Phenolic Composition and Biological Properties of Cynara cardunculus L. var. altilis Petioles: Influence of the Maturity Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction Procedure
2.3. Phenolic Compound Identification and Quantification
2.4. Evaluation of the Bioactive Potential
2.4.1. Antioxidant Activity
2.4.2. Anti-Inflammatory Activity
2.4.3. Cytotoxic and Hepatotoxic Activities
2.4.4. Antimicrobial Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Compounds Composition
3.2. Bioactive Properties
3.2.1. Antioxidant Potential
3.2.2. Anti-Inflammatory Activity
3.2.3. Cytotoxic Effects against Tumor and Nontumor Cells
3.2.4. Antimicrobial Activity
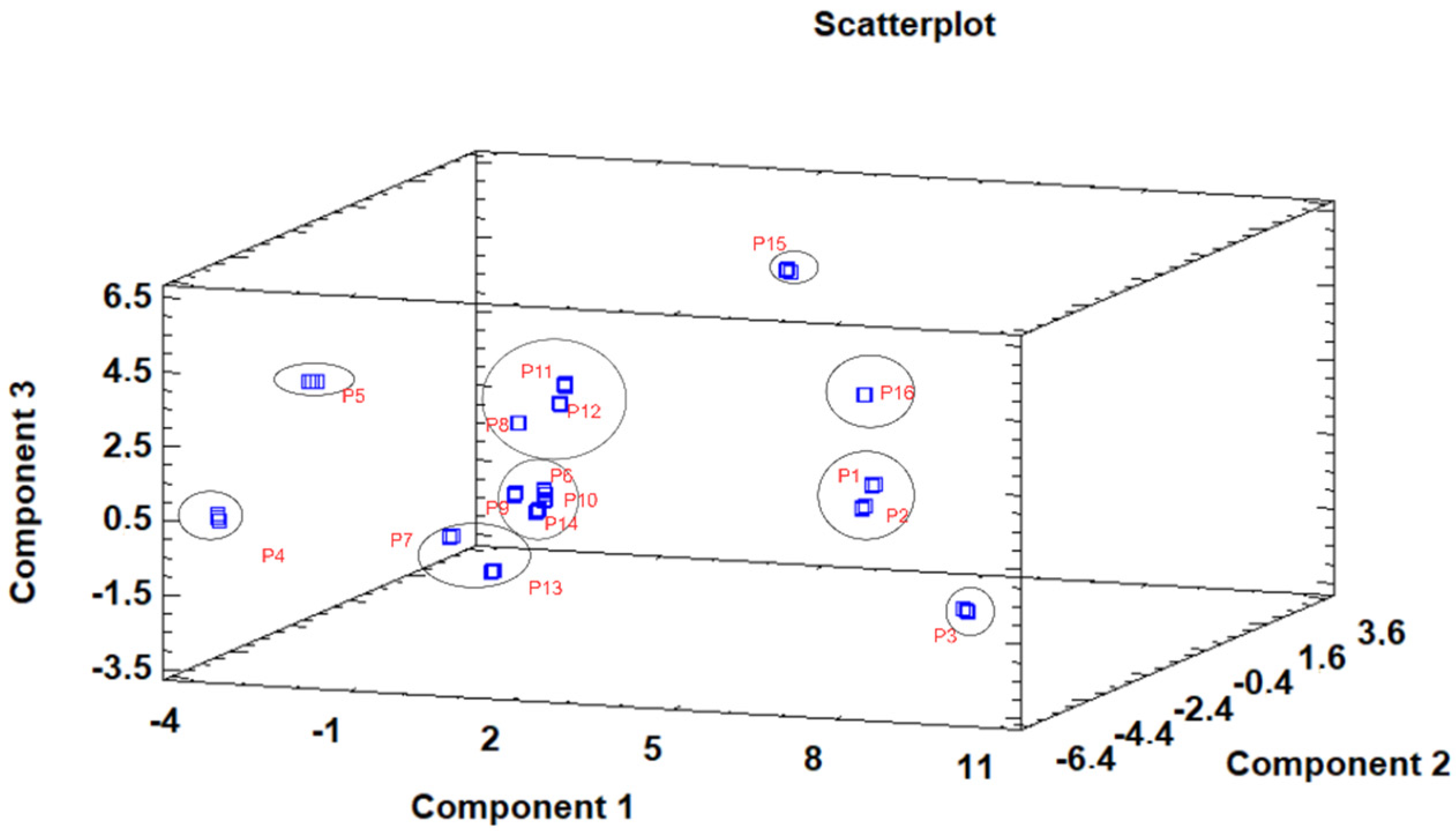
3.3. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Oliveira, P.; Barral, M.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Otero, P.; Prieto, M.A.; Simal-Gandara, J. Traditional plants from Asteraceae family as potential candidates for functional food industry. Food Funct. 2021, 12, 2850–2873. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; The International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Song, J.; Kong, L.; Yuan, T.; Li, W.; Zhang, W.; Hou, B.; Lu, Y.; Du, G. The strategies and techniques of drug discovery from natural products. Pharmacol. Ther. 2020, 216, 107686. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Biological Potential and Medical Use of Secondary Metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Karakaya, S.; Koca, M.; Sytar, O.; Duman, H. The natural phenolic compounds and their antioxidant and anticholinesterase potential of herb Leiotulus dasyanthus (K. Koch) Pimenov & Ostr. Nat. Prod. Res. 2020, 34, 1303–1305. [Google Scholar] [CrossRef]
- Conceição, C.; Martins, P.; Alvarenga, N.; Dias, J.; Lamy, E.; Garrido, L.; Gomes, S.; Freitas, S.; Belo, A.; Brás, T.; et al. Cynara cardunculus: Use in Cheesemaking and Pharmaceutical Applications. In Technological Approaches for Novel Applications in Dairy Processing; IntechOpen: London, UK, 2018; pp. 73–107. [Google Scholar]
- Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostic, M.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Seasonal variation in bioactive properties and phenolic composition of cardoon (Cynara cardunculus var. altilis) bracts. Food Chem. 2021, 336, 127744. [Google Scholar] [CrossRef]
- de Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Ben Amira, A.; Besbes, S.; Attia, H.; Blecker, C. Milk-clotting properties of plant rennets and their enzymatic, rheological, and sensory role in cheese making: A review. Int. J. Food Prop. 2017, 20, S76–S93. [Google Scholar] [CrossRef] [Green Version]
- Folgado, A.; Pires, A.S.; Figueiredo, A.C.; Pimentel, C.; Abranches, R. Toward alternative sources of milk coagulants for cheese manufacturing: Establishment of hairy roots culture and protease characterization from Cynara cardunculus L. Plant Cell Rep. 2020, 39, 89–100. [Google Scholar] [CrossRef]
