Hypoxic and Thermal Stress: Many Ways Leading to the NOS/NO System in the Fish Heart
Abstract
1. Introduction
2. The NOS/NO System and the Fish Heart
2.1. Hypoxia
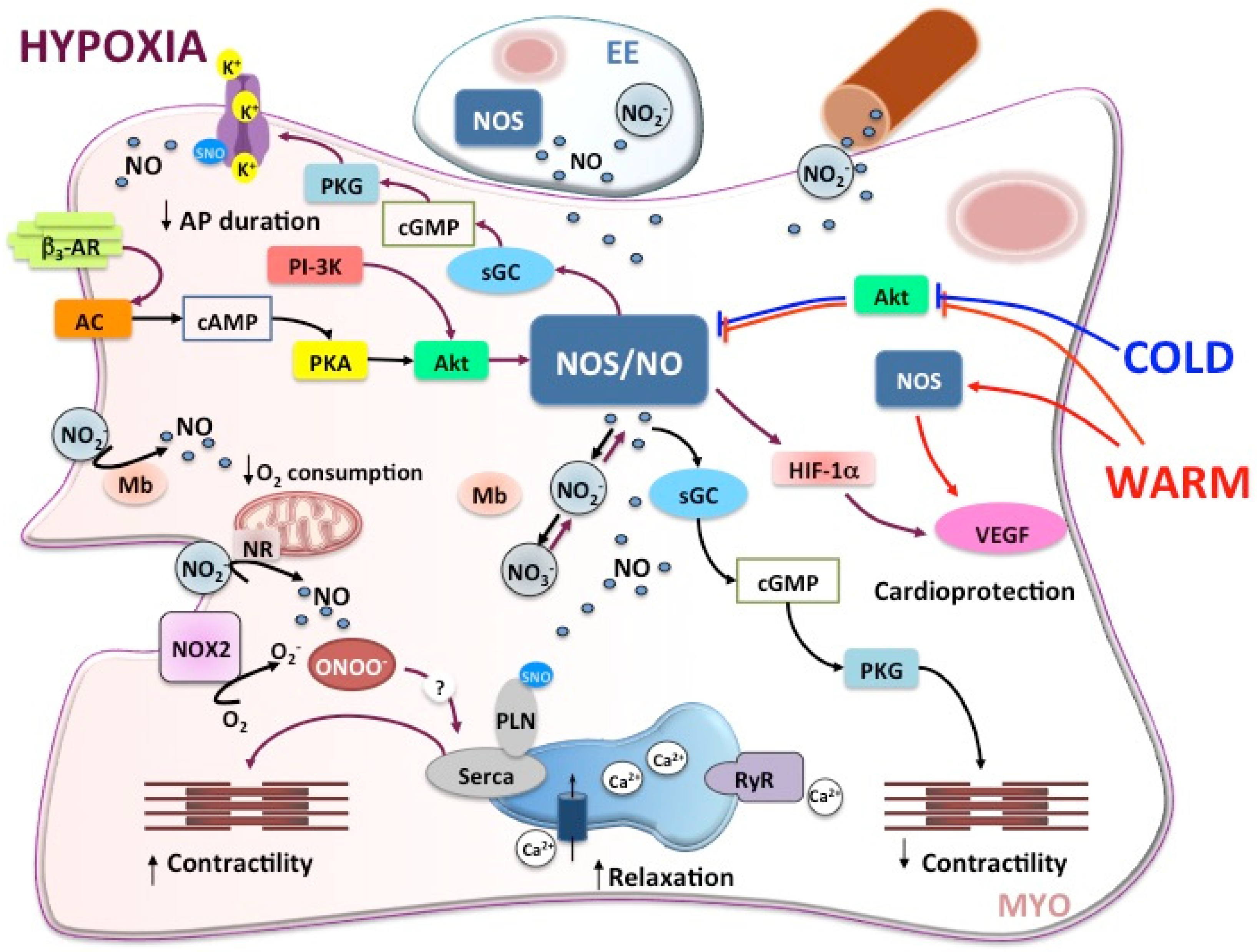
2.2. The NOS/NO System in the Fish Heart under Hypoxia
2.3. Temperature
2.4. The NOS/NO System in the Fish Heart under Temperature Challenges
2.5. Single Stress to Multiple Stress: There Is Room for the Nitrergic System in the Fish Heart
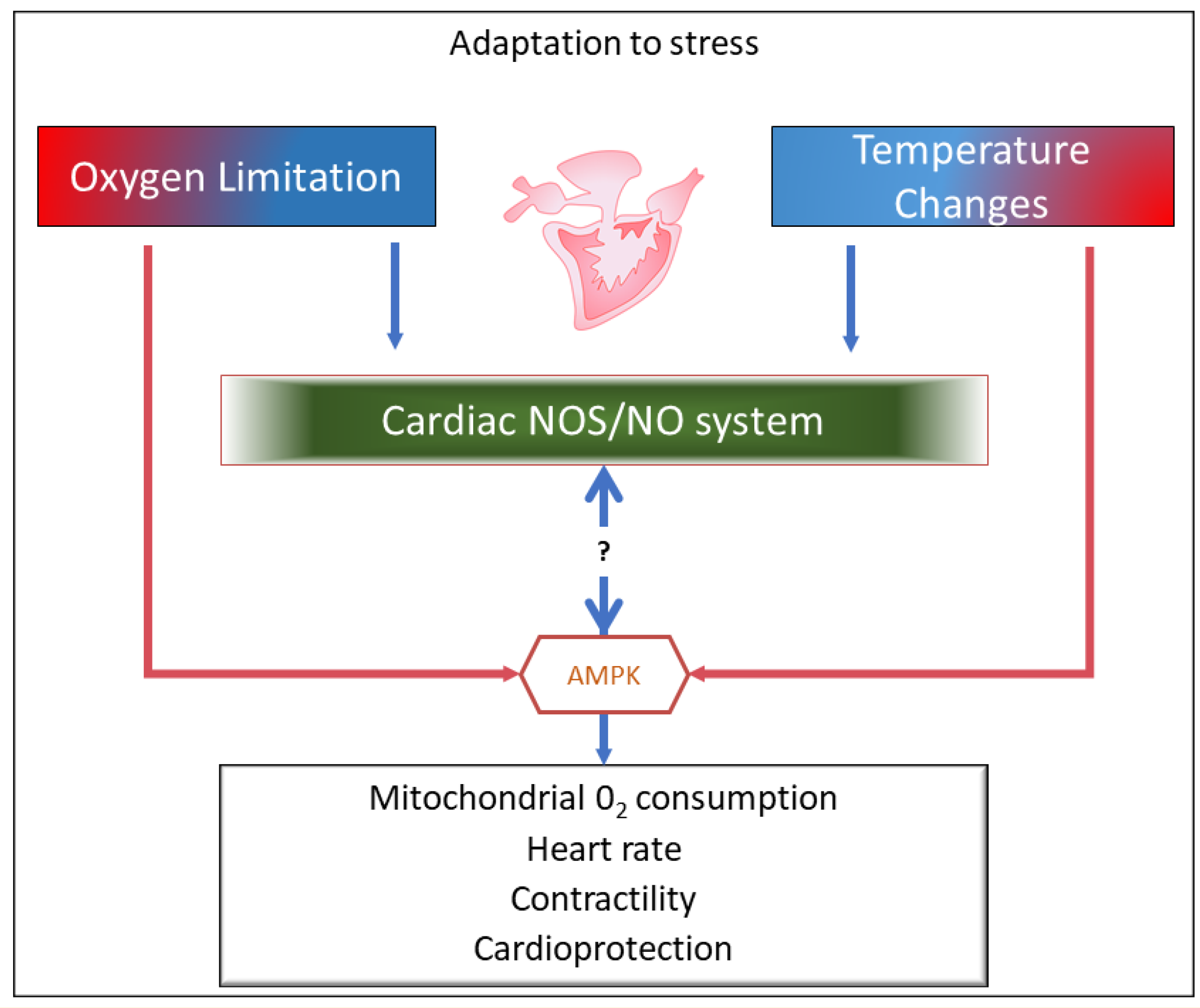
3. Upstream and Downstream the NOS/NO System: AMP-Activated Protein Kinase (AMPK) as a Candidate in Fish
4. Conclusions
Funding
Conflicts of Interest
References
- Selye, H. The Stress of Life; McGraw-Hill: Oxford, UK, 1978. [Google Scholar]
- Selye, H. Stress without Distress. In Psychopathology of Human Adaptation; Serban, G., Ed.; Springer: Boston, MA, USA, 1976; pp. 137–146. [Google Scholar]
- Balasch, J.C.; Tort, L. Netting the Stress Responses in Fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Portner, H.O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Axelsson, M.; Chabot, D.; Claireaux, G.; Cooke, S.J.; Corner, R.A.; De Boeck, G.; Domenici, P.; Guerreiro, P.M.; Hamer, B.; et al. Conservation physiology of marine fishes: State of the art and prospects for policy. Conserv. Physiol. 2016, 4, cow046. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.R.; Brandt, S.B.; Houde, E.D.; Pierson, J.J. Interactive Effects of Hypoxia and Temperature on Coastal Pelagic Zooplankton and Fish. Front. Mar. Sci. 2019, 6, 139. [Google Scholar] [CrossRef]
- Allmon, E.; Serafin, J.; Chen, S.; Rodgers, M.L.; Griffitt, R.; Bosker, T.; de Guise, S.; Sepulveda, M.S. Effects of polycyclic aromatic hydrocarbons and abiotic stressors on Fundulus grandis cardiac transcriptomics. Sci. Total. Environ. 2021, 752, 142156. [Google Scholar] [CrossRef]
- Cameron, J.S.; Hoffmann, K.E.; Zia, C.; Hemmett, H.M.; Kronsteiner, A.; Lee, C.M. A role for nitric oxide in hypoxia-induced activation of cardiac KATP channels in goldfish (Carassius auratus). J. Exp. Biol. 2003, 206, 4057–4065. [Google Scholar] [CrossRef]
- Cameron, J.S.; DeWitt, J.P.; Ngo, T.T.; Yajnik, T.; Chan, S.; Chung, E.; Kang, E. Cardiac K(ATP) channel alterations associated with acclimation to hypoxia in goldfish (Carassius auratus L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Amelio, D.; Garofalo, F.; Wong, W.P.; Chew, S.F.; Ip, Y.K.; Cerra, M.C.; Tota, B. Nitric oxide synthase-dependent “on/off” switch and apoptosis in freshwater and aestivating lungfish, Protopterus annectens: Skeletal muscle versus cardiac muscle. Nitric Oxide 2013, 32, 1–12. [Google Scholar] [CrossRef]
- Imbrogno, S.; Capria, C.; Tota, B.; Jensen, F.B. Nitric oxide improves the hemodynamic performance of the hypoxic goldfish (Carassius auratus) heart. Nitric Oxide 2014, 42, 24–31. [Google Scholar] [CrossRef]
- Gattuso, A.; Garofalo, F.; Cerra, M.C.; Imbrogno, S. Hypoxia Tolerance in Teleosts: Implications of Cardiac Nitrosative Signals. Front. Physiol. 2018, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Eddy, F.B. Role of nitric oxide in larval and juvenile fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 221–230. [Google Scholar] [CrossRef]
- Hill, B.G.; Dranka, B.P.; Bailey, S.M.; Lancaster, J.R., Jr.; Darley-Usmar, V.M. What part of NO don’t you understand? Some answers to the cardinal questions in nitric oxide biology. J. Biol. Chem. 2010, 285, 19699–19704. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Jensen, F.B. The role of nitrite in nitric oxide homeostasis: A comparative perspective. Biochim. Biophys. Acta 2009, 1787, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Seddon, M.; Shah, A.M.; Casadei, B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc. Res. 2007, 75, 315–326. [Google Scholar] [CrossRef]
- Balligand, J.L.; Feron, O.; Dessy, C. eNOS activation by physical forces: From short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 2009, 89, 481–534. [Google Scholar] [CrossRef]
- Agnisola, C. Role of nitric oxide in the control of coronary resistance in teleosts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Donald, J.A. Nervous control of circulation-the role of gasotransmitters, NO, CO, and H2S. Acta Histochem. 2009, 111, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Imbrogno, S.; Tota, B.; Gattuso, A. The evolutionary functions of cardiac NOS/NO in vertebrates tracked by fish and amphibian paradigms. Nitric Oxide 2011, 25, 1–10. [Google Scholar] [CrossRef]
- Imbrogno, S.; Filice, M.; Cerra, M.C.; Gattuso, A. NO, CO and H2S: What about gasotransmitters in fish and amphibian heart? Acta Physiol. 2018, 223, e13035. [Google Scholar] [CrossRef] [PubMed]
