Quercetin Alleviates the Immunotoxic Impact Mediated by Oxidative Stress and Inflammation Induced by Doxorubicin Exposure in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Compounds
2.2. Animals and Study Design
2.3. Sampling and Analysis
2.4. Hematological Parameters
2.5. Assessment of Immunological Parameters in Serum
2.6. Antioxidants in Spleen Tissue
2.7. DNA Damage (Comet Assay)
2.8. Transcription Levels of Antioxidant, Inflammatory Response, and Apoptosis-Related Genes by Real-Time qPCR
2.9. Light Microscopy
2.10. Data Analysis
3. Results
3.1. Effect on Hematological Variables
3.2. Antioxidants in Spleen Tissue
3.3. Immunological Parameters in Serum
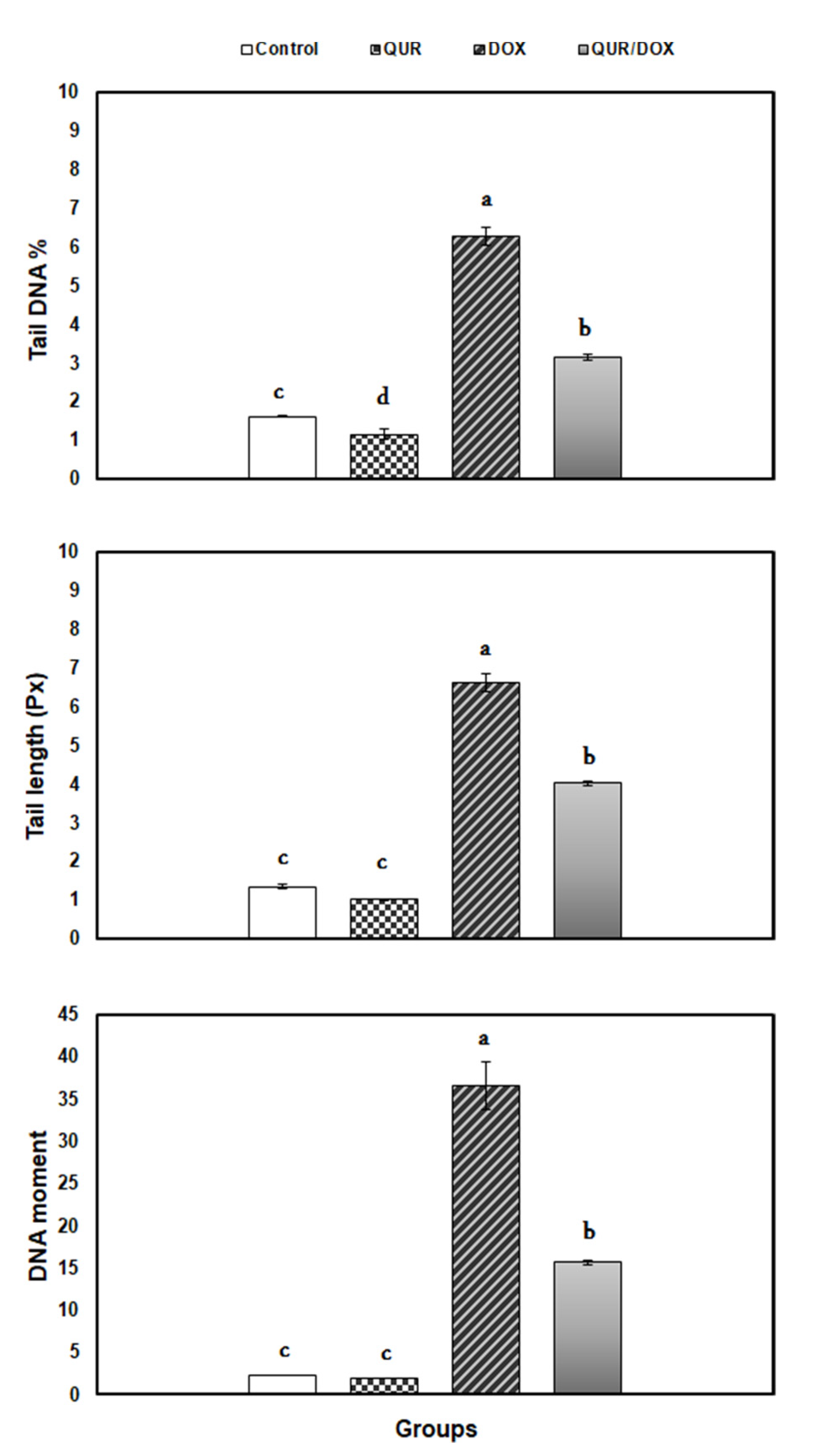
3.4. DNA Damage (Comet Assay)
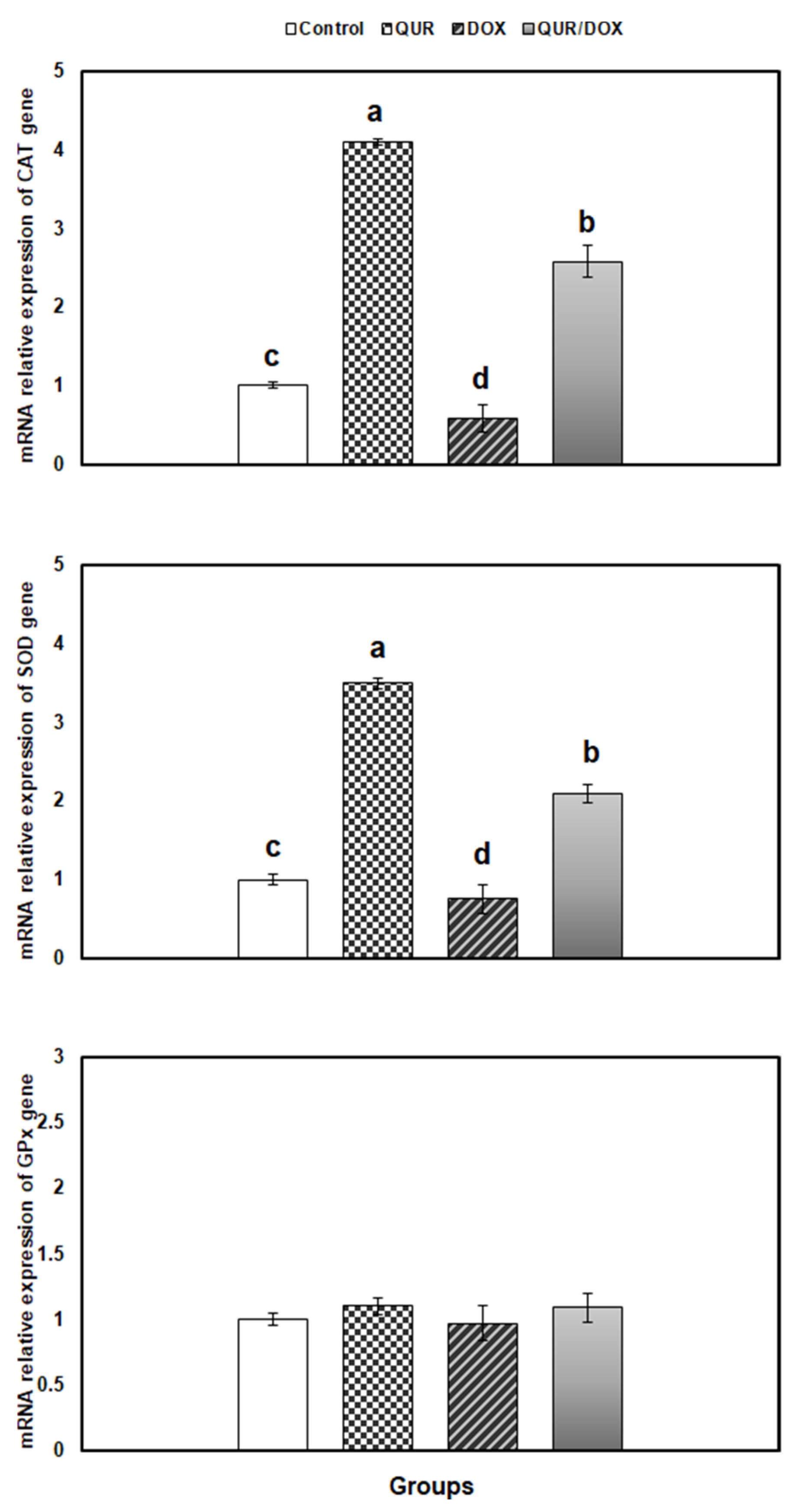
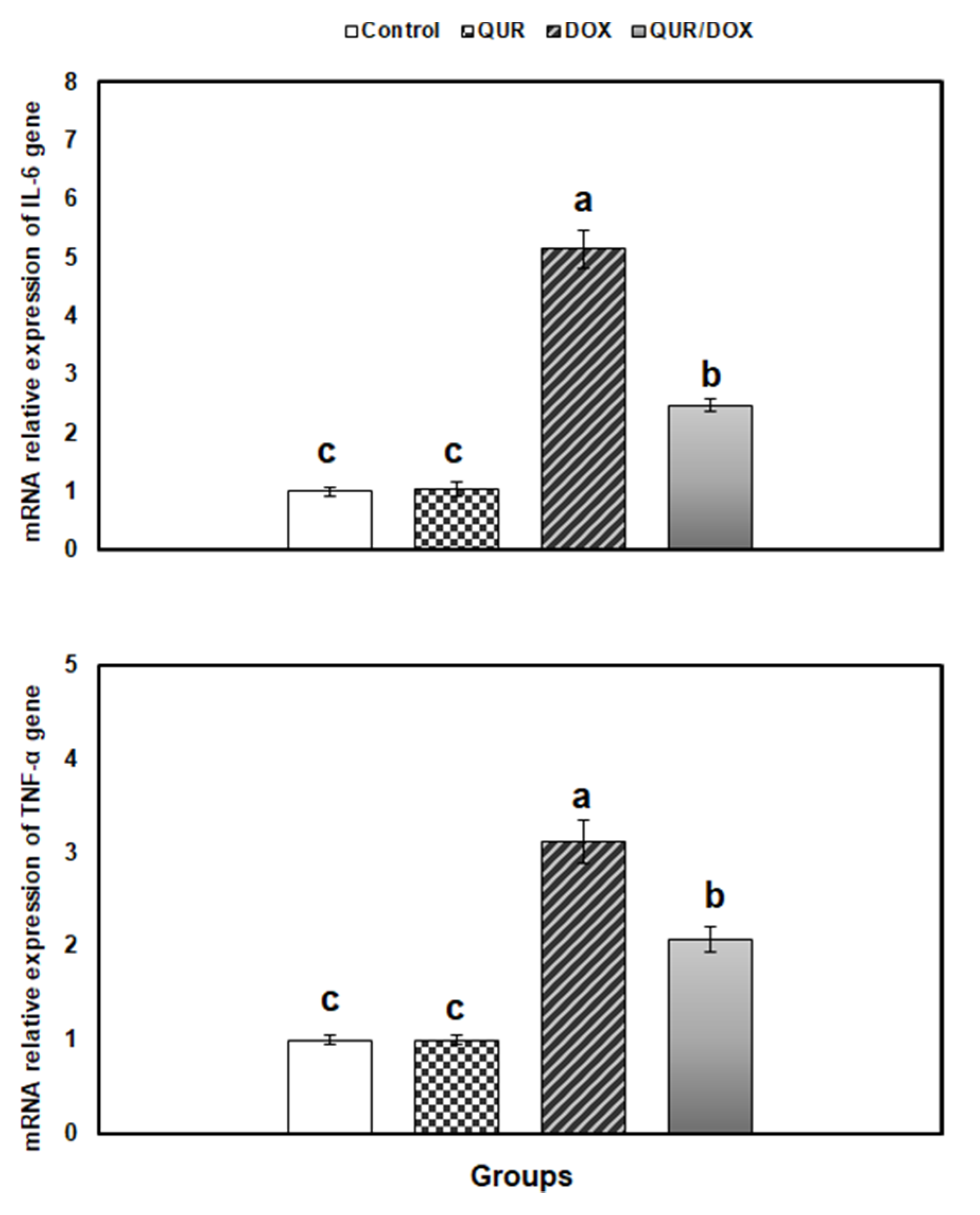
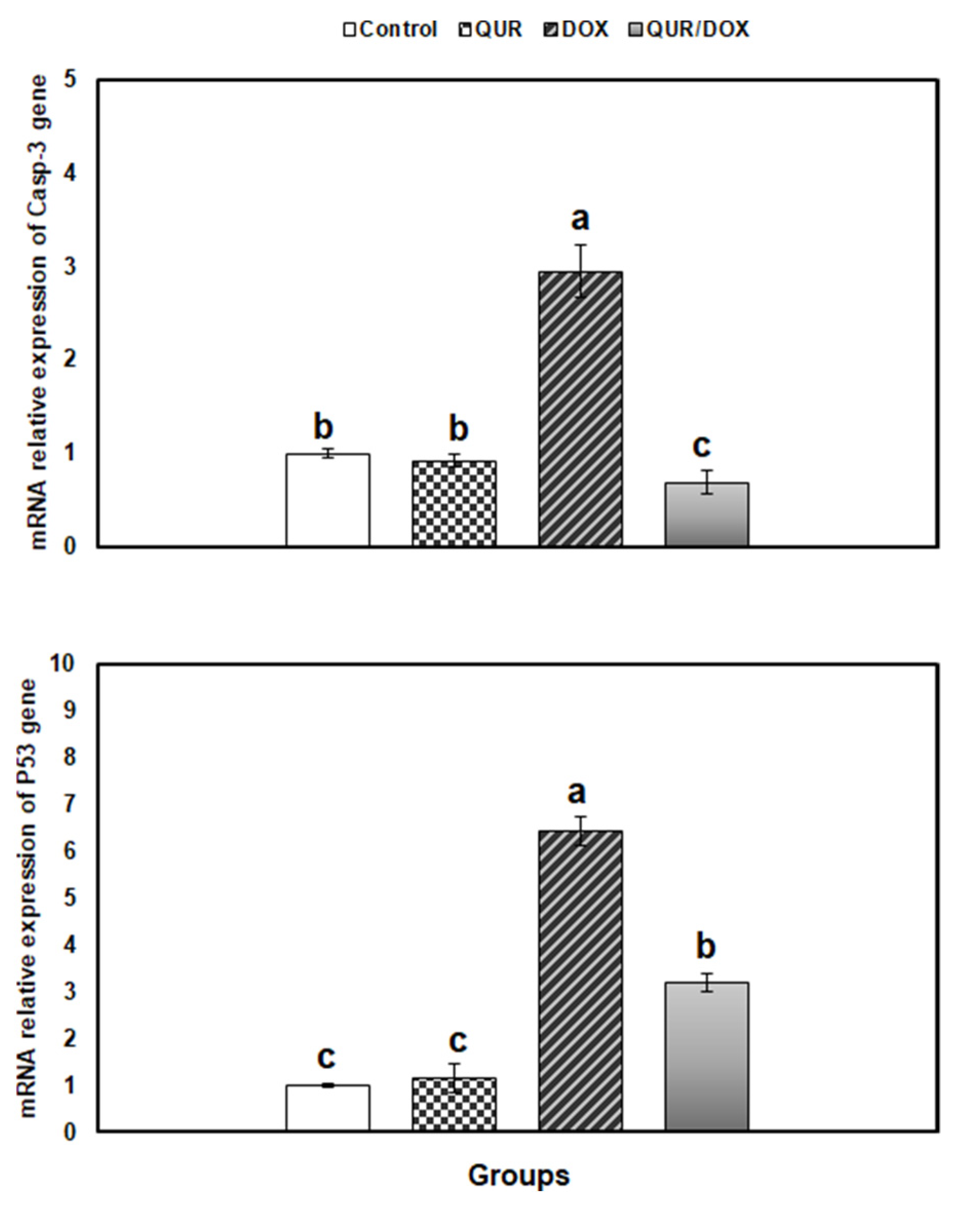
3.5. Transcriptional Profile of Antioxidants, Inflammatory and Apoptosis-Related Genes in the Spleen Tissue
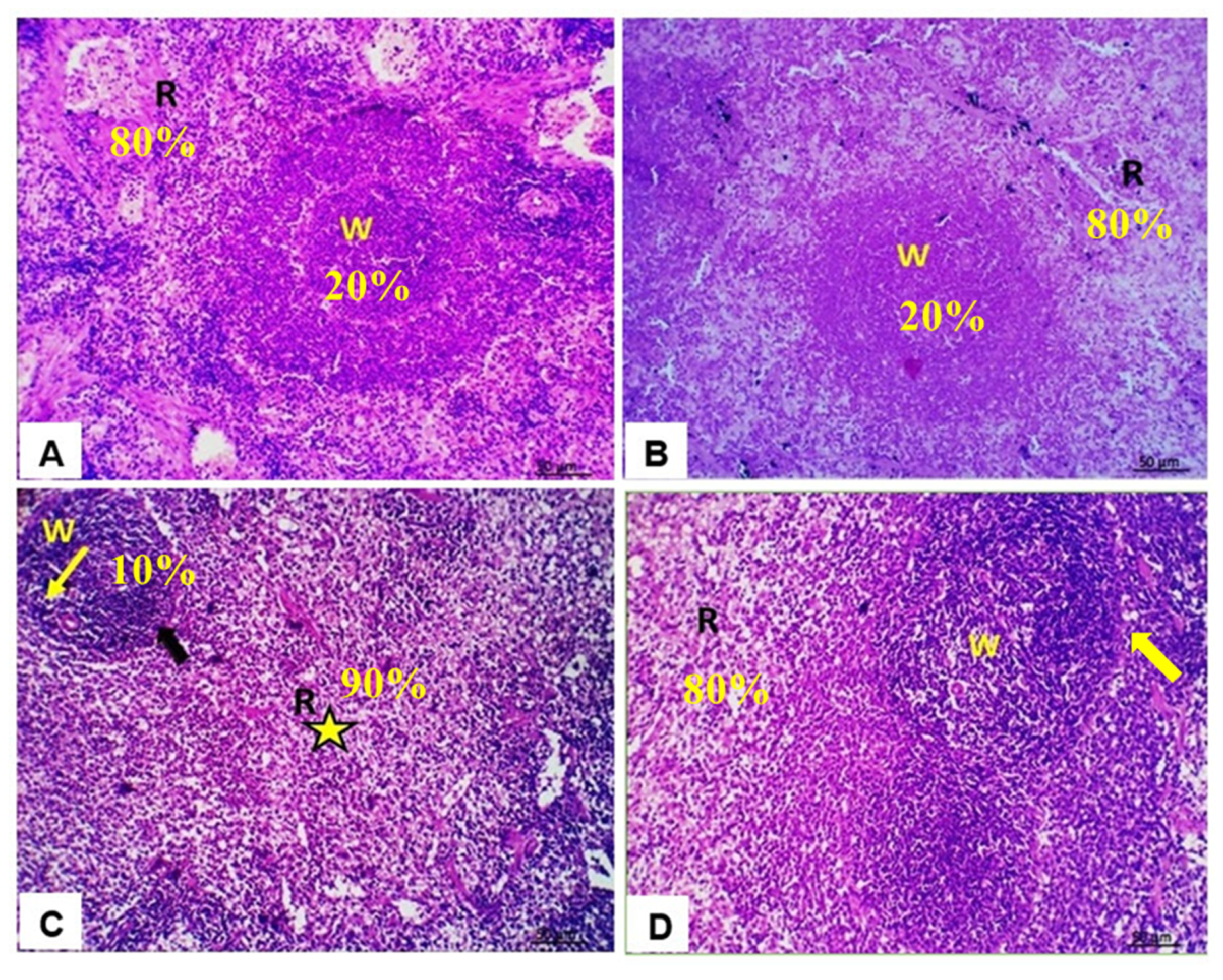
3.6. Histopathological Findings in Spleen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Gopalakrishnan, A.V. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef] [PubMed]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Hmingthazuali, V.L. Protection of Doxorubicin-Induced Biochemical Injury in the Rat Bone Marrow by a Dietary Bioflavonoid Naringin. Ann. Clin. Lab. Res. 2018, 6, 224. [Google Scholar] [CrossRef]
- Lubis, M.R.; Haryani, R.; Safriana, S.; Satria, D. Ethanolic Extract of Herb Pugun Tanoh (Picria fel-terrae Lour.) Modulates TCD4+ and TCD8+ Cell Profile of Doxorubicin-Induced Immuno-Suppressed Rats. Open Access Maced. J. Med. Sci. 2019, 7, 3774–3776. [Google Scholar] [CrossRef]
- Owumi, S.E.; Nwozo, S.O.; Arunsi, U.O.; Oyelere, A.K.; Odunola, O.A. Co-administration of Luteolin mitigated toxicity in rats’ lungs associated with doxorubicin treatment. Toxicol. Appl. Pharmacol. 2021, 411, 115380. [Google Scholar] [CrossRef]
- Shaldoum, F.; El-Kott, A.F.; Ouda, M.M.A.; Abd-Ella, E.M. Immunomodulatory effects of bee pollen on doxorubicin-induced bone marrow/spleen immunosuppression in rat. J. Food Biochem. 2021, 45, e13747. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, A.E.; Hermawan, A.; Nastiti, K.; Suven, S.; Elisa, P.; Hadibarata, T.; Meiyanto, E. Immunomodulatory effects of hexane insoluble fraction of Ficus septica Burm. F. in doxorubicin-treated rats. Asian Pac. J. Cancer Prev. 2012, 13, 5785–5790. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.Y.; Li, W.G.; Wu, Y.J.; Gao, M.T. Amelioration of doxorubicin-induced myocardial oxidative stress and immunosuppression by grape seed proanthocyanidins in tumour-bearing mice. J. Pharm. Pharmacol. 2005, 57, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Jadapalli, J.K.; Wright, G.W.; Kain, V.; Sherwani, M.A.; Sonkar, R.; Yusuf, N.; Halade, G.V. Doxorubicin triggers splenic contraction and irreversible dysregulation of COX and LOX that alters the inflammation-resolution program in the myocardium. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1091–H1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; You, Z.Q.; Yang, L.; Li, L.L.; Wu, Y.P.; Gu, L.Q.; Xin, Y.F. Protective effect of Shenmai injection on doxorubicin-induced cardiotoxicity via regulation of inflammatory mediators. BMC Complement. Altern. Med. 2019, 19, 317. [Google Scholar] [CrossRef]
- Guo, J.; Cao, W.; Chen, C.; Chen, X. Peroxiredoxin 6 overexpression regulates adriamycin-induced myocardial injury, oxidative stress and immune response in rats. Ann. Transl. Med. 2020, 8, 1320. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Abdel-Motal, S.M.; Abd-Elsalam, M.; Abd El-Hameed, N.E.; Awad, A. Restoring strategy of ethanolic extract of Moringa oleifera leaves against Tilmicosin-induced cardiac injury in rats: Targeting cell apoptosis-mediated pathways. Gene 2020, 730, 144272. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Salem, H.F.A.; Metwally, M.M.M.; Emad, R.M.; Elbohi, K.M.; Ali, S.A. Protective effect of Spirulina platensis against physiological, ultrastructural and cell proliferation damage induced by furan in kidney and liver of rat. Ecotoxicol. Environ. Saf. 2020, 192, 110256. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, S.M.; Ahmed, A.I.; Awad, A.; Mohammed, W.A.; Metwally, M.M.M.; Almeer, R.; Abdel-Daim, M.M.; Khalil, S.R. Moringa oleifera ethanolic extract attenuates tilmicosin-induced renal damage in male rats via suppression of oxidative stress, inflammatory injury, and intermediate filament proteins mRNA expression. Biomed. Pharmacother. 2021, 133, 110997. [Google Scholar] [CrossRef] [PubMed]
- El Bohi, K.M.; Abdel-Motal, S.M.; Khalil, S.R.; Abd-Elaal, M.M.; Metwally, M.M.M.; El Hady, W.M. The efficiency of pomegranate (Punica granatum) peel ethanolic extract in attenuating the vancomycin-triggered liver and kidney tissues injury in rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 7134–7150. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Taha, H.S.A.; Ismail, T.A.; Khalil, S.R.; Abou-Zeid, S.M. Immune response and susceptibility of Nile tilapia fish to Aeromonas hydrophila infection following the exposure to Bifenthrin and/or supplementation with Petroselinum crispum essential oil. Ecotoxicol. Environ. Saf. 2021, 216, 112205. [Google Scholar] [CrossRef] [PubMed]
