Effects of Remote Ischaemic Preconditioning on the Internal Thoracic Artery Nitric Oxide Synthase Isoforms in Patients Undergoing Coronary Artery Bypass Grafting
Abstract
:1. Introduction
2. Materials and Methods
2.1. RIPC Protocol Description
2.2. Patient Selection
2.3. Sample Collection
2.4. SDS-PAGE and Western Blotting
2.5. Immunohistochemistry
2.6. Light and Electron Microscopy
2.7. Statistical Analysis
3. Results
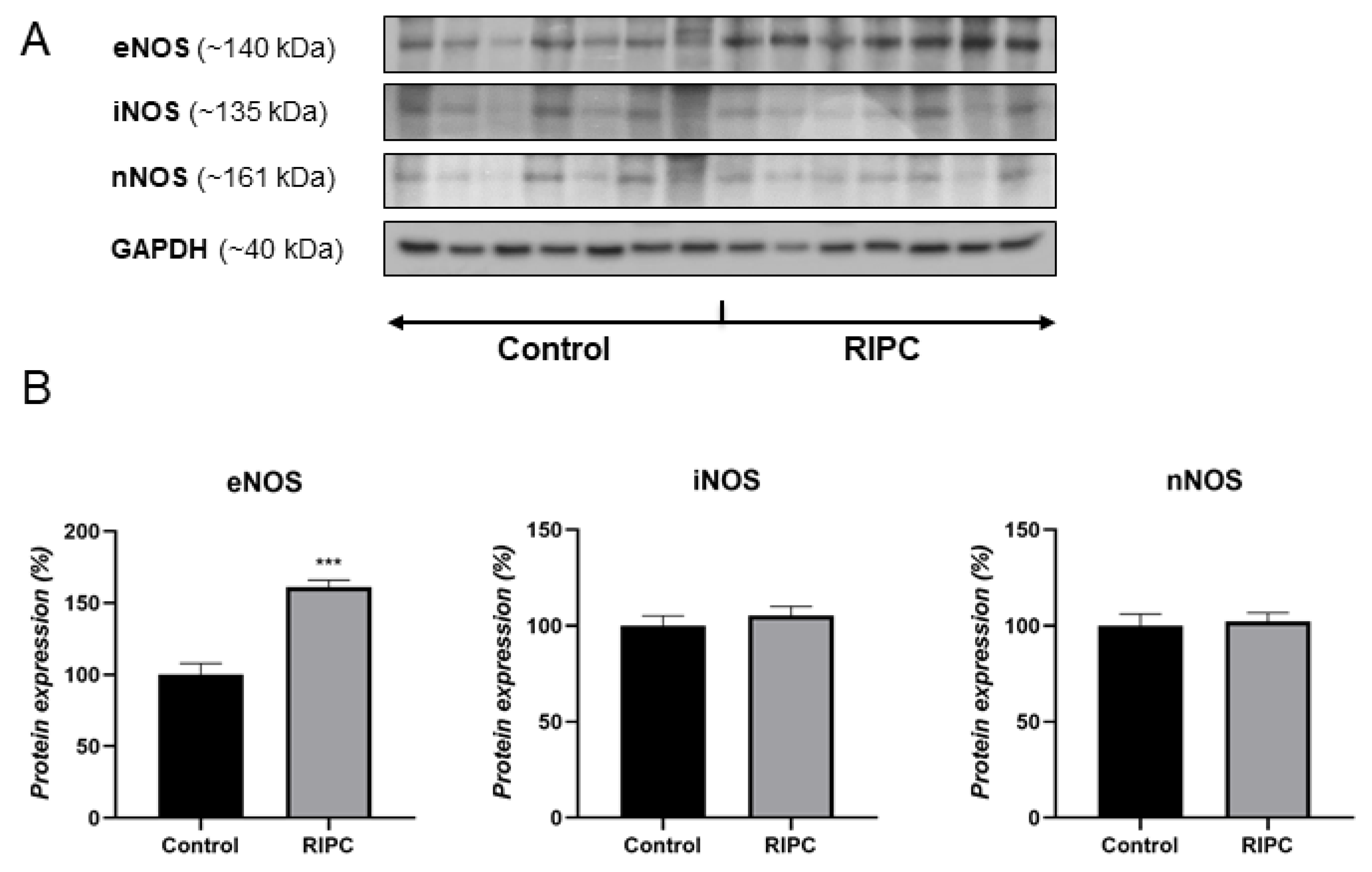
3.1. Protein Levels of NOS Isoforms
3.2. Immunohistochemical Analysis
3.3. Light and Electron Microscopy Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, S.Y.; Hausenloy, D.J. Remote ischemic conditioning: From bench to bedside. Front. Physiol. 2012, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahaleh, B.; Mulligan-Kehoe, M.J. Mechanisms of vascular disease. In Scleroderma; Springer: Boston, MA, USA, 2012. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.Y.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A.; Andreadou, I.; Oelze, M.; Davidson, S.M.; Hausenloy, D.J. Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radic. Biol. Med. 2021, 163, 325–343. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Remote ischaemic preconditioning: Underlying mechanisms and clinical application. Cardiovasc. Res. 2008, 79, 377–386. [Google Scholar] [CrossRef]
- Xiong, J.; Liao, X.; Xue, F.S.; Yuan, Y.J.; Wang, Q.; Liu, J.H. Remote ischemia conditioning-an endogenous cardioprotective strategy from outside the heart. Chin. Med. J. 2011, 124, 2209–2215. [Google Scholar] [CrossRef]
- Przyklenk, K. Reduction of myocardial infarct size with ischemic “conditioning”: Physiologic and technical considerations. Anesth. Analg. 2013, 117, 891–901. [Google Scholar] [CrossRef]
- Lau, J.K.; Roy, P.; Javadzadegan, A.; Moshfegh, A.; Fearon, W.F.; Ng, M.; Lowe, H.; Brieger, D.; Kritharides, L.; Yong, A.S. Remote Ischemic Preconditioning Acutely Improves Coronary Microcirculatory Function. J. Am. Heart Assoc. 2018, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, D.; Young, R.; Cialdella, P.; McCartney, P.; Bajrangee, A.; Hennigan, B.; Collison, D.; Carrick, D.; Shaukat, A.; Good, R.; et al. The effects of remote ischaemic preconditioning on coronary artery function in patients with stable coronary artery disease. Int. J. Cardiol. 2018, 252, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Marsh, R.; Cunniffe, B.; Cardinale, M.; Yellon, D.M.; Davidson, S.M. From Protecting the Heart to Improving Athletic Performance—The Benefits of Local and Remote Ischaemic Preconditioning. Cardiovasc. Drugs Ther. 2015, 29, 573–588. [Google Scholar] [CrossRef] [Green Version]
