Influence of Oxidative Stress Generated by Smoking during Pregnancy on Glutathione Status in Mother-Newborn Pairs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Collection
2.3. Maternal and Cord Blood Cotinine Concentration
2.4. Maternal and Cord Blood Oxidative Status Markers
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Maternal and cord Blood Cotinine Concentrations
3.3. Maternal and Cord Blood Oxidative Status Markers
3.4. Relations between GSH, GSSG Levels and Other Biochemical Parameters
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of oxidative stress on pregnancy. Oxid. Med. Cell Longev. 2020, 15, 6398520. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Laschi, E.; Buonocore, G. Biomarkers of oxidative stress in the fetus and in the newborn. Free Radic. Biol. Med. 2019, 142, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Santacroce, A.; Longini, M.; Proietti, F.; Bazzini, F.; Buonocore, G. The free radical diseases of prematurity: From cellular mechanisms to bedside. Oxid. Med. Cell Longev. 2018, 2018, 7483062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laforgia, N.; Di Mauro, A.; Favia Guarnieri, G.; Varvara, D.; De Cosmo, L.; Panza, R.; Capozza, M.; Baldassarre, M.E.; Resta, N. The role of oxidative stress in the pathomechanism of congenital malformations. Oxid. Med. Cell Longev. 2018, 30, 7404082. [Google Scholar] [CrossRef]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Tataranno, M.L.; Perrone, S.; Longini, M.; Buonocore, G. New antioxidant drugs for neonatal brain injury. Oxid. Med. Cell Longev. 2015, 2015, 108251. [Google Scholar] [CrossRef] [Green Version]
- Kida, N.; Matsuo, Y.; Hashimoto, Y.; Nishi, K.; Tsuzuki-Nakao, T.; Bono, H.; Maruyama, T.; Hirota, K.; Okada, H. Cigarette smoke extract activates hypoxia-inducible factors in a reactive oxygen species-dependent manner in stroma cells from human endometrium. Antioxidants 2021, 10, 48. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Bizoń, A.; Milnerowicz, H.; Kowalska-Piastun, K.; Milnerowicz-Nabzdyk, E. The impact of early pregnancy and exposure to tobacco smoke on blood antioxidant status and copper, zinc, cadmium concentration-a pilot study. Antioxidants 2021, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Pizent, A.; Lazarus, M.; Kovačić, J.; Tariba Lovaković, B.; Brčić Karačonji, I.; Živković Semren, T.; Sekovanić, A.; Orct, T.; Branović-Čakanić, K.; Brajenović, N.; et al. Cigarette smoking during pregnancy: Effects on antioxidant enzymes, metallothionein and trace elements in mother-newborn pairs. Biomolecules 2020, 10, 892. [Google Scholar] [CrossRef]
- Ermis, B.; Ors, R.; Yildirim, A.; Tastekin, A.; Kardes, F.; Akcay, F. Influence of smoking on maternal and neonatal serum malondialdehyde, superoxide dismutase, and glutathione peroxidase levels. Ann. Clin. Lab. Sci. 2004, 34, 405–409. [Google Scholar] [PubMed]
- Chelchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Laskowska-Klita, T. The effect of tobacco smoking during pregnancy on plasma oxidant and antioxidant status in mother and newborn. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Chełchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Rowicka, G.; Maciejewski, T.M.; Mazur, J. Cord blood adiponectin and visfatin concentrations in relation to oxidative stress markers in neonates exposed and nonexposed in utero to tobacco smoke. Oxid. Med. Cell Longev. 2016, 2016, 4569108. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, D.; Megha, K.; Mishra, R.; Mandal, P.K. Glutathione in brain: Overview of its conformations, functions, biochemical characteristics, quantitation and potential therapeutic role in brain disorders. Neurochem. Res. 2020, 45, 1461–1480. [Google Scholar] [CrossRef]
- Mari, M.; Morales, A.; Colell, A.; Garcia-Ruiz, C.; Kaplowitz, N.; Fernandez-Checa, J.C. Mitochondrial glutathione: Features, regulation and role in disease. Biochim. Biophys. Acta 2013, 1830, 3317–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, S.; Laschi, E.; Buonocore, G. Oxidative stress biomarkers in the perinatal period: Diagnostic and prognostic value. Semin. Fetal Neonatal Med. 2020, 5, 101087. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [Green Version]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Aydogan, U.; Durmaz, E.; Ercan, C.M.; Eken, A.; Ulutas, O.K.; Kavuk, S.; Gursel, O.; Alanbay, I.; Akay, C.; Kurekci, A.E.; et al. Effects of smoking during pregnancy on DNA damage and ROS level consequences in maternal and newborns’ blood. Arh. Hig. Rada Toksikol. 2013, 64, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Napierala, M.; Merritt, T.A.; Miechowicz, I.; Mielnik, K.; Mazela, J.; Florek, E. The effect of maternal tobacco-smoking and second-hand tobacco smoke exposure on human milk oxidant-antioxidant status. Environ. Res. 2019, 170, 110–121. [Google Scholar] [CrossRef]
- Bizoń, A.; Milnerowicz-Nabzdyk, E.; Zalewska, M.; Zimmer, M.; Milnerowicz, H. Changes in pro/antioxidant balance in smoking and non-smoking pregnant women with intrauterine growth restriction. Reprod. Toxicol. 2011, 32, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Bizoń, A.; Milnerowicz, H. The effect of passive and active exposure to tobacco smoke on lipid profile parameters and the activity of certain membrane enzymes in the blood of women in the first trimester of pregnancy. Environ. Toxicol. Pharmacol. 2017, 53, 74–80. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Garavaglia, M.L.; Colombo, G.; Astori, E.; Lionetti, M.C.; La Porta, C.A.M.; Santucci, A.; Rossi, R.; Giustarini, D.; Milzani, A. Cigarette smoke and glutathione: Focus on in vitro cell models. Toxicol. In Vitro 2020, 65, 104818. [Google Scholar] [CrossRef] [PubMed]
- Chełchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Lewandowski, L.; Reśko-Zachara, M.; Maciejewski, T.M. Influence of active exposure to tobacco smoke on nitric oxide status of pregnant women. Int. J. Environ. Res. Public Health. 2018, 15, 2719. [Google Scholar] [CrossRef] [Green Version]
