Red Wine Oxidation Characterization by Accelerated Ageing Tests and Cyclic Voltammetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Model Wine Solution
2.3. Red Wines
2.4. Wine Global Chemical Characterization
2.4.1. Wine Global Chemical Characterization
2.4.2. Quantitative Analysis of Free SO2 Levels (GC-MS)
2.5. Accelerated Ageing Tests
2.6. Determination of Phenolic Composition
2.6.1. Determination of Total Phenolic Content (TPC)
2.6.2. Total Tannins Content (TC)
2.6.3. Total Pigments Index (TP)
2.6.4. SO2 Bleaching Resistant Pigments Index (RP)
2.7. Electrochemical Apparatus and Measurements
2.8. Statistical Analysis
3. Results and Discussion
3.1. Wines Phenolic Characterization
3.2. Accelerated Ageing Tests: Oxygen Consumption Parameters
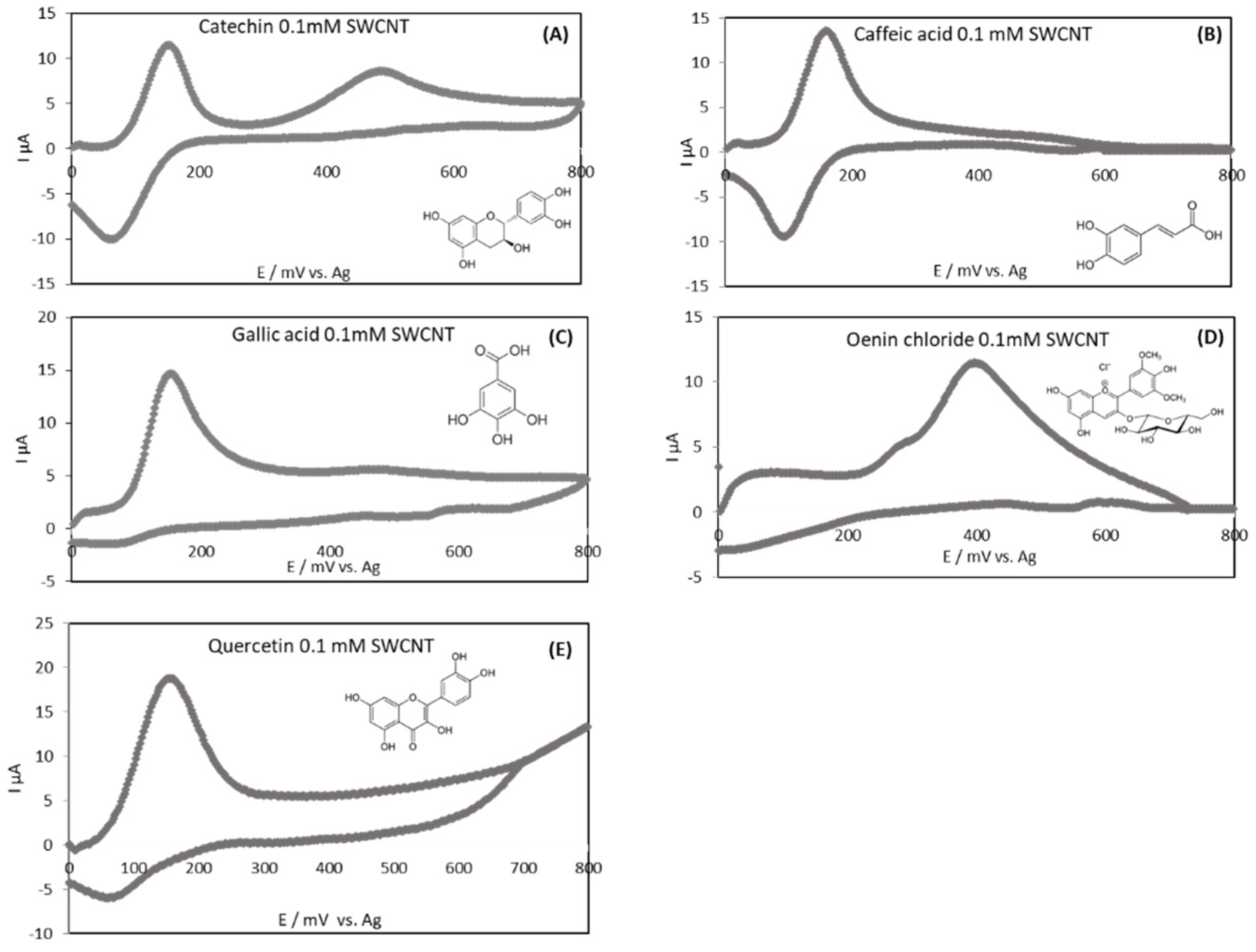
3.3. Electrochemical Behavior of Standard Polyphenols and Wine Samples Oxidized with the Three Different Protocols
3.3.1. Electrochemical Behavior of Standard Polyphenols
