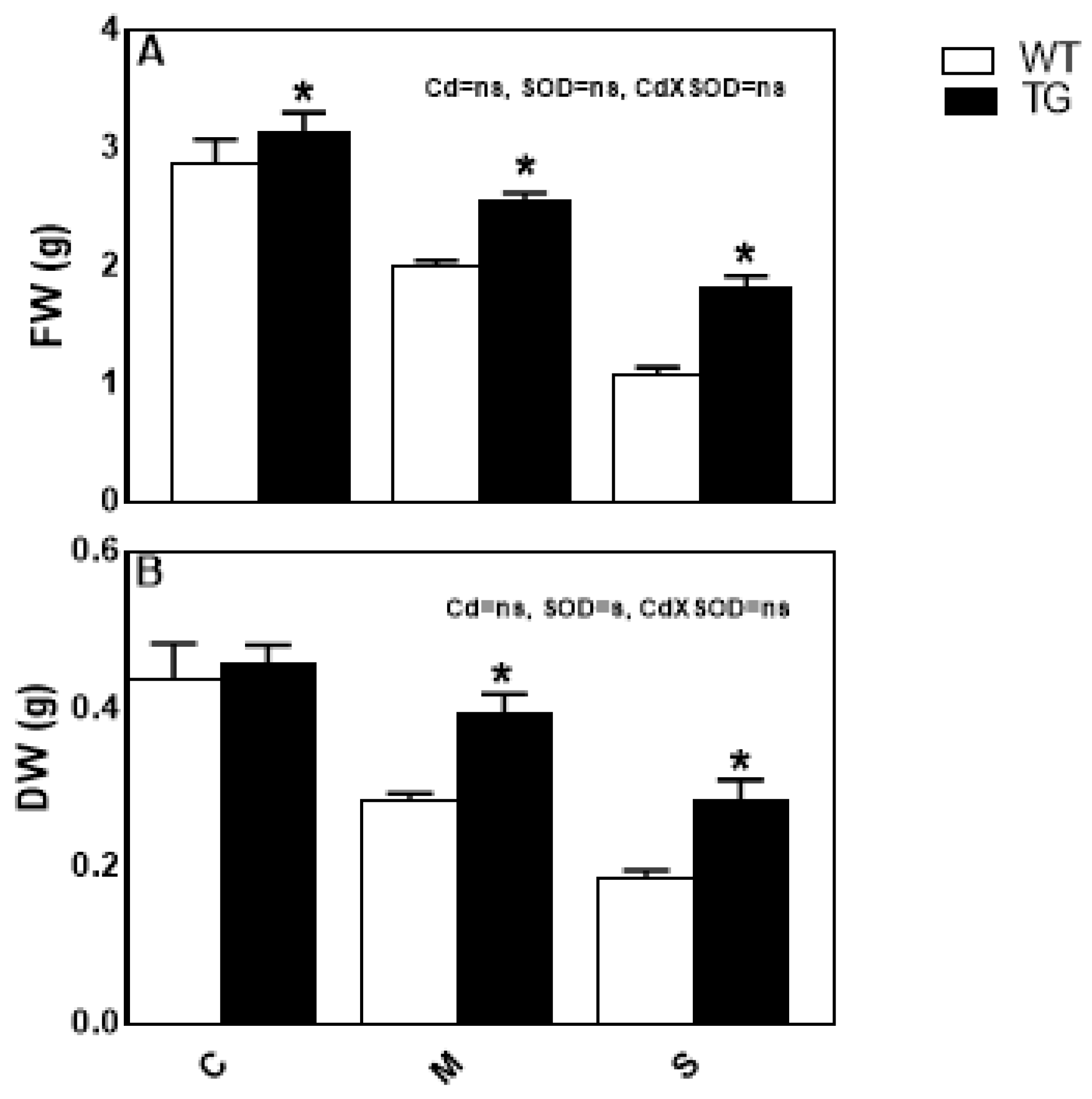
3.5. SOD Overexpression Increased Cd-Stress Tolerance
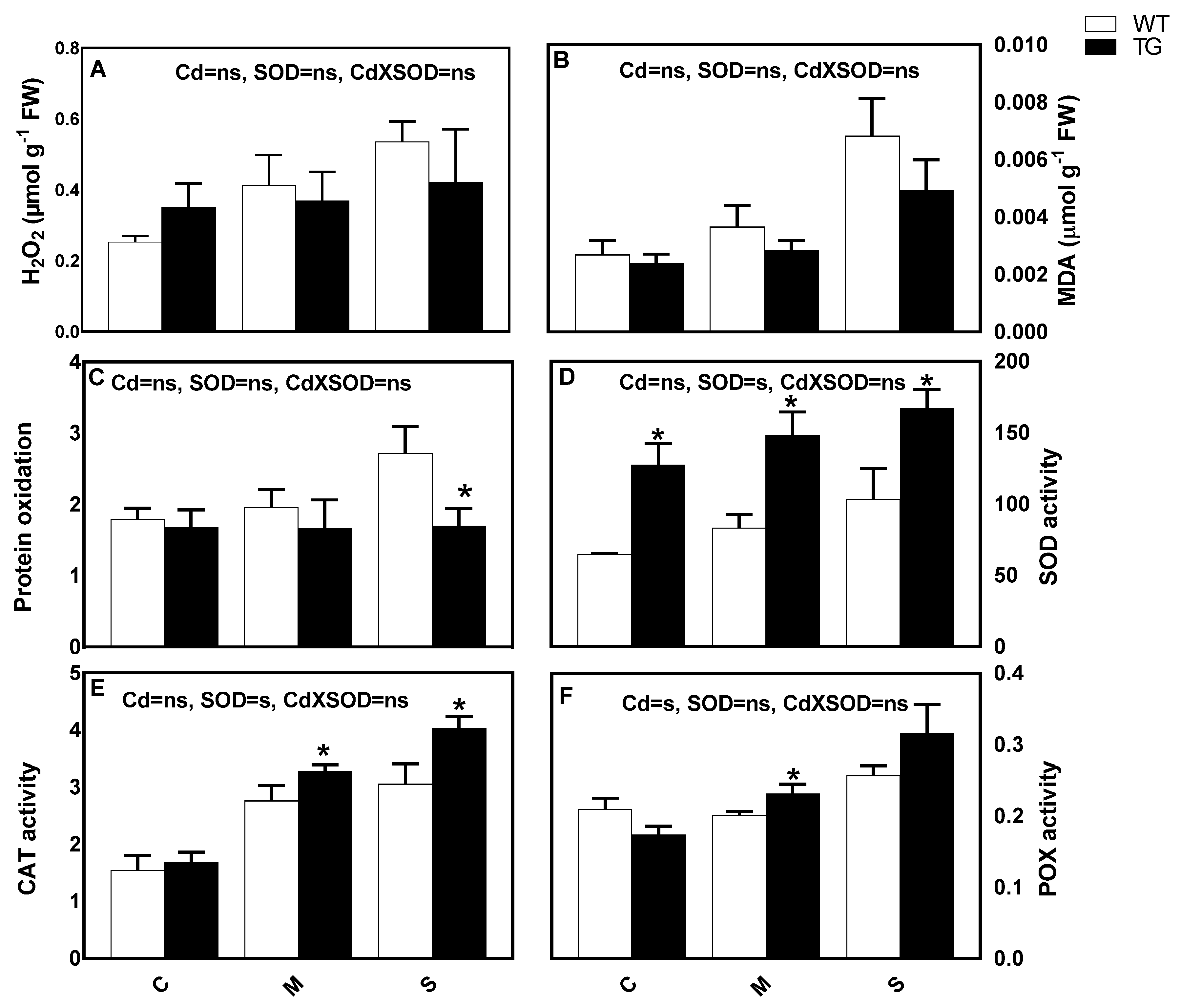
3.6. Stimulation of SOD Activity Is Associated with Lower Cd-Induced Oxidative Stress and Stimulation of Antioxidant Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.; Sebastian, A.; Prasad, M.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2020, 1–52. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, J.; Hayat, K.; Abdelfattah Elateeq, A.; Salam, U.; Yu, B.; Ma, Y.; Wang, H.; Tang, Z.-H. Comparative study of growth, cadmium accumulation and tolerance of three chickpea (Cicer arietinum L.) cultivars. Plants 2020, 9, 310. [Google Scholar] [CrossRef] [Green Version]
- Shanmugaraj, B.M.; Malla, A.; Ramalingam, S. Cadmium stress and toxicity in plants: An overview. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M., Fujita, M., Eds.; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2019; pp. 1–17. [Google Scholar]
- Thakur, S.; Singh, L.; Ab Wahid, Z.; Siddiqui, M.F.; Atnaw, S.M.; Din, M.F.M. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 2016, 188, 206. [Google Scholar] [CrossRef]
- Bertels, J.; Huybrechts, M.; Hendrix, S.; Bervoets, L.; Cuypers, A.; Beemster, G.T. Cadmium inhibits cell cycle progression and specifically accumulates in the maize leaf meristem. J. Exp. Bot. 2020, 71, 6418–6428. [Google Scholar] [CrossRef]
- Luo, Z.-B.; He, J.; Polle, A.; Rennenberg, H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: Paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef]
- Adil, M.F.; Sehar, S.; Han, Z.; Lwalaba, J.L.W.; Jilani, G.; Zeng, F.; Chen, Z.-H.; Shamsi, I.H. Zinc alleviates cadmium toxicity by modulating photosynthesis, ROS homeostasis, and cation flux kinetics in rice. Environ. Pollut. 2020, 265, 114979. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.-J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Zhang, C.-h.; WU, Z.-y.; Ting, J.; Ying, G. Purification and identification of glutathione S-transferase in rice root under cadmium stress. Rice Sci. 2013, 20, 173–178. [Google Scholar] [CrossRef]
- Asad, S.A.; Farooq, M.; Afzal, A.; West, H. Integrated phytobial heavy metal remediation strategies for a sustainable clean environment—A review. Chemosphere 2019, 217, 925–941. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Zhang, S.; Yin, Z.; Zhu, W.; Li, J.; Meng, L.; Zhong, H.; Xu, N.; Wu, Y. Rootstock alleviates salt stress in grafted mulberry seedlings: Physiological and PSII function responses. Front. Plant Sci. 2018, 9, 1806. [Google Scholar] [CrossRef]