- Andreakis, N.; D’Aniello, S.; Albalat, R.; Patti, F.P.; Garcia-Fernandez, J.; Procaccini, G.; Sordino, P.; Palumbo, A. Evolution of the nitric oxide synthase family in metazoans. Mol. Biol. Evol. 2011, 28, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Lepiller, S.; Franche, N.; Solary, E.; Chluba, J.; Laurens, V. Comparative analysis of zebrafish nos2a and nos2b genes. Gene 2009, 445, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tota, B.; Amelio, D.; Pellegrino, D.; Ip, Y.K.; Cerra, M.C. NO modulation of myocardial performance in fish hearts. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 142, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Amelio, D.; Garofalo, F.; David, S.; Fucarino, A.; Jensen, F.B.; Imbrogno, S.; Cerra, M.C. Angiotensin II dependent cardiac remodeling in the eel Anguilla anguilla involves the NOS/NO system. Nitric Oxide 2017, 65, 50–59. [Google Scholar] [CrossRef]
- Amelio, D.; Garofalo, F.; Pellegrino, D.; Giordano, F.; Tota, B.; Cerra, M.C. Cardiac expression and distribution of nitric oxide synthases in the ventricle of the cold-adapted Antarctic teleosts, the hemoglobinless Chionodraco hamatus and the red-blooded Trematomus bernacchii. Nitric Oxide 2006, 15, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, F.; Amelio, D.; Cerra, M.C.; Tota, B.; Sidell, B.D.; Pellegrino, D. Morphological and physiological study of the cardiac NOS/NO system in the Antarctic (Hb-/Mb-) icefish Chaenocephalus aceratus and in the red-blooded Trematomus bernacchii. Nitric Oxide 2009, 20, 69–78. [Google Scholar] [CrossRef]
- Amelio, D.; Garofalo, F.; Brunelli, E.; Loong, A.M.; Wong, W.P.; Ip, Y.K.; Tota, B.; Cerra, M.C. Differential NOS expression in freshwater and aestivating Protopterus dolloi (lungfish): Heart vs. kidney readjustments. Nitric Oxide 2008, 18, 1–10. [Google Scholar] [CrossRef]
- Tota, B.; Imbrogno, S.; Mazza, R.; Gattuso, A. NOS distribution and NO control of cardiac performance in fish and amphibian hearts. Adv. Exp. Biol. 2007, 1, 311–338. [Google Scholar] [CrossRef]
- Carnevale, C.; Syme, D.A.; Gamperl, A.K. Effects of hypoxic acclimation, muscle strain, and contraction frequency on nitric oxide-mediated myocardial performance in steelhead trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R588–R610. [Google Scholar] [CrossRef]
- Imbrogno, S.; De Iuri, L.; Mazza, R.; Tota, B. Nitric oxide modulates cardiac performance in the heart of Anguilla anguilla. J. Exp. Biol. 2001, 204, 1719–1727. [Google Scholar] [CrossRef]
- Gattuso, A.; Mazza, R.; Imbrogno, S.; Sverdrup, A.; Tota, B.; Nylund, A. Cardiac performance in Salmo salar with infectious salmon anaemia (ISA): Putative role of nitric oxide. Dis. Aquat. Organ. 2002, 52, 11–20. [Google Scholar] [CrossRef]
- Garofalo, F.; Parisella, M.L.; Amelio, D.; Tota, B.; Imbrogno, S. Phospholamban S-nitrosylation modulates Starling response in fish heart. Proc. Biol. Sci. 2009, 276, 4043–4052. [Google Scholar] [CrossRef]
- Imbrogno, S.; Cerra, M.C.; Tota, B. Angiotensin II-induced inotropism requires an endocardial endothelium-nitric oxide mechanism in the in-vitro heart of Anguilla anguilla. J. Exp. Biol. 2003, 206, 2675–2684. [Google Scholar] [CrossRef]
- Imbrogno, S.; Angelone, T.; Corti, A.; Adamo, C.; Helle, K.B.; Tota, B. Influence of vasostatins, the chromogranin A-derived peptides, on the working heart of the eel (Anguilla anguilla): Negative inotropy and mechanism of action. Gen. Comp. Endocrinol. 2004, 139, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Imbrogno, S.; Angelone, T.; Adamo, C.; Pulera, E.; Tota, B.; Cerra, M.C. Beta3-adrenoceptor in the eel (Anguilla anguilla) heart: Negative inotropy and NO-cGMP-dependent mechanism. J. Exp. Biol. 2006, 209, 4966–4973. [Google Scholar] [CrossRef]
- Hansen, M.N.; Lundberg, J.O.; Filice, M.; Fago, A.; Christensen, N.M.; Jensen, F.B. The roles of tissue nitrate reductase activity and myoglobin in securing nitric oxide availability in deeply hypoxic crucian carp. J. Exp. Biol. 2016, 219, 3875–3883. [Google Scholar] [CrossRef] [PubMed]
- Sandvik, G.K.; Nilsson, G.E.; Jensen, F.B. Dramatic increase of nitrite levels in hearts of anoxia-exposed crucian carp supporting a role in cardioprotection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R468–R477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jensen, F.B. Nitric oxide formation from nitrite in zebrafish. J. Exp. Biol. 2007, 210, 3387–3394. [Google Scholar] [CrossRef]
- Cerra, M.C.; Angelone, T.; Parisella, M.L.; Pellegrino, D.; Tota, B. Nitrite modulates contractility of teleost (Anguilla anguilla and Chionodraco hamatus, i.e., the Antarctic hemoglobinless icefish) and frog (Rana esculenta) hearts. Biochim. Biophys. Acta 2009, 1787, 849–855. [Google Scholar] [CrossRef][Green Version]