- Gominho, J.; Lourenco, A.; Palma, P.; Lourenço, M.E.; Curt, M.d.; Fernández, J.; Pereira, H. Large scale cultivation of Cynara cardunculus L. for biomass production—A case study. Ind. Crop. Prod. 2010, 33, 1–6. [Google Scholar] [CrossRef]
- Ben Amira, A.; Blecker, C.; Richel, A.; Arias, A.A.; Fickers, P.; Francis, F.; Besbes, S.; Attia, H. Influence of the ripening stage and the lyophilization of wild cardoon flowers on their chemical composition, enzymatic activities of extracts and technological properties of cheese curds. Food Chem. 2018, 245, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.T.; Montiel-Rozas, M.M.; Madejón, P.; Diaz, M.J.; Madejón, E. The potential of native species as bioenergy crops on trace-element contaminated Mediterranean lands. Sci. Total. Environ. 2017, 590–591, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenergy 2018, 109, 257–275. [Google Scholar] [CrossRef]
- Barracosa, P.; Oliveira, J.; Barros, M.; Pires, E. Morphological evaluation of cardoon (Cynara cardunculus L.): Assessing biodiversity for applications based on tradition, innovation and sustainability. Genet. Resour. Crop. Evol. 2018, 65, 17–28. [Google Scholar] [CrossRef]
- Cabiddu, A.; Contini, S.; Gallo, A.; Lucini, L.; Bani, P.; Decandia, M.; Molle, G.; Piluzza, G.; Sulas, L. In vitro fermentation of cardoon seed press cake—A valuable byproduct from biorefinery as a novel supplement for small ruminants. Ind. Crop. Prod. 2019, 130, 420–427. [Google Scholar] [CrossRef]
- Chihoub, W.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C. Valorisation of the green waste parts from turnip, radish and wild cardoon: Nutritional value, phenolic profile and bioactivity evaluation. Food Res. Int. 2019, 126, 108651. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.I.; Barros, L.; Barreira, J.; Alves, M.J.; Barracosa, P.; Ferreira, I.C. Phenolic profile and bioactivity of cardoon (Cynara cardunculus L.) inflorescence parts: Selecting the best genotype for food applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Mandim, F.; Petropoulos, S.A.; Giannoulis, K.D.; Dias, M.I.; Fernandes, Â.; Pinela, J.; Kostic, M.; Soković, M.; Barros, L.; Santos-Buelga, C.; et al. Seasonal variation of bioactive properties and phenolic composition of Cynara cardunculus var. altilis. Food Res. Int. 2020, 134, 109281. [Google Scholar] [CrossRef]
- Ongaro, A.; Povolo, C.; Zagotto, G.; Ribaudo, G. HPLC and NMR quantification of bioactive compounds in flowers and leaves of Brassica rapa: The influence of aging. Nat. Prod. Res. 2019, 34, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Mandim, F.; Petropoulos, S.A.; Fernandes, Â.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Chemical Composition of Cynara Cardunculus, L. var. altilis Heads: The Impact of Harvesting Time. Agronomy 2020, 10, 1088. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Karkanis, A.; Ntatsi, G.; Barros, L.; Ferreira, I.C. Successive harvesting affects yield, chemical composition and antioxidant activity of Cichorium spinosum L. Food Chem. 2017, 237, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R. Chemical composition, nutritional value and antioxidant properties of Mediterranean okra genotypes in relation to harvest stage. Food Chem. 2018, 242, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Archontoulis, S.V.; Struik, P.C.; Vos, J.; Danalatos, N.G. Phenological growth stages of Cynara cardunculus: Codification and description according to the BBCH scale. Ann. Appl. Biol. 2010, 156, 253–270. [Google Scholar] [CrossRef]
- Bessada, S.; Barreira, J.; Barros, L.; Ferreira, I.C.; Oliveira, B. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crop. Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Mandim, F.; Barros, L.; Calhelha, R.C.; Abreu, R.M.V.; Pinela, J.; Alves, M.J.; Heleno, S.; Santos, P.F.; Ferreira, I.C.F.R. Calluna vulgaris (L.) Hull: Chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019, 119, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSnidentification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value and Bioactive Compounds Characterization of Plant Parts from Cynara cardunculus L. (Asteraceae) Cultivated in Central Greece. Front. Plant Sci. 2018, 9, 459. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.A.; Pereira, C.; Ntatsi, G.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and chemical composition of Greek artichoke genotypes. Food Chem. 2018, 267, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Graça, V.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Ferreira, I.C.F.R.; Santos, P. Bio-guided fractionation of extracts of Geranium robertianum L.: Relationship between phenolic profile and biological activity. Ind. Crop. Prod. 2017, 108, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Shallan, M.A.; Ali, M.A.; Meshrf, W.A.; Marrez, D.A. In vitro antimicrobial, antioxidant and anticancer activities of globe artichoke (Cynara cardunculus var. scolymus L.) bracts and receptacles ethanolic extract. Biocatal. Agric. Biotechnol. 2020, 29, 101774. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Graziani, G.; Docimo, T.; Palma, M.; Sparvoli, F.; Izzo, L.; Tucci, M.; Ritieni, A. Changes in Phenolics and Fatty Acids Composition and Related Gene Expression during the Development from Seed to Leaves of Three Cultivated Cardoon Genotypes. Antioxidants 2020, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Mauromicale, G. Choice of time of harvest influences the polyphenol profile of globe artichoke. J. Funct. Foods 2013, 5, 1822–1828. [Google Scholar] [CrossRef]
- Mandim, F.; Dias, M.I.; Pinela, J.; Barracosa, P.; Ivanov, M.; Stojkovic, D.; Sokovic, M.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and in vitro biological activities of cardoon (Cynara cardunculus L. var. altilis DC.) seeds as influenced by viability. Food Chem. 2020, 323, 126838. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Pagano, I.; Piccinelli, A.L.; Celano, R.; Campone, L.; Gazzerro, P.; De Falco, E.; Rastrelli, L. Chemical profile and cellular antioxidant activity of artichoke by-products. Food Funct. 2016, 7, 4841–4850. [Google Scholar] [CrossRef]