- Pagliano, C.; Raviolo, M.; Dalla Vecchia, F.; Gabbrielli, R.; Gonnelli, C.; Rascio, N.; Barbato, R.; La Rocca, N. Evidence for PSII donor-side damage and photoinhibition induced by cadmium treatment on rice (Oryza sativa L.). J. Photochem. Photobiol. B Biol. 2006, 84, 70–78. [Google Scholar] [CrossRef]
- Geiken, B.; Masojidek, J.; Rizzuto, M.; Pompili, M.; Giardi, M. Incorporation of [35S] methionine in higher plants reveals that stimulation of the D1 reaction centre II protein turnover accompanies tolerance to heavy metal stress. Plant Cell Environ. 1998, 21, 1265–1273. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic response of plants under different abiotic stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Gallie, D.R.; Chen, Z. Chloroplast-localized iron superoxide dismutases FSD2 and FSD3 are functionally distinct in Arabidopsis. PLoS ONE 2019, 14, e0220078. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Li, H.; Zhang, X.; Fu, J. Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne L.) under cadmium stress. Ecotoxicology 2011, 20, 770–778. [Google Scholar] [CrossRef]
- Smeets, K.; Ruytinx, J.; Semane, B.; Van Belleghem, F.; Remans, T.; Van Sanden, S.; Vangronsveld, J.; Cuypers, A. Cadmium-induced transcriptional and enzymatic alterations related to oxidative stress. Environ. Exp. Bot. 2008, 63, 1–8. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, S.; Lu, Q.; Yang, Z.; Wen, X.; Zhang, L.; Lu, C. Characterization of photosystem II in transgenic tobacco plants with decreased iron superoxide dismutase. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, C.; Zhao, X.; Li, R.; Deng, W. Involvement of an antioxidant defense system in the adaptive response to cadmium in maize seedlings (Zea mays L.). Bull. Environ. Contam. Toxicol. 2014, 93, 618–624. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Bonavia, D. Maize: Origin, Domestication, and Its Role in the Development of Culture; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Dowswell, C.; Paliwal, R.; Cantrell, R. Maize in the Third World; Westview Press: Boulder, CO, USA, 1996. [Google Scholar]
- Wuana, R.; Okieimen, F. Phytoremediation potential of maize (Zea mays L.). A review. Afr. J. Gen. Agric. 2010, 6, 275–287. [Google Scholar]
- Tian, Y.; Zhang, H.; Guo, W.; Chen, Z.; Wei, X.; Zhang, L.; Han, L.; Dai, L. Assessment of the phytoremediation potential of bioenergy crop maize (Zea mays) in soil contaminated by cadmium: Morphology, photosynthesis and accumulation. Fresenius Environ. Bull. 2012, 21, 3575–3581. [Google Scholar]