- Kaiserova, H.; Simunek, T.; van der Vijgh, W.J.; Bast, A.; Kvasnickova, E. Flavonoids as protectors against doxorubicin cardiotoxicity: Role of iron chelation, antioxidant activity and inhibition of carbonyl reductase. Biochim. Biophys. Acta 2007, 1772, 1065–1074. [Google Scholar] [CrossRef]
- Karimi, A.; Naeini, F.; Azar, V.A.; Hasanzadeh, M.; Ostadrahimi, A.; Niazkar, H.R.; Mobasseri, M.; Tutunchi, H. A comprehensive systematic review of the therapeutic effects and mechanisms of action of quercetin in sepsis. Phytomedicine 2021, 86, 153567. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Khalil, S.R.; Awad, A.; Khairy, G.M. Quercetin Reverses Altered Energy Metabolism in the Heart of Rats Receiving Adriamycin Chemotherapy. Cardiovasc. Toxicol. 2018, 18, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Mohammed, A.T.; Abd El-Fattah, A.H.; Zaglool, A.W. Intermediate filament protein expression pattern and inflammatory response changes in kidneys of rats receiving doxorubicin chemotherapy and quercetin. Toxicol. Lett. 2018, 288, 89–98. [Google Scholar] [CrossRef]
- Willard-Mack, C.L.; Elmore, S.A.; Hall, W.C.; Harleman, J.; Kuper, C.F.; Losco, P.; Rehg, J.E.; Ruhl-Fehlert, C.; Ward, J.M.; Weinstock, D.; et al. Nonproliferative and Proliferative Lesions of the Rat and Mouse Hematolymphoid System. Toxicol. Pathol. 2019, 47, 665–783. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, P.D.; Lopez-Hernandez, F.J.; Perez-Barriocanal, F.; Morales, A.I.; Lopez-Novoa, J.M. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol. Dial. Transplant. 2011, 26, 3484–3495. [Google Scholar] [CrossRef] [PubMed]
- Iskender, H.; Dokumacioglu, E.; Sen, T.M.; Ince, I.; Kanbay, Y.; Saral, S. The effect of hesperidin and quercetin on oxidative stress, NF-kappaB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed. Pharmacother. 2017, 90, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF-kappaB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 2020, 20, 280. [Google Scholar] [CrossRef]
- Siveski-Iliskovic, N.; Kaul, N.; Singal, P.K. Probucol promotes endogenous antioxidants and provides protection against adriamycin-induced cardiomyopathy in rats. Circulation 1994, 89, 2829–2835. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Chinde, S.; Grover, P. Toxicological assessment of nano and micron-sized tungsten oxide after 28days repeated oral administration to Wistar rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 819, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.; Mossa, A.-T.H. Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pestic. Biochem. Physiol. 2009, 93, 34–39. [Google Scholar] [CrossRef]