- Docimo, T.; De Stefano, R.; Cappetta, E.; Piccinelli, A.L.; Celano, R.; De Palma, M.; Tucci, M. Physiological, Biochemical, and Metabolic Responses to Short and Prolonged Saline Stress in Two Cultivated Cardoon Genotypes. Plants 2020, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.M.; Zhou, H.Z.; Yang, J.Y.; Li, R.; Song, H.; Wu, H.X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef] [Green Version]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) Byproducts as a Potential Source of Health-Promoting Antioxidant Phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Scavo, A.; Pandino, G.; Restuccia, C.; Parafati, L.; Cirvilleri, G.; Mauromicale, G. Antimicrobial activity of cultivated cardoon (Cynara cardunculus L. var. altilis DC.) leaf extracts against bacterial species of agricultural and food interest. Ind. Crop. Prod. 2019, 129, 206–211. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Pereira, C.; Barros, L.; Ferreira, I.C.F.R. Leaf parts from Greek artichoke genotypes as a good source of bioactive compounds and antioxidants. Food Funct. 2017, 8, 2022–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Nakhel, C.; Petropoulos, S.A.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; Colla, G.; Troise, A.D.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. The bioactive profile of lettuce produced in a closed soilless system as configured by combinatorial effects of genotype and macrocation supply composition. Food Chem. 2019, 309, 125713. [Google Scholar] [CrossRef]



| Peak | Rt (min) | λmax (nm) | [M-H]− (m/z) | MS2 (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 4.18 | 321 | 353 | 191 (100), 179 (33), 173 (5), 135 (5) | 3-O-Caffeoylquinic acid |
| 2 | 6.14 | 266 | 153 | 109 (100) | Protocatechuic acid |
| 3 | 6.52 | 321 | 353 | 173 (100), 179 (11), 191 (10), 161 (5), 135 (5) | 4-O-Caffeoylquinic acid |
| 4 | 6.63 | 326 | 353 | 191 (100), 179 (7), 173 (5), 135 (5) | cis-5-O-Caffeoylquinic acid |
| 5 | 7.10 | 326 | 353 | 191 (100), 179 (7), 173 (5), 135 (5) | trans-5-O-Caffeoylquinic acid |
| 6 | 15.97 | 285/sh324 | 463 | 287 (100) | Eriodictyol-O-hexuronoside |
| 7 | 16.69 | 322 | 515 | 353 (100), 335 (25), 191 (62), 179 (15) | 1,3-di-O-caffeoylquinic acid |
| 8 | 18.61 | 266/343 | 461 | 285 (100) | Luteolin-O-hexuronoside derivative I |
| 9 | 18.86 | 267/343 | 461 | 285 (100) | Luteolin-O-hexuronoside derivative II |
| 10 | 19.01 | 334 | 515 | 353 (100), 179 (10), 173 (29), 353 (10), 191 (10), 135 (8), 161 (5) | O-Dicaffeyolquinic acid |
| 11 | 20.39 | 324 | 515 | 353 (100), 191 (12), 335 (10) | 1,5-di-O-cafffeoylquinic acid |
| 12 | 22.66 | 329 | 515 | 353 (100), 335 (5), 229 (2), 255 (2), 203 (2), 191 (75), 179 (13), 173 (5, MS3 base peak) | 3,4-di-O-cafffeoylquinic acid |
| 13 | 23.69 | 268/332 | 533 | 489 (100), 285 (20) | Luteolin-O-malonyl hexoside derivative I |