- Angelone, T.; Gattuso, A.; Imbrogno, S.; Mazza, R.; Tota, B. Nitrite is a positive modulator of the Frank-Starling response in the vertebrate heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1271–R1281. [Google Scholar] [CrossRef][Green Version]
- Mu, J.; Chernick, M.; Dong, W.; Di Giulio, R.T.; Hinton, D.E. Early life co-exposures to a real-world PAH mixture and hypoxia result in later life and next generation consequences in medaka (Oryzias latipes). Aquat. Toxicol. 2017, 190, 162–173. [Google Scholar] [CrossRef]
- Keeling, R.F.; Garcia, H.E. The change in oceanic O(2) inventory associated with recent global warming. Proc. Natl. Acad. Sci. USA 2002, 99, 7848–7853. [Google Scholar] [CrossRef]
- Bograd, S.J.; Castro, C.G.; Di Lorenzo, E.; Palacios, D.M.; Bailey, H.; Gilly, W.; Chavez, F.P. Oxygen declines and the shoaling of the hypoxic boundary in the California Current. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Chapman, L.J. Low-Oxygen Lifestyles. In Extremophile Fishes: Ecology, Evolution, and Physiology of Teleosts in Extreme Environments; Riesch, R., Tobler, M., Plath, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 9–33. [Google Scholar]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Gregoire, M.; Chavez, F.P.; Conley, D.J.; Garcon, V.; Gilbert, D.; Gutierrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Galic, N.; Hawkins, T.; Forbes, V.E. Adverse impacts of hypoxia on aquatic invertebrates: A meta-analysis. Sci. Total Environ. 2019, 652, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Jonz, M.G.; Buck, L.T.; Perry, S.F.; Schwerte, T.; Zaccone, G. Sensing and surviving hypoxia in vertebrates. Ann. N. Y. Acad. Sci. 2016, 1365, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, P.G.; Brill, R.W.; Bourke, R.E. Cardiorespiratory responses of skipjack tuna (Katsuwonus pelamis), yellowfin tuna (Thunnus albacares), and bigeye tuna (Thunnus obesus) to acute reductions of ambient oxygen. Can. J. Zool. 1990, 68, 1857–1865. [Google Scholar] [CrossRef]
- Gesser, H. The effects of hypoxia and reoxygenation on force development in myocardia of carp and rainbow trout: Protective effects of CO2/HCO3. J. Exp. Biol. 1977, 69, 199–206. [Google Scholar] [CrossRef]
- Axelsson, M.; Farrell, A.P.; Nilsson, S. Effects of Hypoxia and Drugs on the Cardiovascular Dynamics of the Atlantic Hagfish Myxine Glutinosa. J. Exp. Biol. 1990, 151, 297–316. [Google Scholar] [CrossRef]
- Bailey, J.R.; Val, A.L.; Almeida-Val, V.; Driedzic, W.R. Anoxic cardiac performance in Amazonian and north-temperate-zone teleosts. Can. J. Zool. 1999, 77, 683–689. [Google Scholar] [CrossRef]
- Bickler, P.E.; Buck, L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.G. Physiological, behavioral and biochemical adaptations of intertidal fishes to hypoxia. J. Exp. Biol. 2011, 214, 191–199. [Google Scholar] [CrossRef]
- Agnisola, C.; McKenzie, D.J.; Pellegrino, D.; Bronzi, P.; Tota, B.; Taylor, E.W. Cardiovascular responses to hypoxia in the Adriatic sturgeon (Acipenser naccarii). J. Appl. Ichthyol. 1999, 15, 67–72. [Google Scholar] [CrossRef]
- Pedersen, C.L.; Faggiano, S.; Helbo, S.; Gesser, H.; Fago, A. Roles of nitric oxide, nitrite and myoglobin on myocardial efficiency in trout (Oncorhynchus mykiss) and goldfish (Carassius auratus): Implications for hypoxia tolerance. J. Exp. Biol. 2010, 213, 2755–2762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carnevale, C.; Roberts, J.C.; Syme, D.A.; Gamperl, A.K. Hypoxic acclimation negatively impacts the contractility of steelhead trout (Oncorhynchus mykiss) spongy myocardium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R214–R226. [Google Scholar] [CrossRef]
- Stecyk, J.A.; Stenslokken, K.O.; Farrell, A.P.; Nilsson, G.E. Maintained cardiac pumping in anoxic crucian carp. Science 2004, 306, 77. [Google Scholar] [CrossRef] [PubMed]
- Imbrogno, S.; Aiello, D.; Filice, M.; Leo, S.; Mazza, R.; Cerra, M.C.; Napoli, A. MS-Based proteomic analysis of cardiac response to hypoxia in the goldfish (Carassius auratus). Sci. Rep. 2019, 9, 18953. [Google Scholar] [CrossRef]
- Shoubridge, E.A.; Hochachka, P.W. Ethanol: Novel end product of vertebrate anaerobic metabolism. Science 1980, 209, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Fagernes, C.E.; Stenslokken, K.O.; Rohr, A.K.; Berenbrink, M.; Ellefsen, S.; Nilsson, G.E. Extreme anoxia tolerance in crucian carp and goldfish through neofunctionalization of duplicated genes creating a new ethanol-producing pyruvate decarboxylase pathway. Sci. Rep. 2017, 7, 7884. [Google Scholar] [CrossRef]