- Kanoria, S.; Jalan, R.; Seifalian, A.M.; Williams, R.; Davidson, B.R. Protocols and mechanisms for remote ischemic preconditioning: A novel method for reducing ischemia reperfusion injury. Transplantation 2007, 84, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Przyklenk, K.; Whittaker, P. Remote ischemic preconditioning: Current knowledge, unresolved questions, and future priorities. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Billah, M.; Ridiandries, A.; Allahwala, U.; Mudaliar, H.; Dona, A.; Hunyor, S.; Khachigian, L.M.; Bhindi, R. Circulating mediators of remote ischemic preconditioning: Search for the missing link between non-lethal ischemia and cardioprotection. Oncotarget 2019, 10, 216–244. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Skyschally, A.; Heusch, G. Cardioprotection by remote ischemic conditioning and its signal transduction. Pflugers Arch. Eur. J. Physiol. 2017, 469, 159–181. [Google Scholar] [CrossRef]
- Huang, P.L. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol. Metab. 2009, 20, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Feil, R.; Lohmann, S.M.; De Jonge, H.; Walter, U.; Hofmann, F. Cyclic GMP-Dependent Protein Kinases and the Cardiovascular System: Insights from Genetically Modified Mice. Circ. Res. 2003, 93, 907–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Z.; Li, H.; Irwin, M.G. Myocardial ischaemia reperfusion injury: The challenge of translating ischaemic and anaesthetic protection from animal models to humans. Br. J. Anaesth. 2016, 117, ii44–ii62. [Google Scholar] [CrossRef] [Green Version]
- Abu-Amara, M.; Yang, S.Y.; Quaglia, A.; Rowley, P.; De Mel, A.; Tapuria, N.; Seifalian, A.; Davidson, B.; Fuller, B. Nitric oxide is an essential mediator of the protective effects of remote ischaemic preconditioning in a mouse model of liver ischaemia/reperfusion injury. Clin. Sci. 2011, 121, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Rastaldo, R.; Cappello, S.; Folino, A.; Di Stilo, A.; Chegaev, K.; Tritto, I.; Pagliaro, P.; Losano, G. Low concentrations of an nitric oxide-donor combined with a liposoluble antioxidant compound enhance protection against reperfusion injury in isolated rat hearts. J. Physiol. Pharmacol. 2010, 61, 21–27. [Google Scholar]
- Bilińska, M.; Mązewski, M.; Beręsewicz, A. Donors of nitric oxide mimic effects of ischaemic preconditioning on reperfusion induced arrhythmias in isolated rat heart. Mol. Cell Biochem. 1996, 160–161, 265–271. [Google Scholar] [CrossRef]
- Kanno, S.; Lee, P.C.; Zhang, Y.; Ho, C.; Griffith, B.P.; Shears, L.L.; Billiar, T.R. Attenuation of myocardial ischemia/reperfusion injury by superinduction of inducible nitric oxide synthase. Circulation 2000, 101, 2742–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andelová, E.; Barteková, M.; Pancza, D.; Styk, J.; Ravingerová, T. The role of NO in ischemia/reperfusion injury in isolated rat heart. Gen. Physiol. Biophys. 2005, 24, 411–426. [Google Scholar]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Biosynthesis of nitric oxide from l-arginine. A pathway for the regulation of cell function and communication. Biochem. Pharmacol. 1989, 38, 1709–1715. [Google Scholar] [CrossRef]