- Hayat, K.; Menhas, S.; Bundschuh, J.; Zhou, P.; Niazi, N.K.; Amna; Hussain, A.; Hayat, S.; Ali, H.; Wang, J. Plant growth promotion and enhanced uptake of Cd by combinatorial application of Bacillus pumilus and EDTA on Zea mays L. Int. J. Phytoremediat. 2020, 22, 1372–1384. [Google Scholar] [CrossRef]
- Ekmekçi, Y.; Tanyolac, D.; Ayhan, B. Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J. Plant Physiol. 2008, 165, 600–611. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ullah, E.; Wang, L.; Khan, I.; Samad, R.A.; Tung, S.A.; Anam, M.; Shahzad, B. Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. CLEAN–Soil Air Water 2016, 44, 29–36. [Google Scholar] [CrossRef]
- Wang, M.; Zou, J.; Duan, X.; Jiang, W.; Liu, D. Cadmium accumulation and its effects on metal uptake in maize (Zea mays L.). Bioresour. Technol. 2007, 98, 82–88. [Google Scholar] [CrossRef]
- Yue, R.; Lu, C.; Qi, J.; Han, X.; Yan, S.; Guo, S.; Liu, L.; Fu, X.; Chen, N.; Yin, H. Transcriptome analysis of cadmium-treated roots in maize (Zea mays L.). Front. Plant Sci. 2016, 7, 1298. [Google Scholar] [CrossRef] [Green Version]
- AbdElgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Slooten, L.; Stassart, J.-M.; Moens, T.; Botterman, J.; Van Montagu, M.; Inzé, D. Overproduction of Arabidopsis thaliana FeSOD confers oxidative stress tolerance to transgenic maize. Plant Cell Physiol. 1999, 40, 515–523. [Google Scholar] [CrossRef]
- Cottenie, A.; Verloo, M.; Kiekens, L.; Velghe, G.; Camerlynck, R. Chemical Analysis of Plants and Soils; Laboratory Agrochemistry State University: Gent, Belgium, 1982. [Google Scholar]
- Soltanpour, P. Determination of nutrient availability and elemental toxicity by AB-DTPA soil test and ICPS. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1991; Volume 16, pp. 165–190. [Google Scholar]
- Zacchini, M.; Pietrini, F.; Mugnozza, G.S.; Iori, V.; Pietrosanti, L.; Massacci, A. Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut. 2009, 197, 23–34. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- de Knecht, J.A.; Koevoets, P.L.; Verkleij, J.A.; Ernst, W.H. Evidence against a role for phytochelatins in naturally selected increased cadmium tolerance in Silene vulgaris (Moench) Garcke. New Phytol. 1992, 122, 681–688. [Google Scholar] [CrossRef]
- Avramova, V.; AbdElgawad, H.; Zhang, Z.; Fotschki, B.; Casadevall, R.; Vergauwen, L.; Knapen, D.; Taleisnik, E.; Guisez, Y.; Asard, H. Drought induces distinct growth response, protection, and recovery mechanisms in the maize leaf growth zone. Plant Physiol. 2015, 169, 1382–1396. [Google Scholar] [CrossRef]