- Alderman, S.L.; Harter, T.S.; Wilson, J.M.; Supuran, C.T.; Farrell, A.P.; Brauner, C.J. Evidence for a plasma-accessible carbonic anhydrase in the lumen of salmon heart that may enhance oxygen delivery to the myocardium. J. Exp. Biol. 2016, 219, 719–724. [Google Scholar] [CrossRef]
- Farrell, A.P.; Farrell, N.D.; Jourdan, H.; Cox, G.K. A Perspective on the Evolution of the Coronary Circulation in Fishes and the Transition to Terrestrial Life. In Ontogeny and Phylogeny of the Vertebrate Heart; Sedmera, D., Wang, T., Eds.; Springer: New York, NY, USA, 2012; pp. 75–102. [Google Scholar]
- Tota, B.; Cimini, V.; Salvatore, G.; Zummo, G. Comparative study of the arterial and lacunary systems of the ventricular myocardium of elasmobranch and teleost fishes. Am. J. Anat. 1983, 167, 15–32. [Google Scholar] [CrossRef]
- Icardo, J.M. 1—Heart Morphology and Anatomy. In Fish Physiology; Gamperl, A.K., Gillis, T.E., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 36, pp. 1–54. [Google Scholar]
- Imbrogno, S.; Filice, M.; Cerra, M.C. Exploring cardiac plasticity in teleost: The role of humoral modulation. Gen. Comp. Endocrinol. 2019, 283, 113236. [Google Scholar] [CrossRef]
- Holeton, G.F.; Randall, D.J. The Effect of Hypoxia Upon the Partial Pressure of Gases in the Blood and Water Afferent and Efferent to the Gills of Rainbow Trout. J. Exp. Biol. 1967, 46, 317–327. [Google Scholar] [CrossRef]
- Thomas, S.; Fritsche, R.; Perry, S.F. Pre-and post-branchial blood respiratory status during acute hypercapnia or hypoxia in rainbow trout, Oncorhynchus mykiss. J. Comp. Physiol. B 1994, 164, 451–458. [Google Scholar] [CrossRef]
- Gamperl, A.; Pinder, A.; Grant, R.; Boutilier, R. Influence of hypoxia and adrenaline administration on coronary blood flow and cardiac performance in seawater rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 1994, 193, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.C.; Carnevale, C.; Gamperl, A.K.; Syme, D.A. Effects of hypoxic acclimation on contractile properties of the spongy and compact ventricular myocardium of steelhead trout (Oncorhynchus mykiss). J. Comp. Physiol. B 2021, 191, 99–111. [Google Scholar] [CrossRef]
- Erusalimsky, J.D.; Moncada, S. Nitric oxide and mitochondrial signaling: From physiology to pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2524–2531. [Google Scholar] [CrossRef]
- Shen, W.; Tian, R.; Saupe, K.W.; Spindler, M.; Ingwall, J.S. Endogenous nitric oxide enhances coupling between O2 consumption and ATP synthesis in guinea pig hearts. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H838–H846. [Google Scholar] [CrossRef]
- Misfeldt, M.; Fago, A.; Gesser, H. Nitric oxide increases myocardial efficiency in the hypoxia-tolerant turtle Trachemys scripta. J. Exp. Biol. 2009, 212, 954–960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Filice, M.; Mazza, R.; Leo, S.; Gattuso, A.; Cerra, M.C.; Imbrogno, S. The Hypoxia Tolerance of the Goldfish (Carassius auratus) Heart: The NOS/NO System and Beyond. Antioxidants 2020, 9, 555. [Google Scholar] [CrossRef]
- Strijdom, H.; Chamane, N.; Lochner, A. Nitric oxide in the cardiovascular system: A simple molecule with complex actions. Cardiovasc. J. Afr. 2009, 20, 303–310. [Google Scholar]
- Angelone, T.; Quintieri, A.M.; Pasqua, T.; Filice, E.; Cantafio, P.; Scavello, F.; Rocca, C.; Mahata, S.K.; Gattuso, A.; Cerra, M.C. The NO stimulator, Catestatin, improves the Frank-Starling response in normotensive and hypertensive rat hearts. Nitric Oxide 2015, 50, 10–19. [Google Scholar] [CrossRef]
- Hess, D.T.; Matsumoto, A.; Kim, S.O.; Marshall, H.E.; Stamler, J.S. Protein S-nitrosylation: Purview and parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef]
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009, 15, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Sips, P.Y.; Irie, T.; Zou, L.; Shinozaki, S.; Sakai, M.; Shimizu, N.; Nguyen, R.; Stamler, J.S.; Chao, W.; Kaneki, M.; et al. Reduction of cardiomyocyte S-nitrosylation by S-nitrosoglutathione reductase protects against sepsis-induced myocardial depression. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1134–H1146. [Google Scholar] [CrossRef]
- Radi, R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Ischiropoulos, H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun. 2003, 305, 776–783. [Google Scholar] [CrossRef]
- Bigelow, D.J. Nitrotyrosine-modified SERCA2: A cellular sensor of reactive nitrogen species. Pflugers. Arch. 2009, 457, 701–710. [Google Scholar] [CrossRef]