| 14 | 23.77 | 267/346 | 533 | 285 (100), 489 (50), 447 (5) | Luteolin-O-malonyl-hexoside derivative II |
| 15 | 25.58 | 330 | 515 | 353 (100), 191 (12, MS3 base peak) | 3,5-di-O-caffeolyquinic acid |
| Peak | Quantification (mg Equivalents of the Corresponding Standard Used for Quantification Per g of Extract) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | |
| 1 | n.d. | n.d. | n.d. | 1.22 ± 0.03 a | 0.83 ± 0.01 b | 0.52 ± 0.01 ef | 0.48 ± 0.02 g | 0.35 ± 0.01 i | 0.51 ± 0.01 f | 0.568 ± 0.002 c | 0.32 ± 0.01 j | 0.42 ± 0.02 h | 0.559 ± 0.004 cd | 0.54 ± 0.01 de | n.d. | n.d. |
| 2 | n.d. | n.d. | n.d. | 1.38 ± 0.01 b | 2.7 ± 0.1 a | 1.22 ± 0.02 c | 0.42 ± 0.01 d | 0.290 ± 0.002 e | 0.32 ± 0.01 e | 0.297 ± 0.005 e | 0.342 ± 0.001 e | 0.064 ± 0.003 f | 0.279 ± 0.002 e | 0.058 ± 0.001 fg | n.d. | n.d. |
| 3 | n.d. | n.d. | n.d. | 37.2 ± 0.3 a | 33.9 ± 0.3 b | 12.18 ± 0.05 fg | 24.9 ± 0.4 c | 19.2 ± 0.1 d | 16.4 ± 0.2 e | 11.64 ± 0.02 h | 11.9 ± 0.2 gh | 6.1 ± 0.1 k | 8.7 ± 0.2 j | 9.7 ± 0.1 i | n.d. | n.d. |
| 4 | 3.6 ± 0.1 b | 3.70 ± 0.03 b | 5.4 ± 0.2 a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.32 ± 0.03 d | 2.02 ± 0.1 c |
| 5 | 8.0 ± 0.2 c | 8.65 ± 0.05 b | 16.0 ± 0.5 a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.524 ± 0.003 e | 4.17 ± 0.04 d |
| 6 | n.d. | n.d. | n.d. | 0.450 ± 0.001 d | 0.4143 ± 0.0001 g | 0.398 ± 0.001 h | 0.598 ± 0.04 a | 0.50 ± 0.01 e | 0.451 ± 0.001 d | 0.54 ± 0.01 b | 0.43 ± 0.01 f | 0.42 ± 0.01 fg | 0.44 ± 0.01 r | 0.444 ± 0.004 de | n.d. | n.d. |
| 7 | n.d. | n.d. | n.d. | 0.80 ± 0.01 a | 0.72 ± 0.01 b | 0.61 ± 0.01 de | 0.62 ± 0.01 cd | 0.393 ± 0.003 g | 0.60 ± 0.01 e | 0.457 ± 0.004 f | 0.348 ± 0.004 i | 0.313 ± 0.005 j | 0.631 ± 0.002 c | 0.38 ± 0.01 h | n.d. | n.d. |
| 8 | n.d. | n.d. | n.d. | 1.58 ± 0.01 a | 0.68 ± 0.02 e | 0.69 ± 0.01 e | 0.95 ± 0.01 b | 0.89 ± 0.03 c | 0.90 ± 0.03 c | 0.756 ± 0.002 d | 0.439 ± 0.001 h | 0.589 ± 0.001 f | 0.54 ± 0.01 g | 0.5411 ± 0.0004 g | n.d. | n.d. |
| 9 | n.d. | n.d. | n.d. | 0.471 ± 0.001 gh | 0.41 ± 0.01 hi | 0.395 ± 0.004 i | 0.96 ± 0.05 e | 0.49 ± 0.01 g | 1.10 ± 0.02 d | 1.26 ± 0.05 c | 0.75 ± 0.01 f | 0.76 ± 0.02 f | 3.49 ± 0.02 a | 2.6 ± 0.1 b | n.d. | n.d. |
| 10 | 3.6 ± 0.1 a | 1.29 ± 0.03 d | 1.71 ± 0.02 b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.02 ± 0.04 e | 1.5 ± 0.1 c |
| 11 | 30.1 ± 0.4 a | 15.0 ± 0.1 e | 13.1 ± 0.4 g | 23.7 ± 0.4 b | 8.4 ± 0.3 j | 11.5 ± 0.3 h | 16 ± 1 d | 5.0 ± 0.1 l | 12.9 ± 0.3 g | 14.78 ± 0.02 ef | 6.9 ± 0.2 k | 5.42 ± 0.05 l | 22 ± 1 c | 13.1 ± 0.1 g | 9.6 ± 0.2i | 14.25 ± 0.03 f |
| 12 | 2.4 ± 0.1 h | 3.2 ± 0.1 f | 3.5 ± 0.1 e | 4.58 ± 0.02 c | 1.63 ± 0.03 k | 2.8 ± 0.1 g | 3.03 ± 0.04 f | 1.69 ± 0.01 k | 3.6 ± 0.1 e | 4.4 ± 0.1 d | 1.60 ± 0.04 k | 2.2 ± 0.1 i | 6.3 ± 0.2 a | 5.30 ± 0.02 b | 1.54 ± 0.03k | 1.90 ± 0.03 j |
| 13 | 1.48 ± 0.01 d | 3.05 ± 0.05 b | 10.8 ± 0.1 a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.01 ± 0.04c | 1.27 ± 0.02 e |