- Markwell, J.P.; Danko, S.J.; Bauwe, H.; Osterman, J.; Gorz, H.J.; Haskins, F.A. A temperature-sensitive chlorophyll b-deficient mutant of sweetclover (Melilotus alba). Plant Physiol. 1986, 81, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta (BBA)-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–297. [Google Scholar]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. In Plant Stress Tolerance. Methods in Molecular Biology; Sunkar, R., Ed.; Human Press: Totowa, NJ, USA, 2010; pp. 273–280. [Google Scholar]
- Kumar, K.; Khan, P. Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [Green Version]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate–glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Potters, G.; Horemans, N.; Bellone, S.; Caubergs, R.J.; Trost, P.; Guisez, Y.; Asard, H. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiol. 2004, 134, 1479–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Hou, D.; O’Connor, D.; Pan, S.; Zhu, J.; Bolan, N.S.; Mulder, J. Exogenous phosphorus treatment facilitates chelation-mediated cadmium detoxification in perennial ryegrass (Lolium perenne L.). J. Hazard. Mater. 2020, 389, 121849. [Google Scholar] [CrossRef]
- Berwal, M.; Ram, C. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- He, S.; He, Z.; Yang, X.; Stoffella, P.J.; Baligar, V.C. Soil biogeochemistry, plant physiology, and phytoremediation of cadmium-contaminated soils. Adv. Agron. 2015, 134, 135–225. [Google Scholar]
- Liu, S.; Ali, S.; Yang, R.; Tao, J.; Ren, B. A newly discovered Cd-hyperaccumulator Lantana camara L. J. Hazard. Mater. 2019, 371, 233–242. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Zhao, Y.; Long, Y.; Liu, S.; Pan, Y. Physiological and biochemical mechanisms preventing Cd toxicity in the new hyperaccumulator Abelmoschus manihot. J. Plant Growth Regul. 2018, 37, 709–718. [Google Scholar] [CrossRef]
- Hosman, M.E.; El-Feky, S.S.; Elshahawy, M.I.; Shaker, E.M. Mechanism of phytoremediation potential of flax (Linum usitatissimum L.) to Pb, Cd and Zn. Asian J. Plant Sci. Res. 2017, 7, 30–40. [Google Scholar]
- Wang, L.; Zhang, Q.; Liao, X.; Li, X.; Zheng, S.; Zhao, F. Phytoexclusion of heavy metals using low heavy metal accumulating cultivars: A green technology. J. Hazard. Mater. 2021, 125427. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Wang, C.; Liao, W. Recent progress in the knowledge on the alleviating effect of nitric oxide on heavy metal stress in plants. Plant Physiol. Biochem. 2020, 147, 161–171. [Google Scholar] [CrossRef]