- Mazza, R.; Gattuso, A.; Imbrogno, S.; Boukhzar, L.; Leo, S.; Mallouki, B.Y.; Filice, M.; Rocca, C.; Angelone, T.; Anouar, Y.; et al. Selenoprotein T as a new positive inotrope in the goldfish, Carassius auratus. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.L.; Hamstra, S.I.; Messner, H.N.; Fajardo, V.A. SERCA2a tyrosine nitration coincides with impairments in maximal SERCA activity in left ventricles from tafazzin-deficient mice. Physiol. Rep. 2019, 7, e14215. [Google Scholar] [CrossRef]
- Cadenas, E. Mitochondrial free radical production and cell signaling. Mol. Asp. Med. 2004, 25, 17–26. [Google Scholar] [CrossRef]
- Viner, R.I.; Ferrington, D.A.; Huhmer, A.F.; Bigelow, D.J.; Schoneich, C. Accumulation of nitrotyrosine on the SERCA2a isoform of SR Ca-ATPase of rat skeletal muscle during aging: A peroxynitrite-mediated process? FEBS Lett. 1996, 379, 286–290. [Google Scholar] [CrossRef]
- Noma, A. ATP-Regulated K+ channels in cardiac muscle. Nature 1983, 305, 147–148. [Google Scholar] [CrossRef]
- Jugdutt, B.I. Nitric oxide and cardioprotection during ischemia-reperfusion. Heart Fail. Rev. 2002, 7, 391–405. [Google Scholar] [CrossRef]
- Ong, S.G.; Hausenloy, D.J. Hypoxia-Inducible factor as a therapeutic target for cardioprotection. Pharmacol. Ther. 2012, 136, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, X.; Hu, X.; Jiang, H.; Chen, L.; Xu, Q. Hypoxia-Inducible factor 1alpha from a high-altitude fish enhances cytoprotection and elevates nitric oxide production in hypoxic environment. Fish. Physiol. Biochem. 2020, 46, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Garcia-Lecea, M.; Cadenas, S.; Hernandez, C.; Moncada, S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem. J. 2003, 376, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O. Integrating climate-related stressor effects on marine organisms: Unifying principles linking molecule to ecosystem-level changes. Mar. Ecol. Prog. Ser. 2012, 470, 273–290. [Google Scholar] [CrossRef]
- Portner, H.O.; Bock, C.; Mark, F.C. Oxygen- and capacity-limited thermal tolerance: Bridging ecology and physiology. J. Exp. Biol. 2017, 220, 2685–2696. [Google Scholar] [CrossRef]
- Ern, R.; Johansen, J.L.; Rummer, J.L.; Esbaugh, A.J. Effects of hypoxia and ocean acidification on the upper thermal niche boundaries of coral reef fishes. Biol. Lett. 2017, 13, 20170135. [Google Scholar] [CrossRef] [PubMed]
- Leeuwis, R.H.J.; Zanuzzo, F.S.; Peroni, E.F.C.; Gamperl, A.K. Research on sablefish (Anoplopoma fimbria) suggests that limited capacity to increase heart function leaves hypoxic fish susceptible to heat waves. Proc. Biol. Sci. 2021, 288, 20202340. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J., Jr.; Sears, M.W.; Pringle, R.M. Spatial dynamics of nesting behavior: Lizards shift microhabitats to construct nests with beneficial thermal properties. Ecology 2009, 90, 2933–2939. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.M.; Healy, T.M.; Fangue, N.A. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 2011, 51, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, F.; Tallis, J.A.; James, R.S. The cost of muscle power production: Muscle oxygen consumption per unit work increases at low temperatures in Xenopus laevis. J. Exp. Biol. 2014, 217, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Beaman, J.E.; White, C.R.; Seebacher, F. Evolution of Plasticity: Mechanistic Link between Development and Reversible Acclimation. Trends Ecol. Evol. 2016, 31, 237–249. [Google Scholar] [CrossRef]
- Little, A.G.; Loughland, I.; Seebacher, F. What do warming waters mean for fish physiology and fisheries? J. Fish. Biol. 2020, 97, 328–340. [Google Scholar] [CrossRef]
- Eliason, E.J.; Anttila, K. 4—Temperature and the Cardiovascular System. In Fish Physiology; Gamperl, A.K., Gillis, T.E., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 36, pp. 235–297. [Google Scholar]
- Mendonca, P.C.; Gamperl, A.K. The effects of acute changes in temperature and oxygen availability on cardiac performance in winter flounder (Pseudopleuronectes americanus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 155, 245–252. [Google Scholar] [CrossRef]
- Farrell, A.P.; Smith, F. 4—Cardiac Form, Function and Physiology. In Fish Physiology; Gamperl, A.K., Gillis, T.E., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 36, pp. 155–264. [Google Scholar]
- Farrell, A.P.F.P.; Eliason, E.J.E.J.; Sandblom, E.S.; Clark, T.D.C.D. Fish cardiorespiratory physiology in an era of climate change. Can. J. Zool. 2009, 87, 835–851. [Google Scholar] [CrossRef]
- Ekstrom, A.; Sundell, E.; Morgenroth, D.; Sandblom, E. Adrenergic tone benefits cardiac performance and warming tolerance in two teleost fishes that lack a coronary circulation. J. Comp. Physiol. B 2021, 191, 701–709. [Google Scholar] [CrossRef]