- Bhinge, K.N.; Gupta, V.; Hosain, S.B.; Satyanarayanajois, S.D.; Meyer, S.A.; Blaylock, B.; Zhang, Q.J.; Liu, Y.Y. The opposite effects of doxorubicin on bone marrow stem cells versus breast cancer stem cells depend on glucosylceramide synthase. Int. J. Biochem. Cell Biol. 2012, 44, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Aroonvilairat, S.; Tangjarukij, C.; Sornprachum, T.; Chaisuriya, P.; Siwadune, T.; Ratanabanangkoon, K. Effects of topical exposure to a mixture of chlorpyrifos, cypermethrin and captan on the hematological and immunological systems in male Wistar rats. Environ. Toxicol. Pharmacol. 2018, 59, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Melghit, K.; Ali, B.H. The acute proinflammatory and prothrombotic effects of pulmonary exposure to rutile TiO2 nanorods in rats. Exp. Biol. Med. 2008, 233, 610–619. [Google Scholar] [CrossRef]
- Tsang, Y.-W.; Chi, K.-H.; Hu, C.-J.; Tseng, C.-L.; Tseng, F.-W.; Wang, Y.-S. Chemotherapy-induced immunosuppression is restored by a fermented soybean extract: A proof of concept clinical trial. Nutr. Res. 2007, 27, 679–684. [Google Scholar] [CrossRef]
- Steele, T.A. Chemotherapy-induced immunosuppression and reconstitution of immune function. Leuk. Res. 2002, 26, 411–414. [Google Scholar] [CrossRef]
- Ferraro, C.; Quemeneur, L.; Prigent, A.F.; Taverne, C.; Revillard, J.P.; Bonnefoy-Berard, N. Anthracyclines trigger apoptosis of both G0-G1 and cycling peripheral blood lymphocytes and induce massive deletion of mature T and B cells. Cancer Res. 2000, 60, 1901–1907. [Google Scholar] [CrossRef]
- Katoch, O.; Kumar, A.; Adhikari, J.S.; Dwarakanath, B.S.; Agrawala, P.K. Sulforaphane mitigates genotoxicity induced by radiation and anticancer drugs in human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 758, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.T.; Zeid, E.H.; Imam, T.S. Protective role of quercetin against hematotoxic and immunotoxic effects of furan in rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 3780–3789. [Google Scholar] [CrossRef]
- Donmez, H.H.; Donmez, N.; Kisadere, I.; Undag, I. Protective effect of quercetin on some hematological parameters in rats exposed to cadmium. Biotech. Histochem. 2019, 94, 381–386. [Google Scholar] [CrossRef]