- Rassaf, T.; Totzeck, M.; Hendgen-Cotta, U.B.; Shiva, S.; Heusch, G.; Kelm, M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ. Res. 2014, 114, 1601–1610. [Google Scholar] [CrossRef]
- Aggarwal, S.; Randhawa, P.K.; Singh, N.; Jaggi, A.S. Preconditioning at a distance: Involvement of endothelial vasoactive substances in cardioprotection against ischemia-reperfusion injury. Life Sci. 2016, 151, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Miličić, M.; Soldatović, I.; Nežić, D.; Jović, M.; Stojković, V.M.; Vuković, P.; Milojević, P. Remote ischemic preconditioning in patients undergoing coronary bypass grafting following acute coronary syndrome without ST elevation. Vojnosanit. Pregl. 2020, 77, 1017–1023. [Google Scholar] [CrossRef]
- Petrović, V.; Korać, A.; Buzadžić, B.; Korać, B. The effects of L-arginine and L-NAME supplementation on redox-regulation and thermogenesis in interscapular brown adipose tissue. J. Exp. Biol. 2005, 208, 4263–4271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, M.; Goyeneche, M.A.; Garces, M.; Marchini, T.; Pérez, V.; del Mauro, J.; Höcht, C.; Rodríguez, M.; Evelson, P.; Gelpi, R.J. Myocardial triggers involved in activation of remote ischaemic preconditioning. Exp. Physiol. 2016, 101, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Bloch, W.; Mehlhorn, U.; Krahwinkel, A.; Reiner, M.; Dittrich, M.; Schmidt, A.; Addicks, K. Ischemia increases detectable endothelial nitric oxide synthase in rat and human myocardium. Nitric Oxide 2001, 5, 317–333. [Google Scholar] [CrossRef]
- Totzeck, M.; Hendgen-Cotta, U.B.; Rassaf, T. Nitrite-nitric oxide signaling and cardioprotection. Adv. Exp. Med. Biol. 2017, 982, 335–346. [Google Scholar] [CrossRef]
- Vlasov, T.D.; Korzhevskii, D.É.; Polyakova, E.A. Ischemic preconditioning of the rat brain as a method of endothelial protection from ischemic/repercussion injury. Neurosci. Behav. Physiol. 2005, 35, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Küntscher, M.V.; Kastell, T.; Altmann, J.; Menke, H.; Gebhard, M.M.; Germann, G. Acute remote ischemic preconditioning II: The role of nitric oxide. Microsurgery 2002, 22, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Berges, A.; Van Nassauw, L.; Bosmans, J.; Timmermans, J.P.; Vrints, C. Role of nitric oxide and oxidative stress in ischaemic myocardial injury and preconditioning. Acta Cardiol. 2003, 58, 119–132. [Google Scholar] [CrossRef]
- Tapuria, N.; Kumar, Y.; Habib, M.M.; Amara, M.A.; Seifalian, A.M.; Davidson, B.R. Remote Ischemic Preconditioning: A Novel Protective Method from Ischemia Reperfusion Injury—A Review. J. Surg. Res. 2008, 150, 304–330. [Google Scholar] [CrossRef] [PubMed]