- Keen, A.N.; Klaiman, J.M.; Shiels, H.A.; Gillis, T.E. Temperature-Induced cardiac remodelling in fish. J. Exp. Biol. 2017, 220, 147–160. [Google Scholar] [CrossRef]
- Badr, A.; El-Sayed, M.F.; Vornanen, M. Effects of seasonal acclimatization on temperature dependence of cardiac excitability in the roach, Rutilus rutilus. J. Exp. Biol. 2016, 219, 1495–1504. [Google Scholar] [CrossRef]
- Haverinen, J.; Vornanen, M. Temperature acclimation modifies sinoatrial pacemaker mechanism of the rainbow trout heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1023–R1032. [Google Scholar] [CrossRef]
- Aho, E.; Vornanen, M. Cold acclimation increases basal heart rate but decreases its thermal tolerance in rainbow trout (Oncorhynchus mykiss). J. Comp. Physiol. B 2001, 171, 173–179. [Google Scholar] [CrossRef]
- Ekström, A.; Hellgren, K.; Gräns, A.; Pichaud, N.; Sandblom, E. Dynamic changes in scope for heart rate and cardiac autonomic control during warm acclimation in rainbow trout. J. Exp. Biol. 2016, 219, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, R.L.; Li, S.; Gilbert, M.J.H.; Schulte, P.M.; Miller, K.M.; Farrell, A.P. A rapid intrinsic heart rate resetting response with thermal acclimation in rainbow trout, Oncorhynchus mykiss. J. Exp. Biol. 2020, 223, jeb215210. [Google Scholar] [CrossRef] [PubMed]
- Vornanen, M. The temperature dependence of electrical excitability in fish hearts. J. Exp. Biol. 2016, 219, 1941–1952. [Google Scholar] [CrossRef]
- Haverinen, J.; Vornanen, M. Reduced ventricular excitability causes atrioventricular block and depression of heart rate in fish at critically high temperatures. J. Exp. Biol. 2020, 223, jeb225227. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Farrell, A.P. Cardiac plasticity in fishes: Environmental influences and intraspecific differences. J. Exp. Biol. 2004, 207, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Klaiman, J.M.; Fenna, A.J.; Shiels, H.A.; Macri, J.; Gillis, T.E. Cardiac remodeling in fish: Strategies to maintain heart function during temperature Change. PLoS ONE 2011, 6, e24464. [Google Scholar] [CrossRef]
- Nyboer, E.A.; Chapman, L.J. Cardiac plasticity influences aerobic performance and thermal tolerance in a tropical, freshwater fish at elevated temperatures. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed]
- Tota, B.; Cerra, M.C.; Mazza, R.; Pellegrino, D.; Icardo, J. The heart of the Antarctic icefish as paradigm of cold adaptation. J. Therm. Biol. 1997, 22, 409–417. [Google Scholar] [CrossRef]
- Garofalo, F.; Pellegrino, D.; Amelio, D.; Tota, B. The Antarctic hemoglobinless icefish, fifty five years later: A unique cardiocirculatory interplay of disaptation and phenotypic plasticity. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Ruud, J.T. Vertebrates without erythrocytes and blood pigment. Nature 1954, 173, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Grove, T.J.; Hendrickson, J.W.; Sidell, B.D. Two species of antarctic icefishes (genus Champsocephalus) share a common genetic lesion leading to the loss of myoglobin expression. Polar Biol. 2004, 27, 579–585. [Google Scholar] [CrossRef]
- Beers, J.M.; Borley, K.A.; Sidell, B.D. Relationship among circulating hemoglobin, nitric oxide synthase activities and angiogenic poise in red- and white-blooded Antarctic notothenioid fishes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Murphy, E.J.; Meredith, M.P.; King, J.C.; Peck, L.S.; Barnes, D.K.A.; Smith, R.C. Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 149–166. [Google Scholar] [CrossRef]
- Egginton, S.; Axelsson, M.; Crockett, E.L.; O’Brien, K.M.; Farrell, A.P. Maximum cardiac performance of Antarctic fishes that lack haemoglobin and myoglobin: Exploring the effect of warming on nature’s natural knockouts. Conserv. Physiol. 2019, 7, coz049. [Google Scholar] [CrossRef] [PubMed]
- Amelio, D.; Garofalo, F.; Capria, C.; Tota, B.; Imbrogno, S. Effects of temperature on the nitric oxide-dependent modulation of the Frank–Starling mechanism: The fish heart as a case study. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 356–362. [Google Scholar] [CrossRef]
- Jorgensen, S.M.; Castro, V.; Krasnov, A.; Torgersen, J.; Timmerhaus, G.; Hevroy, E.M.; Hansen, T.J.; Susort, S.; Breck, O.; Takle, H. Cardiac responses to elevated seawater temperature in Atlantic salmon. BMC Physiol. 2014, 14, 2. [Google Scholar] [CrossRef]
- Garofalo, F.; Amelio, D.; Icardo, J.M.; Chew, S.F.; Tota, B.; Cerra, M.C.; Ip, Y.K. Signal molecule changes in the gills and lungs of the African lungfish Protopterus annectens, during the maintenance and arousal phases of aestivation. Nitric Oxide 2015, 44, 71–80. [Google Scholar] [CrossRef]