- Ince, E. The protective effect of quercetin in the alcohol-induced liver and lymphoid tissue injuries in newborns. Mol. Biol. Rep. 2020, 47, 451–459. [Google Scholar] [CrossRef]
- Rastogi, S.; Haldar, C. Comparative effect of melatonin and quercetin in counteracting LPS induced oxidative stress in bone marrow mononuclear cells and spleen of Funambulus pennanti. Food Chem. Toxicol. 2018, 120, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, A.C.; Olaniyan, O.O.; Famusiwa, C.D.; Josiah, S.S.; Olaleye, M.T. Ameliorative effect of quercetin, catechin, and taxifolin on rotenone-induced testicular and splenic weight gain and oxidative stress in rats. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20180230. [Google Scholar] [CrossRef]
- Liu, C.M.; Sun, Y.Z.; Sun, J.M.; Ma, J.Q.; Cheng, C. Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim. Biophys. Acta 2012, 1820, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Foruozandeh, H.; Khodayar, M.J.; Siahpoosh, A.; Saki, N.; Kheradmand, P. Antioxidant and hepatoprotective effects of Capparis spinosa L. fractions and Quercetin on tert-butyl hydroperoxide- induced acute liver damage in mice. J. Tradit. Complement. Med. 2018, 8, 120–127. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Dai, C.; Zhang, M.; Das Gupta, S. Molecular Mechanisms Underlying Protective Role of Quercetin on Copper Sulfate-Induced Nephrotoxicity in Mice. Front. Vet. Sci. 2020, 7, 586033. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Beer, L.A.; Kossenkov, A.V.; Liu, Q.; Luning Prak, E.; Domchek, S.; Speicher, D.W.; Ky, B. Baseline Immunoglobulin E Levels as a Marker of Doxorubicin- and Trastuzumab-Associated Cardiac Dysfunction. Circ. Res. 2016, 119, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Hui-Chou, H.G.; Olenczak, J.B.; Drachenberg, C.B.; Shea, S.M.; Rodriguez, E.D. Short-term application of doxorubicin chemotherapy immunosuppressive side effects for composite tissue allotransplantation. Ann. Plast. Surg. 2012, 68, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Valentova, K.; Sima, P.; Rybkova, Z.; Krizan, J.; Malachova, K.; Kren, V. (Anti)mutagenic and immunomodulatory properties of quercetin glycosides. J. Sci. Food Agric. 2016, 96, 1492–1499. [Google Scholar] [CrossRef]