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Asgher, M.; Per, T.S.; Anjum, S.; Khan, M.I.R.; Masood, A.; Verma, S.; Khan, N.A. Contribution of glutathione in heavy metal stress tolerance in plants. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress, 1st ed.; Khan, M., Khan, N., Eds.; Springer: Singapore, 2017; pp. 297–313. [Google Scholar]
- Herschbach, C.; Scheerer, U.; Rennenberg, H. Redox states of glutathione and ascorbate in root tips of poplar (Populus tremula× P. alba) depend on phloem transport from the shoot to the roots. J. Exp. Bot. 2010, 61, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Vileno, B.; Palacios, Ò.; Peris-Díaz, M.D.; Riegel, G.; Gaiddon, C.; Krężel, A.; Faller, P. Reactivity of Cu (ii)–, Zn (ii)–and Fe (ii)–thiosemicarbazone complexes with glutathione and metallothionein: From stability to dissociation to transmetallation. Metallomics 2019, 11, 994–1004. [Google Scholar] [CrossRef]
- Hider, R.; Aviles, M.V.; Chen, Y.-L.; Latunde-Dada, G.O. The role of GSH in intracellular iron trafficking. Int. J. Mol. Sci. 2021, 22, 1278. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Tiwari, S.; Dubey, N. Adaptation Strategies of Plants against Heavy Metal Stress. In Phytoremediation of Environmental Pollutants; CRC Press: New York, NY, USA, 2017; p. 81. [Google Scholar]
- Tzure-Meng, W.; Yi-Ting, H.; Tse-Min, L. Effects of cadmium on the regulation of antioxidant enzyme activity, gene expression, and antioxidant defenses in the marine macroalga Ulva fasciata. Bot. Stud. 2009, 50, 25–34. [Google Scholar]
- Mishra, P.; Sharma, P. Superoxide Dismutases (SODs) and their role in regulating abiotic stress induced oxidative stress in plants. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 53–88. [Google Scholar]
- Madhu, P.M.; Sadagopan, R.S. Effect of heavy metals on growth and development of cultivated plants with reference to cadmium, chromium and lead—A review. J. Stress Physiol. Biochem. 2020, 16, 84–102. [Google Scholar]
- Luo, J.-S.; Zhang, Z. Mechanisms of cadmium phytoremediation and detoxification in plants. Crop J. 2021, 9, 521–529. [Google Scholar] [CrossRef]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef]
- Dixit, P.; Mukherjee, P.K.; Ramachandran, V.; Eapen, S. Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tabacum. PLoS ONE 2011, 6, e16360. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Li, L.; Duan, Q.; Liu, X.; Chen, M. Progress in our understanding of plant responses to the stress of heavy metal cadmium. Plant Signal. Behav. 2020, 16, 1836884. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Song, J.; Finnegan, P.M.; Liu, W.; Li, X.; Yong, J.W.; Xu, J.; Zhang, Q.; Wen, Y.; Qin, K.; Guo, J. Mechanisms underlying enhanced Cd translocation and tolerance in roots of Populus euramericana in response to nitrogen fertilization. Plant Sci. 2019, 287, 110206. [Google Scholar] [CrossRef]