- Amelio, D.; Garofalo, F. The NOS/NO system in an example of extreme adaptation: The African lungfish. J. Therm. Biol. 2020, 90, 102594. [Google Scholar] [CrossRef]
- Filogonio, R.; Joyce, W.; Wang, T. Nitrergic cardiovascular regulation in the African lungfish, Protopterus aethiopicus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 207, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Ern, R.; Norin, T.; Gamperl, A.K.; Esbaugh, A.J. Oxygen dependence of upper thermal limits in fishes. J. Exp. Biol. 2016, 219, 3376–3383. [Google Scholar] [CrossRef] [PubMed]
- Gollock, M.J.; Currie, S.; Petersen, L.H.; Gamperl, A.K. Cardiovascular and haematological responses of Atlantic cod (Gadus morhua) to acute temperature increase. J. Exp. Biol. 2006, 209, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Iftikar, F.I.; Hickey, A.J. Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS ONE 2013, 8, e64120. [Google Scholar] [CrossRef]
- Hunter-Manseau, F.; Desrosiers, V.; Le Francois, N.R.; Dufresne, F.; Detrich, H.W., 3rd; Nozais, C.; Blier, P.U. From Africa to Antarctica: Exploring the Metabolism of Fish Heart Mitochondria Across a Wide Thermal Range. Front. Physiol. 2019, 10, 1220. [Google Scholar] [CrossRef]
- Christen, F.; Desrosiers, V.; Dupont-Cyr, B.A.; Vandenberg, G.W.; Le Francois, N.R.; Tardif, J.C.; Dufresne, F.; Lamarre, S.G.; Blier, P.U. Thermal tolerance and thermal sensitivity of heart mitochondria: Mitochondrial integrity and ROS production. Free Radic. Biol. Med. 2018, 116, 11–18. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Rix, A.S.; Egginton, S.; Farrell, A.P.; Crockett, E.L.; Schlauch, K.; Woolsey, R.; Hoffman, M.; Merriman, S. Cardiac mitochondrial metabolism may contribute to differences in thermal tolerance of red- and white-blooded Antarctic notothenioid fishes. J. Exp. Biol. 2018, 221, jeb177816. [Google Scholar] [CrossRef]
- Michaelsen, J.; Fago, A.; Bundgaard, A. High temperature impairs mitochondrial function in rainbow trout cardiac mitochondria. J. Exp. Biol. 2021, 224, jeb242382. [Google Scholar] [CrossRef]
- Gerber, L.; Clow, K.A.; Mark, F.C.; Gamperl, A.K. Improved mitochondrial function in salmon (Salmo salar) following high temperature acclimation suggests that there are cracks in the proverbial ‘ceiling’. Sci. Rep. 2020, 10, 21636. [Google Scholar] [CrossRef]
- Gerber, L.; Clow, K.A.; Gamperl, A.K. Acclimation to warm temperatures has important implications for mitochondrial function in Atlantic salmon (Salmo salar). J. Exp. Biol. 2021, 224, jeb236257. [Google Scholar] [CrossRef]
- Cook, D.G.; Iftikar, F.I.; Baker, D.W.; Hickey, A.J.R.; Herbert, N.A. Low-O2 acclimation shifts the hypoxia avoidance behaviour of snapper (Pagrus auratus) with only subtle changes in aerobic and anaerobic function. J. Exp. Biol. 2013, 216, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.J.; Renshaw, G.M.; Speers-Roesch, B.; Richards, J.G.; Wang, Y.; Farrell, A.P.; Brauner, C.J. A radical approach to beating hypoxia: Depressed free radical release from heart fibres of the hypoxia-tolerant epaulette shark (Hemiscyllum ocellatum). J. Comp. Physiol. B 2012, 182, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.; Clow, K.A.; Katan, T.; Emam, M.; Leeuwis, R.H.J.; Parrish, C.C.; Gamperl, A.K. Cardiac mitochondrial function, nitric oxide sensitivity and lipid composition following hypoxia acclimation in sablefish. J. Exp. Biol. 2019, 222, jeb208074. [Google Scholar] [CrossRef]
- Fuller, S.J.; Osborne, S.A.; Leonard, S.J.; Hardyman, M.A.; Vaniotis, G.; Allen, B.G.; Sugden, P.H.; Clerk, A. Cardiac protein kinases: The cardiomyocyte kinome and differential kinase expression in human failing hearts. Cardiovasc. Res. 2015, 108, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Rocca, C.; Scavello, F.; Granieri, M.C.; Pasqua, T.; Amodio, N.; Imbrogno, S.; Gattuso, A.; Mazza, R.; Cerra, M.C.; Angelone, T. Phoenixin-14: Detection and novel physiological implications in cardiac modulation and cardioprotection. Cell Mol. Life Sci. 2018, 75, 743–756. [Google Scholar] [CrossRef]
- Zaha, V.G.; Young, L.H. AMP-Activated protein kinase regulation and biological actions in the heart. Circ. Res. 2012, 111, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D. The AMP-activated protein kinase-fuel gauge of the mammalian cell? Eur. J. Biochem. 1997, 246, 259–273. [Google Scholar] [CrossRef]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Dengler, F. Activation of AMPK under Hypoxia: Many Roads Leading to Rome. Int. J. Mol. Sci. 2020, 21, 2428. [Google Scholar] [CrossRef]