- Günaydin, B.; Çakici, I.; Soncul, H.; Kalaycioglu, S.; Çevik, C.; Sancak, B.; Kanzik, I.; Karadenizli, Y. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol. Res. 2000, 41, 493–496. [Google Scholar] [CrossRef]
- Kharbanda, R.K.; Mortensen, U.M.; White, P.A.; Kristiansen, S.B.; Schmidt, M.R.; Hoschtitzky, J.A.; Vogel, M.; Sorensen, K.; Redington, A.N.; MacAllister, R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002, 106, 2881–2883. [Google Scholar] [CrossRef] [Green Version]
- Loukogeorgakis, S.P.; Panagiotidou, A.T.; Broadhead, M.W.; Donald, A.; Deanfield, J.E.; MacAllister, R.J. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: Role of the autonomic nervous system. J. Am. Coll. Cardiol. 2005, 46, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Randhawa, P.K.; Bali, A.; Jaggi, A.S. RIPC for multiorgan salvage in clinical settings: Evolution of concept, evidences and mechanisms. Eur. J. Pharmacol. 2015, 746, 317–332. [Google Scholar] [CrossRef]
- Webb, G.D.; Lim, L.H.; Oh, V.M.S.; El Oakley, R.; Lee, C.N.; Wong, P.S.; Aye, W.M.M.; Chan, E.S.Y.; Moore, P.K. Expression of neuronal nitric oxide synthase in the internal thoracic artery and saphenous vein. J. Thorac. Cardiovasc. Surg. 2006, 132, 1131–1136. [Google Scholar] [CrossRef] [Green Version]
- Buchwalow, I.B.; Podzuweit, T.; Böcker, W.; Samoilova, V.E.; Thomas, S.; Wellner, M.; Baba, H.A.; Robenek, H.; Schnekenburger, J.; Lerch, M.M. Vascular smooth muscle and nitric oxide synthase. FASEB J. 2002, 16, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Gaudino, M.; Toesca, A.; Maggiano, N.; Pragliola, C.; Possati, G. Localization of nitric oxide synthase type III in the internal thoracic and radial arteries and the great saphenous vein: A comparative immunohistochemical study. J. Thorac. Cardiovasc. Surg. 2003, 125, 1510–1515. [Google Scholar] [CrossRef]
- He, G.W.; Liu, Z.G. Comparison of nitric oxide release and endothelium-derived hyperpolarizing factor-mediated hyperpolarization between human radial and internal mammary arteries. Circulation 2001, 104, I-344. [Google Scholar] [CrossRef]
- Tai, S.C.; Robb, G.B.; Marsden, P.A. Endothelial Nitric Oxide Synthase: A New Paradigm for Gene Regulation in the Injured Blood Vessel. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Triggle, C.R.; Samuel, S.M.; Ravishankar, S.; Marei, I.; Arunachalam, G.; Ding, H. The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 2012, 90, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, M.; Ohara, Y.; Navas, J.P.; Nishida, K.; Murphy, T.J.; Alexander, R.W.; Nerem, R.M.; Harrison, D.G. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am. J. Physiol. Cell Physiol. 1995, 269, C1371–C1378. [Google Scholar] [CrossRef] [PubMed]