| 14 | n.d. | n.d. | n.d. | 1.08 ± 0.01 b | 0.70 ± 0.01 g | 0.62 ± 0.02 hi | 1.16 ± 0.01 a | 0.679 ± 0.002 g | 0.94 ± 0.01 c | 0.82 ± 0.04 e | 0.59 ± 0.02 i | 0.64 ± 0.02 h | 0.763 ± 0.002 f | 0.91 ± 0.02 d | n.d. | n.d. |
| 15 | n.d. | n.d. | n.d. | 0.79 ± 0.02 a | 0.457 ± 0.004 e | 0.54 ± 0.03 d | 0.38 ± 0.01 f | 0.29 ± 0.01 g | 0.63 ± 0.0 2 b | 0.45 ± 0.02 e | 0.4788 ± 0.0003 e | 0.30 ± 0.01 g | 0.7895 ± 0.0003 a | 0.57 ± 0.02 c | n.d. | n.d. |
| TPA | 47.8 ± 0.3 c | 31.8 ± 0.2 h | 40 ± 1 e | 69.7 ± 0. 1 a | 48.6 ± 0.4 b | 29.3 ± 0.5 i | 46.1 ± 0.4 d | 27.3 ± 0.2 j | 34.9 ± 0.4 f | 32.56 ± 0.04 g | 21.9 ± 0.4 l | 14.9 ± 0.2 n | 39.5 ± 0.2 e | 29.6 ± 0.2 i | 16.1 ± 0.2 m | 23.85 ± 0.03 l |
| TF | 1.48 ± 0.01 k | 3.05 ± 0.05 f | 10.8 ± 0.1 a | 3.59 ± 0.01 d | 2.211 ± 0.003 i | 2.10 ± 0.01 ij | 3.7 ± 0.1 d | 2.55 ± 0.05 g | 3.4 ± 0.1 e | 3.4 ± 0.1 e | 2.202 ± 0.005 i | 2.407 ± 0.001 h | 5.241 ± 0.003 b | 4.5 ± 0.1 c | 2.01 ± 0.04 j | 1.27 ± 0.02 l |
| TPC | 98.5 ± 0.6 b | 69.7 ± 0.3 d | 101 ± 1 a | 73.3 ± 0.1 c | 50.8 ± 0.4 e | 31.4 ± 0.5 k | 49.8 ± 0.3 f | 29.8 ± 0.1 l | 38.3 ± 0.3 h | 33.9 ± 0.1 i | 24.08 ± 0.39 m | 17.3 ± 0.2 n | 44.8 ± 0.2 g | 34.1 ± 0.3 j | 36.2 ± 0.5 l | 50.2 ± 0.1 ef |
| Yield (%) | 25.46 | 27.4 | 30.99 | 30.9 | 34.79 | 39.36 | 33.53 | 37.56 | 22.86 | 30.98 | 20.17 | 15.46 | 14.42 | 11.90 | 14.4 | 14.57 |
| Antioxidant Activity (IC50, µg/mL) | |||
|---|---|---|---|
| Sample | TBARS | OxHLIA (Δt = 60 min) | OxHLIA (Δt = 120 min) |
| P1 | 15.8 ± 0.1 m | 244 ± 5 b | 323 ± 7 e |
| P2 | 22.6 ± 0.4 j | 392 ± 10 a | 563 ± 17 a |
| P3 | 5.0 ± 0.1 o | 386 ± 2 a | 542 ± 7 a |
| P4 | 75.6 ± 0.5 d | 65 ± 4 i | 180 ± 3 h |
| P5 | 61.0 ± 0.5 e | 110 ± 5 h | 245 ± 7 fg |
| P6 | 20.3 ± 0.2 l | 195 ± 5 d | 382 ± 5 c |
| P7 | 20.8 ± 0.5 kl | 224 ± 9 bc | 466 ± 18 b |
| P8 | 56.6 ± 0.5 f | 168 ± 4 e | 370 ± 4 cd |
| P9 | 92 ± 1 b | 122 ± 4 gh | 206 ± 4 gh |
| P10 | 58 ± 2 f | 122 ± 4 gh | 206 ± 4 gh |
| P11 | 83.9 ± 0.4 c | 157 ± 6 e | 289 ± 9 ef |
| P12 | 34.5 ± 0.5 h | 135 ± 5 fg | 266 ± 4 f |
| P13 | 27 ± 2 i | 114 ± 2 gh | 185 ± 4 h |
| P14 | 44.9 ± 0.5 g | 102 ± 4 hj | 201 ± 5 gh |
| P15 | 287 ± 2 a | 208 ± 14 cd | 400 ± 40 c |
| P16 | 21.9 ± 0.4 jk | 150 ± 8 ef | 243 ± 4 ef |
| Trolox | 9.1 ± 0.3 n | 21.2 ± 0.7 k | 41.1 ± 0.8 i |
| Anti-Inflammatory Activity (IC50; µg/mL) | |
|---|---|
| Sample | RAW 246.7 |
| P1 | 154 ± 4 d |
| P2 | 91 ± 2 e |
| P3 | 179 ± 3 c |
| P4 | 222 ± 13 a |
| P5 | >400 |
| P6 | >400 |
| P7 | >400 |
| P8 | 191 ± 10 b |
| P9 | 14.2 ± 0.5 h |
| P10 | 34 ± 4 g |
| P11 | 40 ± 2 g |
| P12 | 36 ± 2 g |
| P13 | 18 ± 2 h |
| P14 | 31 ± 1 g |
| P15 | 80 ± 3 f |
| P16 | 79 ± 3 f |
| Dexamethasone | 16 ± 1 hi |
| Cytotoxic Activity (GI50; µg/mL) | |||||
|---|---|---|---|---|---|
| Sample | MCF-7 | NCI-H460 | HeLa | HepG2 | PLP2 |
| P1 | 150 ± 3 d | 173 ± 14 d | 153 ± 6 d | 155 ± 9 d | 239 ± 16 d |
| P2 | 76 ± 1 e | 80 ± 6 e | 58 ± 5 g | 65 ± 4 e | 143 ± 7 d |
| P3 | 191 ± 4 c | 223 ± 7 c | 141 ± 8 de | 68 ± 2 e | 336 ± 11 a |