- Shao, D.; Oka, S.; Liu, T.; Zhai, P.; Ago, T.; Sciarretta, S.; Li, H.; Sadoshima, J. A redox-dependent mechanism for regulation of AMPK activation by Thioredoxin1 during energy starvation. Cell Metab. 2014, 19, 232–245. [Google Scholar] [CrossRef]
- Cardaci, S.; Filomeni, G.; Ciriolo, M.R. Redox implications of AMPK-mediated signal transduction beyond energetic clues. J. Cell Sci. 2012, 125, 2115–2125. [Google Scholar] [CrossRef]
- Kar, R.; Kellogg, D.L., 3rd; Roman, L.J. Oxidative stress induces phosphorylation of neuronal NOS in cardiomyocytes through AMP-activated protein kinase (AMPK). Biochem. Biophys. Res. Commun. 2015, 459, 393–397. [Google Scholar] [CrossRef]
- Sartoretto, J.L.; Kalwa, H.; Pluth, M.D.; Lippard, S.J.; Michel, T. Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15792–15797. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, T.S.; Kolb, E.M.; Sun, K.; Lu, X.; Sladek, F.M.; Kassab, G.S.; Garland, T., Jr.; Shyy, J.Y. AMP-Activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhu, H.; Wu, X.; Zhou, L.; Song, Y.; Zhu, S.; Hao, M.; Liu, C.; Fan, Y.; et al. Ischemic postconditioning protects the heart against ischemia-reperfusion injury via neuronal nitric oxide synthase in the sarcoplasmic reticulum and mitochondria. Cell Death Dis. 2016, 7, e2222. [Google Scholar] [CrossRef] [PubMed]
- Salt, I.P.; Hardie, D.G. AMP-Activated Protein Kinase: An Ubiquitous Signaling Pathway With Key Roles in the Cardiovascular System. Circ. Res. 2017, 120, 1825–1841. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Panserat, S.; Craig, P.M.; Martyres, D.J.; Plagnes-Juan, E.; Savari, S.; Aris-Brosou, S.; Moon, T.W. The metabolic consequences of hepatic AMP-kinase phosphorylation in rainbow trout. PLoS ONE 2011, 6, e20228. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.M.; Moyes, C.D.; LeMoine, C.M.R. Sensing and responding to energetic stress: Evolution of the AMPK network. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 224, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Causey, D.R.; Kim, J.H.; Devlin, R.H.; Martin, S.A.M.; Macqueen, D.J. The AMPK system of salmonid fishes was expanded through genome duplication and is regulated by growth and immune status in muscle. Sci. Rep. 2019, 9, 9819. [Google Scholar] [CrossRef]
- Stenslokken, K.O.; Ellefsen, S.; Stecyk, J.A.; Dahl, M.B.; Nilsson, G.E.; Vaage, J. Differential regulation of AMP-activated kinase and AKT kinase in response to oxygen availability in crucian carp (Carassius carassius). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1803–R1814. [Google Scholar] [CrossRef] [PubMed]
- Jibb, L.A.; Richards, J.G. AMP-Activated protein kinase activity during metabolic rate depression in the hypoxic goldfish, Carassius auratus. J. Exp. Biol. 2008, 211, 3111–3122. [Google Scholar] [CrossRef]
- Pamenter, M.E. Mitochondria: A multimodal hub of hypoxia tolerance. Can. J. Zool. 2014, 92, 569–589. [Google Scholar] [CrossRef]
- Anttila, K.; Casselman, M.T.; Schulte, P.M.; Farrell, A.P. Optimum temperature in juvenile salmonids: Connecting subcellular indicators to tissue function and whole-organism thermal optimum. Physiol. Biochem. Zool. 2013, 86, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Lu, Y.; Zou, C.; Wang, L.; Zhang, P.; You, F. Insight into AMPK regulation mechanism in vivo and in vitro: Responses to low temperatures in the olive flounder Paralichthys olivaceus. J. Therm. Biol. 2020, 91, 102640. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.E.; Vaage, J.; Stensløkken, K.-O. Oxygen- and temperature-dependent expression of survival protein kinases in crucian carp (Carassius carassius) heart and brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R50–R61. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filice, M.; Imbrogno, S.; Gattuso, A.; Cerra, M.C. Hypoxic and Thermal Stress: Many Ways Leading to the NOS/NO System in the Fish Heart. Antioxidants 2021, 10, 1401. https://doi.org/10.3390/antiox10091401
Filice M, Imbrogno S, Gattuso A, Cerra MC. Hypoxic and Thermal Stress: Many Ways Leading to the NOS/NO System in the Fish Heart. Antioxidants. 2021; 10(9):1401. https://doi.org/10.3390/antiox10091401
Chicago/Turabian StyleFilice, Mariacristina, Sandra Imbrogno, Alfonsina Gattuso, and Maria Carmela Cerra. 2021. "Hypoxic and Thermal Stress: Many Ways Leading to the NOS/NO System in the Fish Heart" Antioxidants 10, no. 9: 1401. https://doi.org/10.3390/antiox10091401
APA StyleFilice, M., Imbrogno, S., Gattuso, A., & Cerra, M. C. (2021). Hypoxic and Thermal Stress: Many Ways Leading to the NOS/NO System in the Fish Heart. Antioxidants, 10(9), 1401. https://doi.org/10.3390/antiox10091401