- Pandey, P.; Dubey, R.S. Metal toxicity in rice and strategies for improving stress tolerance. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.H., Eds.; Elsevier: Cambridge, UK, 2019; pp. 313–339. [Google Scholar]
- Yamaguchi, C.; Khamsalath, S.; Takimoto, Y.; Suyama, A.; Mori, Y.; Ohkama-Ohtsu, N.; Maruyama-Nakashita, A. SLIM1 transcription factor promotes sulfate uptake and distribution to shoot, along with phytochelatin accumulation, under cadmium stress in Arabidopsis thaliana. Plants 2020, 9, 163. [Google Scholar] [CrossRef] [Green Version]
- Song, W.Y.; Mendoza-Cózatl, D.G.; Lee, Y.; Schroeder, J.I.; Ahn, S.N.; Lee, H.S.; Wicker, T.; Martinoia, E. Phytochelatin–metal (loid) transport into vacuoles shows different substrate preferences in barley and Arabidopsis. Plant Cell Environ. 2014, 37, 1192–1201. [Google Scholar] [CrossRef] [Green Version]
- Greger, M.; Kabir, A.H.; Landberg, T.; Maity, P.J.; Lindberg, S. Silicate reduces cadmium uptake into cells of wheat. Environ. Pollut. 2016, 211, 90–97. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Zhang, W.; Zhang, S.; Zhao, F.J. Protein phosphatase 2A alleviates cadmium toxicity by modulating ethylene production in Arabidopsis thaliana. Plant Cell Environ. 2020, 43, 1008–1022. [Google Scholar] [CrossRef]

| Parameter | Cd-Stress | Cultivars | |||
|---|---|---|---|---|---|
| TWC321 | TWC360 | TWC324 | SC128 | ||
| Shoot Cd content (mg kg−1) | Control | 0.20 | 3.70 | 1.20 | 1.80 |
| Mild | 34.3 | 59.3 | 38.0 | 59.3 | |
| Severe | 43.1 | 72.3 | 58.1 | 91.5 | |
| Root Cd content (mg kg−1) | Control | 0.40 | 2.60 | 24.7 | 1.50 |
| Mild | 102.7 | 121.7 | 125.6 | 134.5 | |
| Severe | 187.5 | 240.4 | 172.9 | 234.3 | |
| Bio-concentration factor | Control | 2.14 | 22.5 | 92.5 | 11.85 |
| Mild | 6.21 | 8.61 | 7.71 | 9.81 | |
| Severe | 5.71 | 7.12 | 5.48 | 7.44 | |
| Translocation factor | Control | 0.50 | 1.42 | 0.049 | 1.2 |
| Mild | 0.33 | 0.49 | 0.0302 | 0.441 | |
| Severe | 0.23 | 0.3 | 0.336 | 0.391 | |
| Parameter | Lines | Cadmium Stress | Two-Way ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Control | Mild | Severe | SOD | Cd | SOD × Cd | ||
| Photosynthesis | WT | 0.14 ± 0.024 | 0.055 ± 0.011 | 0.022 ± 0.01 | 0.383 | 0 | 0.993 |
| (mmol CO2 m−2 s−1) | TG | 0.152 ± 0.03 | 0.07 ± 0.02 | 0.03 ± 0.01 | |||
| Chl a + b | WT | 0.24 ± 0.05 | 0.11 ± 0.01 | 0.06 ± 0.002 | 0.94 | 0 | 0.458 |