| P4 | 253 ± 5 b | 238 ± 23 c | 132 ± 5 e | 228 ± 9 c | 307 ± 9 b |
| P5 | >400 | 353 ± 25 a | 20 ± 2 c | 351 ± 14 a | >400 |
| P6 | >400 | >400 | 204 ± 15 a | >400 | 314 ± 19 b |
| P7 | 345 ± 20 a | >400 | 269 ± 8 b | >400 | >400 |
| P8 | 203 ± 15 c | 312 ± 8 b | 82 ± 3 f | 304 ± 11 b | 282 ± 12 c |
| P9 | 12 ± 1 ij | 16.0 ± 0.5 hi | 11.1 ± 0.3 i | 14 ± 1 g | 16 ± 1 i |
| P10 | 43 ± 2 h | 36 ± 2 gh | 17 ± 1 i | 17 ± 2 g | 49 ± 3 jk |
| P11 | 58 ± 4 fg | 55 ± 1 fg | 38 ± 2 h | 62 ± 5 e | 61 ± 2 j |
| P12 | 26 ± 1 i | 35 ± 1 gh | 19 ± 1 i | 16 ± 1 g | 44 ± 1 jk |
| P13 | 23 ± 1 i | 70 ± 1 ef | 17 ± 1 i | 19 ± 2 g | 37 ± 1 h |
| P14 | 51 ± 3 gh | 30 ± 2 h | 20 ± 1 i | 19 ± 2 g | 55 ± 1 j |
| P15 | 66 ± 5 ef | 86 ± 3 e | 54 ± 3 gh | 47 ± 4 f | 112 ± 4 e |
| P16 | 60 ± 2 fg | 72 ± 5 ef | 55 ± 3 gh | 39 ± 3 f | 116 ± 6 e |
| Ellipticine | 1.21 ± 0.02 k | 0.9 ± 0.1 j | 1.03 ± 0.09 j | 1.10 ± 0.09 h | 2.3 ± 0.2 j |
| Antibacterial Activity (mg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. cereus | S. aureus | L. monocytogenes | E. cloacae | E. coli | S.Typhimurium | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| P1 | 1.17 | 2.33 | 2.33 | 4.66 | 2.33 | 4.66 | 2.33 | 4.66 | 2.33 | 4.66 | 2.33 | 4.66 |
| P2 | 2.39 | 4.78 | 4.78 | 9.55 | 4.78 | 9.55 | 1.15 | 2.31 | 2.39 | 4.78 | 4.78 | 9.55 |
| P3 | 2.31 | 4.61 | 4.61 | 9.23 | 2.31 | 4.61 | 4.61 | 9.23 | 4.61 | 9.23 | 4.61 | 9.23 |
| P4 | 1.71 | 3.42 | 6.84 | 6.84 | 3.42 | 6.84 | 1.71 | 3.42 | 1.71 | 3.42 | 3.42 | 6.84 |
| P5 | 0.75 | 1.51 | 1.51 | 3.02 | 1.51 | 3.02 | 1.51 | 3.02 | 0.75 | 1.51 | 1.51 | 3.02 |
| P6 | 1.69 | 3.37 | 3.37 | 6.75 | 1.69 | 6.75 | 3.37 | 6.75 | 1.69 | 3.37 | 6.75 | >6.75 |
| P7 | 1.57 | 3.13 | 3.13 | 6.27 | 1.57 | 3.13 | 1.57 | 3.13 | 0.78 | 1.57 | 1.57 | 3.13 |
| P8 | 1.63 | 3.27 | 1.63 | 3.27 | 1.63 | 3.27 | 1.63 | 3.27 | 0.82 | 1.63 | 1.63 | 3.27 |
| P9 | 1.89 | 3.78 | 1.89 | 3.78 | 1.89 | 3.78 | 1.89 | 3.78 | 0.94 | 1.89 | 1.89 | 3.78 |
| P10 | 1.78 | 3.55 | 3.55 | 7.11 | 1.78 | 3.55 | 1.78 | 3.55 | 1.78 | 3.55 | 3.55 | 7.11 |
| P11 | 1.81 | 1.81 | 3.63 | 7.26 | 3.63 | 7.26 | 3.63 | 7.26 | 1.81 | 3.63 | 3.63 | 7.26 |
| P12 | 0.81 | 1.62 | 3.24 | 6.48 | 3.24 | 6.48 | 3.24 | 6.48 | 3.24 | 6.48 | 3.24 | 6.48 |
| P13 | 1.72 | 1.72 | 3.43 | 3.43 | 3.43 | 6.87 | 3.43 | 6.87 | 3.43 | 6.87 | 3.43 | 6.87 |
| P14 | 0.85 | 1.71 | 1.71 | 3.42 | 1.71 | 3.42 | 1.71 | 3.42 | 1.71 | 3.42 | 1.71 | 3.42 |
| P15 | 1.15 | 2.31 | 2.31 | 4.61 | 2.31 | 4.61 | 2.31 | 4.61 | 2.31 | 4.61 | 2.31 | 4.61 |
| P16 | 1.18 | 2.36 | 4.73 | 9.46 | 4.73 | 9.76 | 2.36 | 4.73 | 2.36 | 4.73 | 2.36 | 4.73 |
| Streptomycin | 0.10 | 0.20 | 0.04 | 0.10 | 0.20 | 0.30 | 0.20 | 0.30 | 0.20 | 0.30 | 0.20 | 0.30 |
| Ampicillin | 0.25 | 0.40 | 0.25 | 0.45 | 0.40 | 0.50 | 0.25 | 0.50 | 0.40 | 0.50 | 0.75 | 1.20 |
| Antifungal Activity (mg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. fumigatus | A. versicolor | A. niger | P. funiculosum | P. ochrochloron | P. verrucosum var. cyclopium | |||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| P1 | 3.71 | 7.42 | 1.86 | 3.71 | 1.86 | 3.71 | 0.93 | 1.86 | 1.86 | 3.71 | 1.86 | 3.71 |