- Singh, D.; Tanwar, H.; Jayashankar, B.; Sharma, J.; Murthy, S.; Chanda, S.; Singh, S.B.; Ganju, L. Quercetin exhibits adjuvant activity by enhancing Th2 immune response in ovalbumin immunized mice. Biomed. Pharmacother. 2017, 90, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Vaid, M.; van Steeg, H.; Meeran, S.M. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev. Res. 2010, 3, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Uspenskaya, Y.A.; Mikhutkina, S.V.; Taksanova, E.I.; Popova, N.N.; Olovyannikova, R.Y.; Salmina, A.B. Induction of apoptosis in bone marrow cells is mediated via purinergic receptors. Bull. Exp. Biol. Med. 2004, 138, 114–118. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Sudheer, A.R.; Nalini, N.; Menon, V.P. Effect of quercetin on nicotine-induced biochemical changes and DNA damage in rat peripheral blood lymphocytes. Redox Rep. 2008, 13, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Oyinloye, B.E.; Adenowo, A.F.; Kappo, A.P. Reactive oxygen species, apoptosis, antimicrobial peptides and human inflammatory diseases. Pharmaceuticals 2015, 8, 151–175. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 during Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef]
- Silva, L.C.; Ortigosa, L.C.; Benard, G. Anti-TNF-alpha agents in the treatment of immune-mediated inflammatory diseases: Mechanisms of action and pitfalls. Immunotherapy 2010, 2, 817–833. [Google Scholar] [CrossRef]
- Kanterman, J.; Sade-Feldman, M.; Baniyash, M. New insights into chronic inflammation-induced immunosuppression. Semin. Cancer Biol. 2012, 22, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.C.; Peng, Y.C.; Yang, F.L.; Subeq, Y.M.; Lee, R.P. Slow infusion rate of doxorubicin induces higher pro-inflammatory cytokine production. Regul. Toxicol. Pharmacol. 2016, 81, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.B.; Sardao, V.A.; Oliveira, P.J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.; Deinzer, A.; Stich, L.; Zinser, E.; Steinkasserer, A.; Knippertz, I. Quercetin induces an immunoregulatory phenotype in maturing human dendritic cells. Immunobiology 2020, 225, 151929. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.Q.; Yang, F.Y.; Wang, M.S.; Shi, B.K.; Chen, D.X.; Chen, D.; Zhou, Q.; He, Q.B.; Ma, L.X.; Cheng, W.L.; et al. Quercetin protects against chronic prostatitis in rat model through NF-κB and MAPK signaling pathways. Prostate 2018, 78, 790–800. [Google Scholar] [CrossRef]
- Yuan, K.; Zhu, Q.; Lu, Q.; Jiang, H.; Zhu, M.; Li, X.; Huang, G.; Xu, A. Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J. Nutr. Biochem. 2020, 84, 108454. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, X.; Gao, H.; Zhai, J.; Tao, L.; Sun, J.; Song, Y.; Zhang, J. Quercetin attenuates NLRP3 inflammasome activation and apoptosis to protect INH-induced liver injury via regulating SIRT1 pathway. Int. Immunopharmacol. 2020, 85, 106634. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Heo, K.S.; Lee, H.; Nigro, P.; Thomas, T.; Le, N.T.; Chang, E.; McClain, C.; Reinhart-King, C.A.; King, M.R.; Berk, B.C.; et al. PKCζ mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation. J. Cell Biol. 2011, 193, 867–884. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, M.; Sharma, R.; Sharma, N. Deltamethrin-Induced Immunotoxicity and its Protection by Quercetin: An Experimental Study. Endocr. Metab. Immune. Disord. Drug Targets 2020, 20, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, Y.; Fang, W.; Wang, D. Regulatory effect of quercetin on hazardous microcystin-LR-induced apoptosis of Carassius auratus lymphocytes in vitro. Fish Shellfish Immunol. 2014, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Karaulov, A.V.; Renieri, E.A.; Smolyagin, A.I.; Mikhaylova, I.V.; Stadnikov, A.A.; Begun, D.N.; Tsarouhas, K.; Djordjevic, A.B.; Hartung, T.; Tsatsakis, A. Long-term effects of chromium on morphological and immunological parameters of Wistar rats. Food Chem. Toxicol. 2019, 133, 110748. [Google Scholar] [CrossRef]
| Gene | Sequence | 5′-3′ Primer Sequence | Accession Number |
|---|---|---|---|
| Tumor necrosis factor-alpha (TNFα) | forward | GTTCTCTTCAAGGGACAAGGC | NM_012675.3 |
| reverse | TGGAAGACTCCTCCCAGGTA | ||
| Interleukin-6 (IL6) | forward | CCACTGCCTTCCCTACTTCA | NM_012589.2 |
| reverse | ACAGTGCATCATCGCTGTTC | ||
| Tumor suppressor P53 | forward | CTGTTTCAAAAAGCAAAAAGATGAC | NM_030989.3 |
| reverse | TAGCAAGGAAAGTCATGAACTGCCA | ||
| Caspase 3, apoptosis-related cysteine peptidase (Caspase 3) | forward | TTTGCGCCATGCTGAAACT | NM_012922.2 |
| reverse | ACGAGTGAGGATGTGCATGAATT | ||
| Superoxide dismutase (SOD) | forward | CATTCCATCATTGGCCGTACT | BC_082800.1 |
| reverse | CCACCTTTGCCCAAGTCATC | ||
| Catalase (CAT) | forward | GTACAGGCCGGCTCTCACA | NM_012520.2 |
| reverse | ACCCGTGCTTTACAGGTTAGCT | ||
| Glutathione peroxidase (GPx) | forward | GCGCTGGTCTCGTCCATT | NM_030826.3 |
| reverse | TGGTGAAACCGCCTTTCTTT | ||
| Beta actin (β-actin) | forward | CACCATGTACCCAGGCATTG | NM_031144.3 |
| reverse | CCTGCTTGCTGATCCACATC |
| Hematological Variables | Experimental Groups | |||
|---|---|---|---|---|
| Control | QUR | DOX | QUR/DOX | |
| RBCs (106/mm3) | 8.19 ± 0.12 a | 8.13 ± 0.41 a | 5.53 ± 0.69 b | 6.56 ± 0.38 b |
| Hb (g/dl) | 13.77 ± 0.03 a | 13.62 ± 0.24 a | 8.46 ± 0.07 c | 9.48 ± 0.20 b |
| PCV (%) | 48.37 ± 2.92 a | 49.86 ± 0.24 a | 32.05 ± 2.66 b | 28.68 ± 2.64 b |
| MCV (fl) | 52.47 ± 1.38 a | 51.96 ± 0.79 a | 36.98 ± 2.41 b | 40.17 ± 0.73 b |