| (μg pigment g−1 FW) | TG | 0.20 ± 0.05 | 0.12 ± 0.01 | 0.1 ± 0.01 * | |||
| Carotenoids | WT | 0.03 ± 0.004 * | 0.04 ± 0.003 * | 0.05 ± 0.004 | 0 | 0 | 0.085 |
| (μg pigment g−1 FW) | TG | 0.014 ± 0.002 * | 0.015 ± 0.001 * | 0.025 ± 0.002 * | |||
| gs | WT | 192.46 ± 15.75 | 63.73 ± 3.96 | 28.48 ± 2.14 | 0.772 | 0 | 0.006 |
| (mmol m−2 s−1) | TG | 151.32 ± 16.42 * | 80.43 ± 7.30 * | 45.83 ± 1.9 * | |||
| Fv/Fm | WT | 0.81 ± 0.01 | 0.67 ± 0.026 | 0.55 ± 0.018 | 0 | 0 | 0.001 |
| TG | 0.82 ± 0.01 | 0.77 ± 0.01 * | 0.70 ± 0.01 * | ||||
| Parameter | Lines | Cadmium Stress | Two-Way ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Control | Mild | Severe | SOD | Cd | SOD × Cd | ||
| Cd | WT | 27.77 ± 2.55 | 40.82 ± 3.50 | 60.61 ± 2.68 | 0.14 | 0 | 0.13 |
| (mg kg−1 FW) | TG | 29.57 ± 2.27 | 38.37 ± 2.51 | 51.41 ± 2.34 * | |||
| PCs | WT | 0.93 ± 0.07 | 1.30 ± 0.22 | 1.96 ± 0.14 | 0.973 | 0.044 | 0.558 |
| (μmol g−1 FW) | TG | 0.73 ± 0.46 | 1.1 ± 0.1 | 1.1 ± 0.14 * | |||
| GPX | WT | 0.016 ± 0.001 | 0.018 ± 0.001 | 0.02 ± 0.003 | 0.031 | 0 | 0.004 |
| (μmol NADPH mg−1 min−1) | TG | 0.015 ± 0.001 * | 0.018 ± 0.001 | 0.04 ± 0.005 * | |||
| GST | WT | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.024 | 0 | 0.335 |
| (μmol CDNB mg−1 protein min−1) | TG | 0.01 ± 0.001 * | 0.01 ± 0 | 0.02 ± 0.001 * | |||
| Parameter | Lines | Cadmium Stress | Two-Way ANOVA | ||||
|---|---|---|---|---|---|---|---|
| Control | Mild | Severe | SOD | Cd | SOD × Cd | ||
| APX | WT | 0.14 ± 0.004 | 0.15 ± 0.01 | 0.17 ± 0.013 | 0.688 | 0.012 | 0.931 |
| (μmol mg−1 protein min−1) | TG | 0.13 ± 0.01 | 0.15 ± 0.01 | 0.17 ± 0.01 | |||
| MDHAR | WT | 0.053 ± 0.003 | 0.06 ± 0.003 | 0.06 ± 0.003 | 0.335 | 0.02 | 0.124 |
| (μmol mg−1 protein min−1) | TG | 0.05 ± 0.003 | 0.06 ± 0.01 | 0.07 ± 0.004 * | |||
| DHAR | WT | 0.01 ± 0.001 | 0.02 ± 0.001 | 0.02 ± 0.002 | 0.912 | 0 | 0.049 |
| (μmol mg−1 protein min−1) | TG | 0.01 ± 0.002 | 0.01 ± 0.001 * | 0.027 ± 0.002 * | |||
| GR | WT | 0.016 ± 0.001 | 0.02 ± 0.001 | 0.03 ± 0.003 | 0.271 | 0 | 0.901 |
| (μmol mg−1 protein min−1) | TG | 0.02 ± 0.001 | 0.02 ± 0.002 | 0.03 ± 0.004 | |||
| FRAP | WT | 14.1 ± 1. 50 | 14.34 ± 1.25 | 15.7 ± 0.78 | 0.007 | 0.036 | 0.183 |
| (μmol g−1 FW) | TG | 17.84 ± 1.78 | 15.7 ± 0.82 | 23.68 ± 3.55 * | |||
| Flavonoids | WT | 0.62 ± 0.032 | 0.83 ± 0.04 | 0.63 ± 0.017 | 0 | 0.001 | 0 |
| (mg quercetin g−1 FW) | TG | 0.71 ± 0.03 | 0.78 ± 0.04 | 1.02 ± 0.03 * | |||
| ASC | WT | 1.39 ± 0.20 | 1.74 ± 0.16 | 1.80 ± 0.19 | 0.216 | 0.083 | 0.603 |
| (μmol g−1 FW) | TG | 1.50 ± 0.19 | 1.82 ± 0.24 | 2.23 ± 0.18 | |||