- van der Vlugt, E.R.; Verburg, P.E.; Leemaqz, S.Y.; McCowan, L.M.E.; Poston, L.; Kenny, L.C.; Myers, J.; Walker, J.J.; Dekker, G.A.; Roberts, C.T.; et al. Sex- and growth-specific characteristics of small for gestational age infants: A prospective cohort study. Biol. Sex Differ. 2020, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.J.; Tunstall-Pedoe, H.; Feyerabend, C.; Vesey, C.; Saloojee, Y. Comparison of tests used to distinguish smokers from non-smokers. Am. J. Public Health 1987, 77, 1435–1438. [Google Scholar] [CrossRef] [Green Version]
- Tatzber, F.; Griebenow, S.; Wonisch, W.; Winkler, R. Dual method for the determination of peroxidase activity and total peroxides-iodide leads to a significant increase of peroxidase activity in human sera. Anal. Biochem. 2003, 316, 147–153. [Google Scholar] [CrossRef]
- Boehm, R.E.; Do Nascimento, S.N.; Cohen, C.R.; Bandiera, S.; Pulcinelli, R.R.; Balsan, A.M.; Fao, N.S.; Peruzzi, C.; Garcia, S.C.; Sekine, L.; et al. Cigarette smoking and antioxidant defences in packed red blood cells prior to storage. Blood Transfus. 2020, 18, 40–48. [Google Scholar]
- Garg, N.; Singh, R.; Dixit, J.; Jain, A.; Tewari, V. Levels of lipid peroxides and antioxidants in smokers and nonsmokers. J. Periodontal Res. 2006, 41, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kaur, M.; Suhalka, M.L.; Shrivastav, C. Correlation of α-lipoic acid and glutathione level with free radical excess in tobacco consumers. J. Clin. Diagn. Res. 2016, 10, CC01–CC04. [Google Scholar] [CrossRef]
- Mons, U.; Muscat, J.E.; Modesto, J.; Richie, J.P., Jr.; Brenner, H. Effect of smoking reduction and cessation on the plasma levels of the oxidative stress biomarker glutathione—Post-hoc analysis of data from a smoking cessation trial. Free Radic. Biol. Med. 2016, 91, 172–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bizon, A.; Milnerowicz, H. Effect of tobacco smoking on glutathione concentration in the blood. Przegl. Lek. 2012, 69, 809–811. [Google Scholar] [PubMed]
- Gould, N.S.; Min, E.; Gauthier, S.; Martin, R.J.; Day, B.J. Lung glutathione adaptive responses to cigarette smoke exposure. Respir. Res. 2011, 12, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohan, S.B.; Buhring, K.; Lauer, A.C.; Friedrich, A.; Lademann, J.; Buss, A.; Sabat, R.; Wolk, K.; Meinke, M.C. Analysis of the