| P2 | 1.83 | 3.66 | 1.83 | 3.66 | 3.66 | 7.39 | 0.92 | 1.83 | 0.92 | 1.83 | 0.92 | 1.83 |
| P3 | 3.71 | 7.42 | 0.93 | 1.86 | 1.86 | 3.71 | 0.93 | 1.86 | 3.71 | 7.42 | 1.86 | 3.71 |
| P4 | 1.14 | 2.28 | 0.57 | 1.14 | >9.12 | > 9.12 | 0.57 | 1.14 | 0.57 | 1.14 | 0.57 | 1.14 |
| P5 | 1.01 | 2.01 | 0.50 | 1.01 | >8.05 | >8.05 | >8.05 | >8.05 | 1.01 | 2.01 | >8.05 | >8.05 |
| P6 | 1.12 | 2.25 | 1.12 | 2.25 | >9 | >9 | >9 | >9 | 5.62 | 1.12 | 9 | >9 |
| P7 | 0.52 | 1.04 | 0.52 | 1.04 | >8.36 | >8.36 | 0.52 | 1.04 | 0.52 | 1.04 | 1.04 | 2.09 |
| P8 | 2.18 | 4.36 | 1.09 | 2.18 | 1.09 | 2.18 | 0.54 | 1.09 | 0.54 | 1.09 | 0.54 | 1.09 |
| P9 | 2.52 | 5.04 | 1.26 | 2.52 | 1.26 | 2.52 | 1.26 | 2.52 | 1.26 | 2.52 | 0.63 | 1.26 |
| P10 | 2.37 | 4.74 | 1.18 | 2.37 | 1.18 | 2.37 | 0.59 | 1.18 | 0.30 | 0.59 | 0.30 | 0.59 |
| P11 | 0.91 | 1.81 | 0.91 | 1.81 | 1.81 | 3.63 | 0.45 | 0.91 | 0.91 | 1.81 | 0.45 | 0.91 |
| P12 | 0.54 | 1.08 | 0.54 | 1.08 | 0.54 | 1.08 | 1.08 | 2.16 | 1.08 | 2.16 | 1.08 | 2.16 |
| P13 | 0.57 | 1.14 | 1.14 | 2.29 | 1.14 | 2.29 | 0.57 | 1.14 | 0.57 | 1.14 | 0.57 | 1.14 |
| P14 | 0.28 | 0.57 | 0.57 | 1.14 | 1.14 | 2.28 | 0.57 | 1.14 | 1.14 | 2.28 | 0.57 | 2.28 |
| P15 | 0.90 | 1.80 | 1.80 | 3.60 | 3.60 | 7.18 | 1.80 | 3.60 | 0.90 | 1.80 | 1.80 | 3.60 |
| P16 | 3.64 | 7.28 | 0.91 | 1.82 | 1.82 | 3.64 | 0.91 | 1.82 | 1.82 | 3.64 | 0.91 | 1.82 |
| Ketoconazole | 0.25 | 0.50 | 0.20 | 0.50 | 0.20 | 0.50 | 0.20 | 0.50 | 1.00 | 1.50 | 0.20 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandim, F.; Petropoulos, S.A.; Dias, M.I.; Pinela, J.; Kostić, M.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Phenolic Composition and Biological Properties of Cynara cardunculus L. var. altilis Petioles: Influence of the Maturity Stage. Antioxidants 2021, 10, 1907. https://doi.org/10.3390/antiox10121907
Mandim F, Petropoulos SA, Dias MI, Pinela J, Kostić M, Soković M, Santos-Buelga C, Ferreira ICFR, Barros L. Phenolic Composition and Biological Properties of Cynara cardunculus L. var. altilis Petioles: Influence of the Maturity Stage. Antioxidants. 2021; 10(12):1907. https://doi.org/10.3390/antiox10121907
Chicago/Turabian StyleMandim, Filipa, Spyridon A. Petropoulos, Maria Inês Dias, José Pinela, Marina Kostić, Marina Soković, Celestino Santos-Buelga, Isabel C. F. R. Ferreira, and Lillian Barros. 2021. "Phenolic Composition and Biological Properties of Cynara cardunculus L. var. altilis Petioles: Influence of the Maturity Stage" Antioxidants 10, no. 12: 1907. https://doi.org/10.3390/antiox10121907
APA StyleMandim, F., Petropoulos, S. A., Dias, M. I., Pinela, J., Kostić, M., Soković, M., Santos-Buelga, C., Ferreira, I. C. F. R., & Barros, L. (2021). Phenolic Composition and Biological Properties of Cynara cardunculus L. var. altilis Petioles: Influence of the Maturity Stage. Antioxidants, 10(12), 1907. https://doi.org/10.3390/antiox10121907