| DHA | WT | 95.73 ± 10.7 | 115.95 ± 7.60 | 167.82 ± 8.02 | 0.63 | 0.00 | 0.29 |
| (μmol g−1 FW) | TG | 102.19 ± 14.77 | 135.80 ± 9.88 | 181.5 ± 8.11 | |||
| TASC | WT | 1.41 ± 0.05 | 117.69 ± 7.75 | 183.29 ± 8.20 | 0.618 | 0 | 0.308 |
| (μmol g−1 FW) | TG | 103 ± 69 | 137.62 ± 10.05 | 170.07 ± 8.01 | |||
| ASC/TASC | WT | 1.41 ± 0.05 | 1.47 ± 0.047 | 0.1 ± 0.1 | 0.321 | 0.014 | 0.044 |
| (μmol g−1 FW) | TG | 1.50 ± 0.04 | 1.3 ± 0.12 | 1.3 ± 0.12 * | |||
| GSH | WT | 0.18 ± 0.02 | 0.19 ± 0.018 | 0.47 ± 0.065 | 0.063 | 0 | 0.292 |
| (μmol g−1 FW) | TG | 0.27 ± 0.04 * | 0.29 ± 0.02 * | 0.46 ± 0.04 | |||
| GSSG | WT | 0.064 ± 0.008 | 0.12 ± 0.003 | 0.20 ± 0.04 | 0.372 | 0.059 | 0.145 |
| (μmol g−1 FW) | TG | 0.08 ± 0.01 | 0.12 ± 0.02 | 0.11 ± 0.05 | |||
| TGSH | WT | 0.24 ± 0.02 | 0.31 ± 0.02 | 0.67 ± 0.09 | 0.339 | 0 | 0.041 |
| (μmol g−1 FW) | TG | 0.36 ± 0.03 * | 0.41 ± 0.01 * | 0.56 ± 0.11 | |||
| GSH/TGSH | WT | 73.66 ± 2.5 | 60.43 ± 2.1 | 70.04 ± 0.04 | 0.339 | 0.12 | 0.593 |
| (μmol g−1 FW) | TG | 75.01 ± 4.8 | 70.17 ± 5.7 | 81.21 ± 3.85 | |||
| Tocopherols | WT | 2.71 ± 0.1 | 3.41 ± 0.11 | 4.37 ± 0.30 | 0.701 | 0.476 | 0.004 |
| (mg g−1 FW) | TG | 4.23 ± 0.5 * | 3.38 ± 0.40 | 3.19 ± 0.15 * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.; AbdElgawad, H.; Hamed, B.A.; Beemster, G.T.S.; El-Shafey, N.M. Differences in Cadmium Accumulation, Detoxification and Antioxidant Defenses between Contrasting Maize Cultivars Implicate a Role of Superoxide Dismutase in Cd Tolerance. Antioxidants 2021, 10, 1812. https://doi.org/10.3390/antiox10111812
Mahmoud A, AbdElgawad H, Hamed BA, Beemster GTS, El-Shafey NM. Differences in Cadmium Accumulation, Detoxification and Antioxidant Defenses between Contrasting Maize Cultivars Implicate a Role of Superoxide Dismutase in Cd Tolerance. Antioxidants. 2021; 10(11):1812. https://doi.org/10.3390/antiox10111812
Chicago/Turabian StyleMahmoud, Aya, Hamada AbdElgawad, Badreldin A. Hamed, Gerrit T.S. Beemster, and Nadia M. El-Shafey. 2021. "Differences in Cadmium Accumulation, Detoxification and Antioxidant Defenses between Contrasting Maize Cultivars Implicate a Role of Superoxide Dismutase in Cd Tolerance" Antioxidants 10, no. 11: 1812. https://doi.org/10.3390/antiox10111812
APA StyleMahmoud, A., AbdElgawad, H., Hamed, B. A., Beemster, G. T. S., & El-Shafey, N. M. (2021). Differences in Cadmium Accumulation, Detoxification and Antioxidant Defenses between Contrasting Maize Cultivars Implicate a Role of Superoxide Dismutase in Cd Tolerance. Antioxidants, 10(11), 1812. https://doi.org/10.3390/antiox10111812