- Arnet, U.A.; McMillan, A.; Dinerman, J.L.; Ballermann, B.; Lowenstein, C.J. Regulation of endothelial nitric-oxide synthase during hypoxia. J. Biol. Chem. 1996, 271, 15069–15073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.X.; Meyrick, B. Hypoxia increases Hsp90 binding to eNOS via PI3K-Akt in porcine coronary artery endothelium. Lab. Investig. 2004, 84, 182–190. [Google Scholar] [CrossRef]
- Jones, S.P.; Girod, W.G.; Palazzo, A.J.; Granger, D.N.; Grisham, M.B.; Jourd’heuil, D.; Huang, P.L.; Lefer, D.J. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H1567–H1573. [Google Scholar] [CrossRef] [Green Version]
- Brunner, F.; Maier, R.; Andrew, P.; Wölkart, G.; Zechner, R.; Mayer, B. Attenuation of myocardial ischemia/reperfusion injury in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Cardiovasc. Res. 2003, 57, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Engel, H.; Friedrich, S.; Schleich, C.; Gebhardt, M.M.; Gross, W.; Germann, G.; Reichenberger, M. Enhancing Nitric Oxide Bioavailability via Exogen Nitric Oxide Synthase and l-Arginine Attenuates Ischemia-Reperfusion-Induced Microcirculatory Alterations. Ann. Plast. Surg. 2017, 79, e25–e29. [Google Scholar] [CrossRef]
- Bell, R.M.; Smith, C.C.T.; Yellon, D.M. Nitric oxide as a mediator of delayed pharmacological (A1 receptor triggered) preconditioning; is eNOS masquerading as iNOS? Cardiovasc. Res. 2002, 53, 405–413. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankovic, A.; Zakic, T.; Milicic, M.; Unic-Stojanovic, D.; Kalezic, A.; Korac, A.; Jovic, M.; Korac, B. Effects of Remote Ischaemic Preconditioning on the Internal Thoracic Artery Nitric Oxide Synthase Isoforms in Patients Undergoing Coronary Artery Bypass Grafting. Antioxidants 2021, 10, 1910. https://doi.org/10.3390/antiox10121910
Jankovic A, Zakic T, Milicic M, Unic-Stojanovic D, Kalezic A, Korac A, Jovic M, Korac B. Effects of Remote Ischaemic Preconditioning on the Internal Thoracic Artery Nitric Oxide Synthase Isoforms in Patients Undergoing Coronary Artery Bypass Grafting. Antioxidants. 2021; 10(12):1910. https://doi.org/10.3390/antiox10121910
Chicago/Turabian StyleJankovic, Aleksandra, Tamara Zakic, Miroslav Milicic, Dragana Unic-Stojanovic, Andjelika Kalezic, Aleksandra Korac, Miomir Jovic, and Bato Korac. 2021. "Effects of Remote Ischaemic Preconditioning on the Internal Thoracic Artery Nitric Oxide Synthase Isoforms in Patients Undergoing Coronary Artery Bypass Grafting" Antioxidants 10, no. 12: 1910. https://doi.org/10.3390/antiox10121910
APA StyleJankovic, A., Zakic, T., Milicic, M., Unic-Stojanovic, D., Kalezic, A., Korac, A., Jovic, M., & Korac, B. (2021). Effects of Remote Ischaemic Preconditioning on the Internal Thoracic Artery Nitric Oxide Synthase Isoforms in Patients Undergoing Coronary Artery Bypass Grafting. Antioxidants, 10(12), 1910. https://doi.org/10.3390/antiox10121910