status of the cutaneous endogenous and exogenous antioxidative system of smokers and the short-term effect of defined smoking thereon. Antioxidants 2020, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- Milnerowicz, H.; Wrześniak, M.; Królik, M.; Kowalska, K. Influence of tobacco smoke on zinc, cadmium, iron, iron-binding proteins, and low-weight anti-oxidant status in pregnancy. Inhal. Toxicol. 2018, 30, 534–541. [Google Scholar] [CrossRef]
- Lee, W.J.; Chen, Y.L.; Chu, Y.W.; Chien, D.S. Comparison of glutathione peroxidase-3 protein expression and enzyme bioactivity in normal subjects and patients with sepsis. Clin. Chim. Acta 2019, 489, 177–182. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Gitto, E.; Reiter, R.J.; Karbownik, M. Causes of oxidative stress in the pre-and perinatal period. Biol. Neonate 2002, 81, 146–157. [Google Scholar] [CrossRef]
- Sherlock, L.G.; Balasubramaniyan, D.; Zheng, L.; Zarate, M.; Sizemore, T.; Delaney, C.; Tipple, T.E.; Wright, C.J.; Nozik-Grayck, E. Neonatal selenoenzyme expression is variably susceptible to duration of maternal selenium deficiency. Antioxidants 2020, 9, 288. [Google Scholar] [CrossRef]
- Sobczak, A.; Wardas, W.; Zielińska-Danch, W.; Pawlicki, K. The influence of smoking on plasma homocysteine and cysteine levels in passive and active smokers. Clin. Chem. Lab. Med. 2004, 42, 408–414. [Google Scholar] [CrossRef]
- Aspera-Werz, R.H.; Ehnert, S.; Heid, D.; Zhu, S.; Chen, T.; Braun, B.; Sreekumar, V.; Arnscheidt, C.; Nussler, A.K. Nicotine and cotinine inhibit catalase and glutathione reductase activity contributing to the impaired osteogenesis of SCP-1 cells exposed to cigarette smoke. Oxid. Med. Cell Longev. 2018, 2018, 3172480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kode, A.; Rajendrasozhan, S.; Caito, S.; Yang, S.R.; Megson, I.L.; Rahman, I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L478–L488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napierala, M.; Olszewski, J.; Miechowicz, I.; Jablecka, A.; Czarnywojtek, A.; Malinger, S.; Florek, E. The influence of tobacco smoke