| MCH (Pg) | 34.25 ± 1.95 a | 35.29 ±1.28 a | 23.30 ± 1.45 b | 26.46 ± 1.11 b |
| MCHC (%) | 8.74 ± 0.13 c | 9.40 ± 0.70 c | 12.90 ±0.03 a | 11.26 ± 0.85 b |
| WBC (103/mm3) | 4.75 ± 0.03 a | 4.71 ± 0.05 a | 3.13 ± 0.49 b | 2.96 ± 0.31 b |
| Lymphocyte (103/mm3) | 4.28 ± 0.39 b | 4.05 ± 0.24 b | 8.14 ± 0.58 a | 7.08 ± 0.41 a |
| Neutrophil (103/mm3) | 0.803 ± 0.00 a | 0.810 ± 0.00 a | 0.383 ± 0.04 b | 0.727 ± 0.05 a |
| Eosinophil (103/mm3) | 0.03 ± 0.00 b | 0.04 ± 0.00 b | 0.05 ± 0.00 a | 0.05 ± 0.00 a |
| Basophil (103/mm3) | 0.11 ± 0.013 b | 0.12 ± 0.013 b | 1.75 ± 0.09 a | 1.723 ± 0.06 a |
| Monocyte (103/mm3) | 8.19 ± 0.12 a | 8.13 ± 0.41 a | 5.53 ± 0.69 b | 6.56 ± 0.38 b |
| Variables | Experimental Groups | |||
|---|---|---|---|---|
| Control | QUR | DOX | QUR/DOX | |
| SOD (U/g tissue) | 12.39 ± 0.44 a | 13.27 ± 0.39 a | 8.29 ± 0.52 c | 9.99 ± 0.19 b |
| CAT (U/g tissue) | 2.51 ± 0.09 a | 2.63 ± 0.15 a | 1.64 ± 0.19 b | 1.64 ± 0.052 b |
| IgG (mg/dl) | 44.91 ± 0.84 a | 46.44 ± 0.42 a | 16.36 ± 0.11 c | 36.97 ± 0.08 b |
| IgM (mg/dl) | 71.73 ± 0.82 a | 72.92 ± 0.72 a | 52.21 ± 0.43 c | 67.94 ± 0.91 b |
| IgE (mg/dl) | 18.07 ± 0.50 a | 17.27 ± 0.04 a | 6.50 ± 0.09 c | 13.13 ± 0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.R.; Moselhy, A.A.A.; El-Mleeh, A.; Aljuaydi, S.H.; Ismail, T.A.; Di Cerbo, A.; Crescenzo, G.; Abou-Zeid, S.M. Quercetin Alleviates the Immunotoxic Impact Mediated by Oxidative Stress and Inflammation Induced by Doxorubicin Exposure in Rats. Antioxidants 2021, 10, 1906. https://doi.org/10.3390/antiox10121906
Farag MR, Moselhy AAA, El-Mleeh A, Aljuaydi SH, Ismail TA, Di Cerbo A, Crescenzo G, Abou-Zeid SM. Quercetin Alleviates the Immunotoxic Impact Mediated by Oxidative Stress and Inflammation Induced by Doxorubicin Exposure in Rats. Antioxidants. 2021; 10(12):1906. https://doi.org/10.3390/antiox10121906
Chicago/Turabian StyleFarag, Mayada R., Attia A. A. Moselhy, Amany El-Mleeh, Samira H. Aljuaydi, Tamer Ahmed Ismail, Alessandro Di Cerbo, Giuseppe Crescenzo, and Shimaa M. Abou-Zeid. 2021. "Quercetin Alleviates the Immunotoxic Impact Mediated by Oxidative Stress and Inflammation Induced by Doxorubicin Exposure in Rats" Antioxidants 10, no. 12: 1906. https://doi.org/10.3390/antiox10121906
APA StyleFarag, M. R., Moselhy, A. A. A., El-Mleeh, A., Aljuaydi, S. H., Ismail, T. A., Di Cerbo, A., Crescenzo, G., & Abou-Zeid, S. M. (2021). Quercetin Alleviates the Immunotoxic Impact Mediated by Oxidative Stress and Inflammation Induced by Doxorubicin Exposure in Rats. Antioxidants, 10(12), 1906. https://doi.org/10.3390/antiox10121906