exposure on selected markers of oxidative stress, kidneys and liver function in the serum of rats with streptozotocin-induced diabetes. Pharmacol. Rep. 2019, 71, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Milnerowicz, H. The influence of age and gender on the pro/antioxidant status in young healthy people. Ann. Clin. Lab. Sci. 2016, 46, 480–488. [Google Scholar] [PubMed]
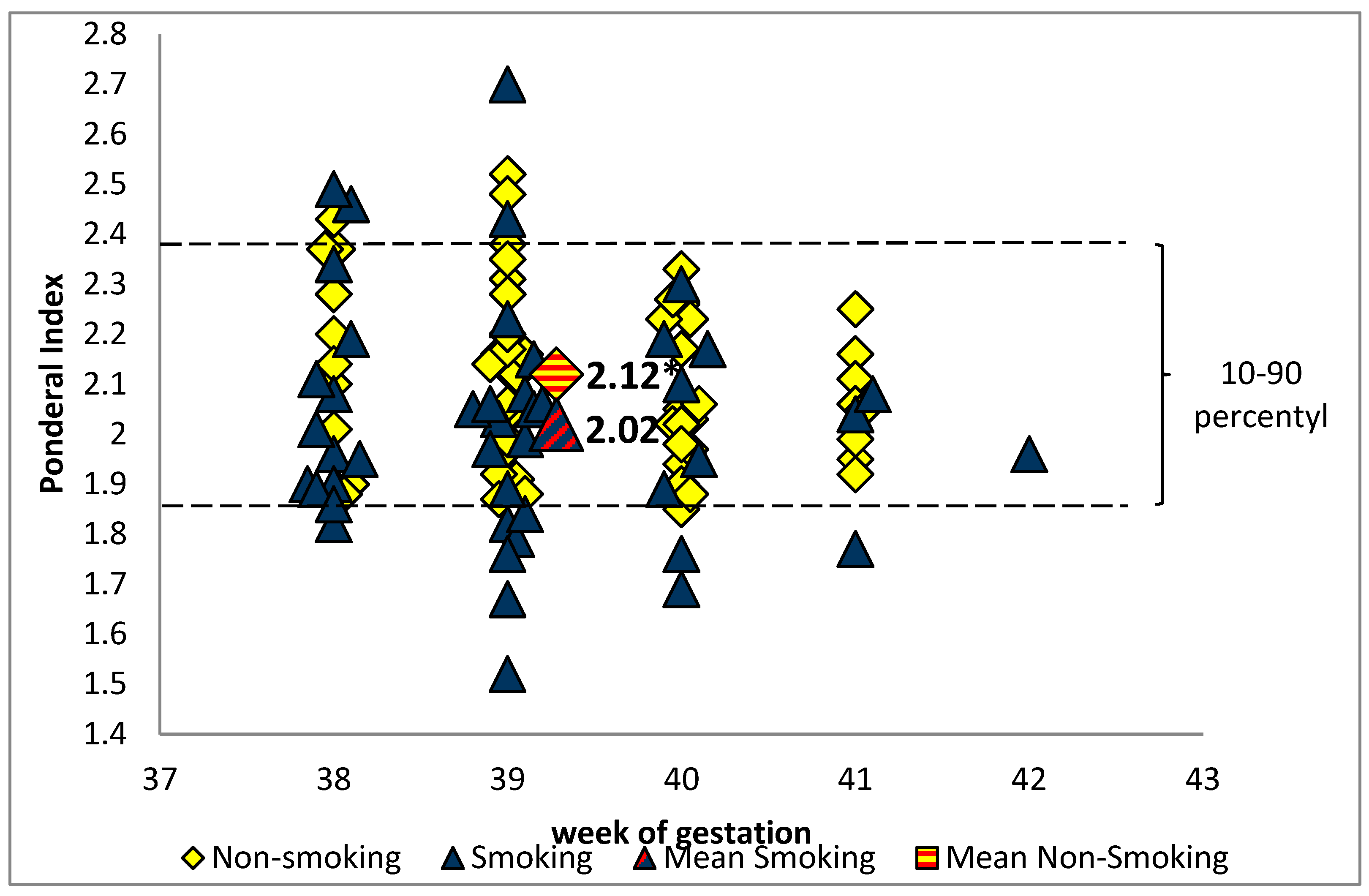
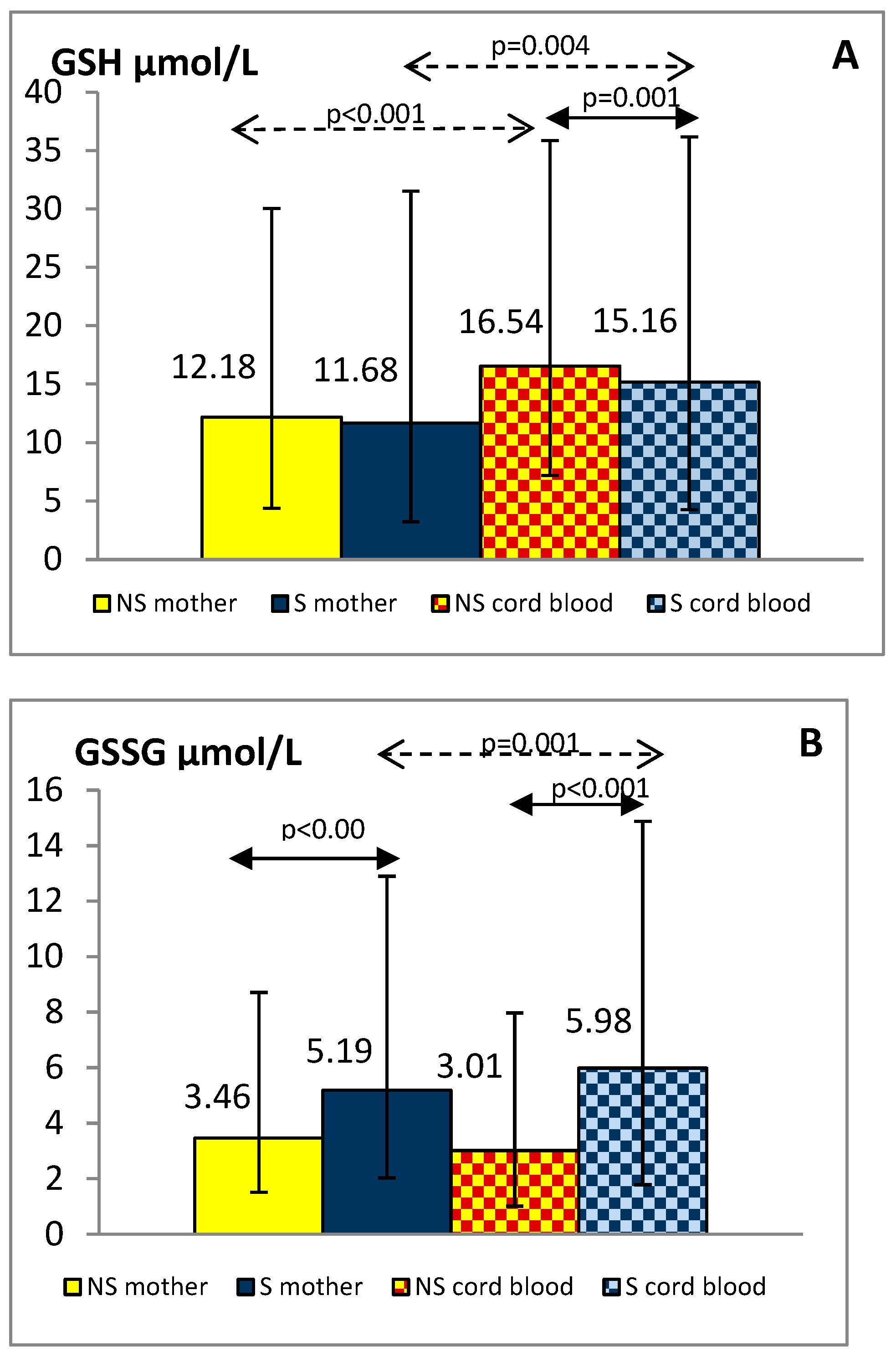
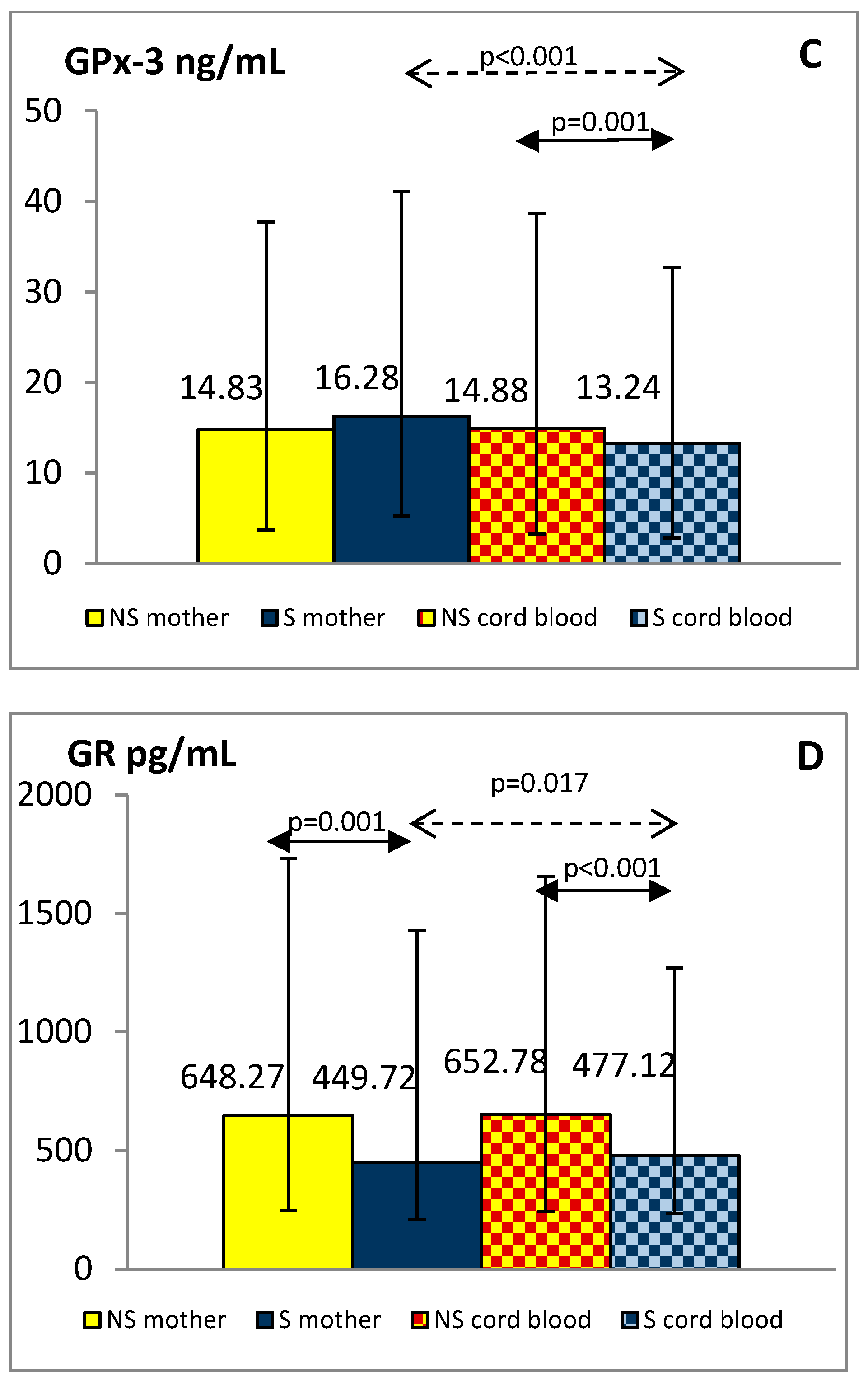
| Biochemical Parameters | GSH (μmol/L) | GSSG (μmol/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mothers | Newborns | Mothers | Newborns | |||||
| Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | |
| Non-smoking Group | ||||||||
| GSH (μmol/L) | - | - | - | - | −0.423 | <0.001 | 0.143 | 0.257 |
| GSSG (μmol/L) | −0.423 | <0.001 | 0.143 | 0.257 | - | - | - | - |
| GPx-3 (ng/mL) | −0.204 | 0.102 | −0.049 | 0.701 | 0.267 | 0.032 | 0.073 | 0.562 |
| GR (pg/mL) | 0.446 | <0.001 | 0.317 | 0.010 | −0.376 | 0.002 | −0.228 | 0.067 |
| TOC (mmol/L) | −0.434 | <0.001 | −0.110 | 0.383 | 0.373 | 0.002 | 0.258 | 0.038 |
| Smoking Group | ||||||||
| GSH (μmol/L) | - | - | - | - | −0.309 | 0.039 | 0.137 | 0.370 |
| GSSG (μmol/L) | −0.309 | 0.039 | 0.137 | 0.370 | - | - | - | - |
| GPx-3 (ng/mL) | −0.510 | <0.001 | −0.173 | 0.255 | 0.407 | 0.006 | 0.248 | 0.101 |
| GR (pg/mL) | 0.408 | 0.005 | 0.229 | 0.134 | −0.498 | 0.001 | −0.378 | 0.011 |
| TOC (mmol/L) | −0.442 | 0.002 | −0.573 | <0.001 | 0.087 | 0.568 | −0.058 | 0.705 |
| Cotinine (μg/L) | −0.480 | 0.001 | −0.532 | <0.001 | 0.275 | 0.068 | 0.187 | 0.219 |
| Mothers n = 110 | Newborns n = 110 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | B | 95%CI | β | p-Value | B | 95%CI | β | p-Value |
| Smoking status No = 0, Yes = 1 | −2.939 | −4.376/−1.502 | −0.383 | <0.001 | 1.211 | −1.019/3.441 | 0.158 | 0.284 |
| GSSG | −0.192 | −0.653/0.270 | −0.089 | 0.412 | 0.626 | 0.124/1.128 | 0.332 | 0.015 |
| GPx-3 | −0.150 | −0.274/−0.025 | −0.196 | 0.019 | −0.051 | −0.218/0.117 | −0.055 | 0.549 |
| GR | 0.005 | 0.002/0.007 | 0.354 | <0.001 | 0.005 | 0.002/0.008 | 0.301 | 0.005 |
| TOC | −6.716 | −10.026/−3.405 | −0.345 | <0.001 | −9.527 | −15.995/−3.060 | −0.313 | 0.004 |
| R2 (%) | 36.3 | 19.8 | ||||||
| Variable | Mothers n = 110 | Newborns n = 110 | ||||||
|---|---|---|---|---|---|---|---|---|
| B | 95%CI | β | p-Value | B | 95%CI | β | p-Value | |
| Smoking status No = 0, Yes = 1 | −1.518 | −2.097/−0.940 | −0.428 | <0.001 | −2.777 | −3.429/−2.125 | −0.683 | <0.001 |
| GSH | −0.034 | −0.115/0.048 | −0.073 | 0.412 | 0.089 | 0.018/0.161 | 0.169 | 0.015 |
| GPx-3 | 0.079 | 0.027/0.130 | 0.223 | 0.003 | 0.041 | −0.022/0.104 | 0.084 | 0.201 |
| GR | −0.002 | −0.003/−0.001 | −0.260 | 0.002 | −0.002 | −0.003/−0.001 | −0.211 | 0.006 |
| TOC | 0.877 | −0.608/2.361 | 0.097 | 0.244 | 0.738 | −1.803/3.280 | 0.046 | 0.566 |
| R2 (%) | 47.6 | 59.2 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chełchowska, M.; Gajewska, J.; Ambroszkiewicz, J.; Mazur, J.; Ołtarzewski, M.; Maciejewski, T.M. Influence of Oxidative Stress Generated by Smoking during Pregnancy on Glutathione Status in Mother-Newborn Pairs. Antioxidants 2021, 10, 1866. https://doi.org/10.3390/antiox10121866
Chełchowska M, Gajewska J, Ambroszkiewicz J, Mazur J, Ołtarzewski M, Maciejewski TM. Influence of Oxidative Stress Generated by Smoking during Pregnancy on Glutathione Status in Mother-Newborn Pairs. Antioxidants. 2021; 10(12):1866. https://doi.org/10.3390/antiox10121866
Chicago/Turabian StyleChełchowska, Magdalena, Joanna Gajewska, Jadwiga Ambroszkiewicz, Joanna Mazur, Mariusz Ołtarzewski, and Tomasz M. Maciejewski. 2021. "Influence of Oxidative Stress Generated by Smoking during Pregnancy on Glutathione Status in Mother-Newborn Pairs" Antioxidants 10, no. 12: 1866. https://doi.org/10.3390/antiox10121866
APA StyleChełchowska, M., Gajewska, J., Ambroszkiewicz, J., Mazur, J., Ołtarzewski, M., & Maciejewski, T. M. (2021). Influence of Oxidative Stress Generated by Smoking during Pregnancy on Glutathione Status in Mother-Newborn Pairs. Antioxidants, 10(12), 1866. https://doi.org/10.3390/antiox10121866