3.3.2. Electrochemical Behavior of Wines Non-Oxidized and Oxidized with Different Oxidation Protocols
3.3.3. Charges of Non-Oxidized Red Wines and Charge Variation after Accelerated Ageing Tests
3.4. Correlation between Phenolic Composition, Oxygen Consumption Rates and Electrochemical Parameters
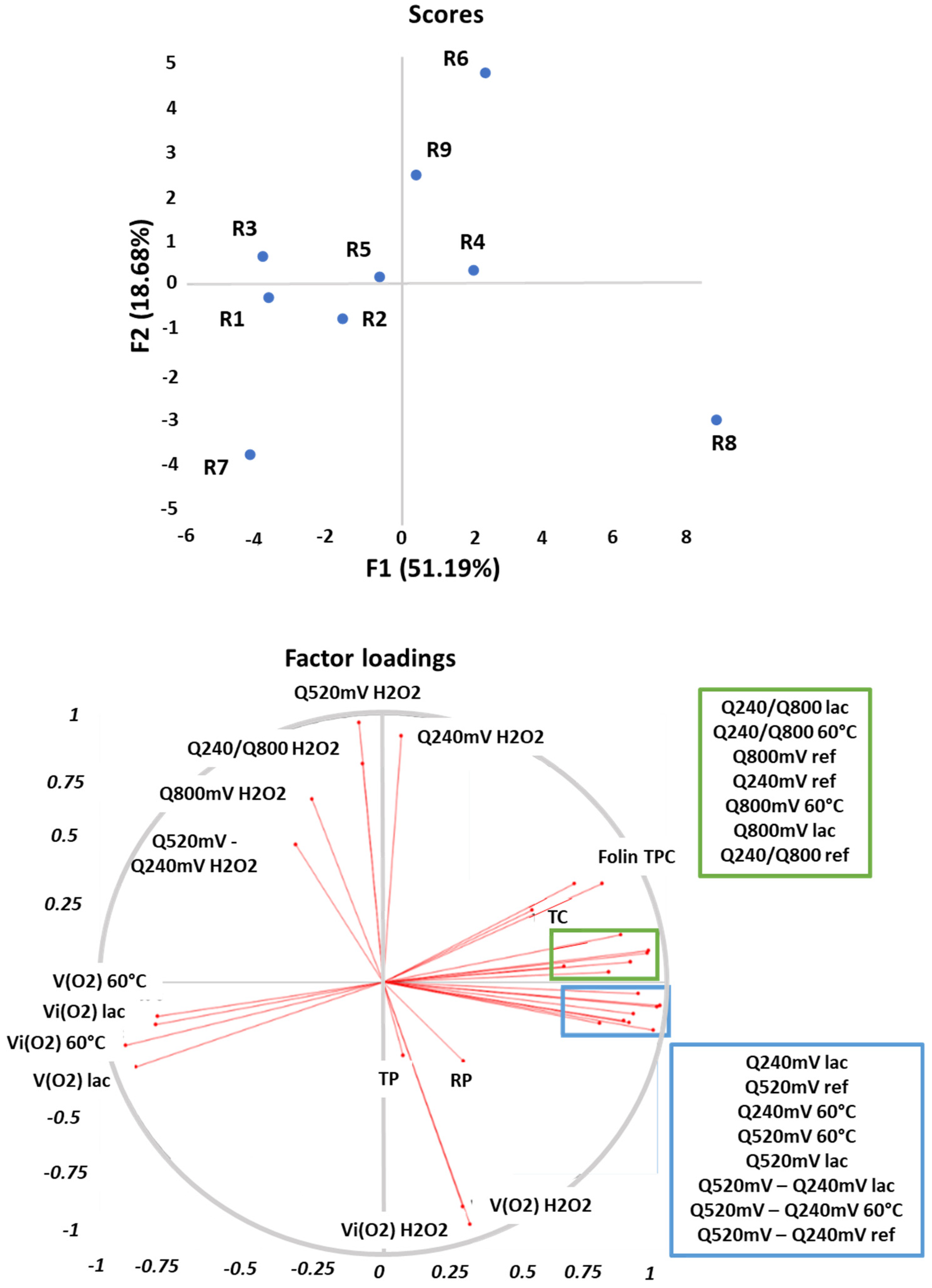
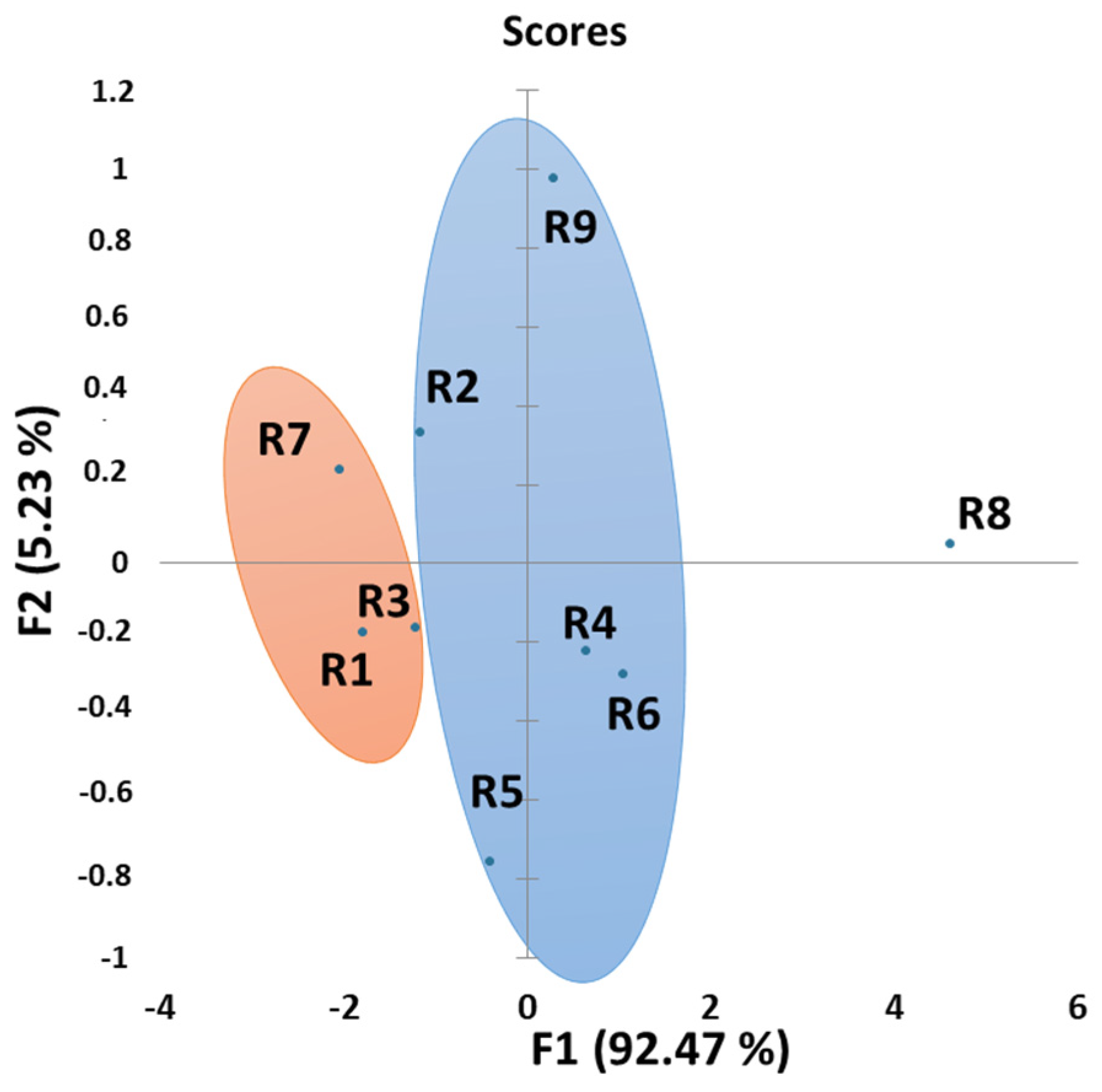
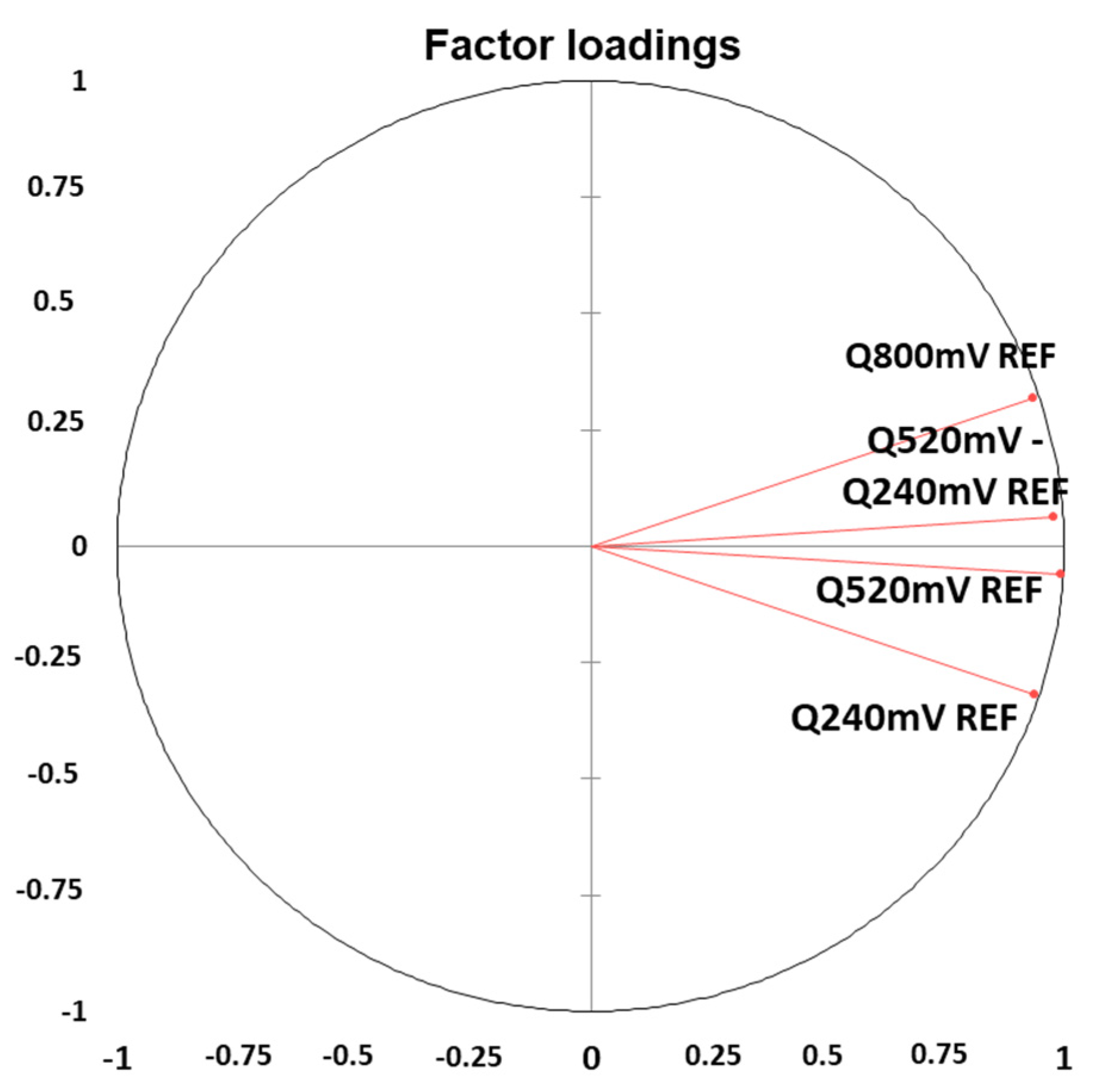
3.5. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Kilmartin, P. The Oxidation of Red and White Wines and Its Impact on Wine Aroma. Chem. N. Z. 2009, 73, 18–22. [Google Scholar]
- Ferreira, V.; Bueno, M.; Franco-Luesma, E.; Culleré, L.; Zurbano, P.F. Key Changes in Wine Aroma Active Compounds during Bottle Storage of Spanish Red Wines under Different Oxygen Levels. J. Agric. Food Chem. 2014, 62, 10015–10027. [Google Scholar] [CrossRef]
- Gambuti, A.; Siani, T.; Picariello, L.; Rinaldi, A.; Lisanti, M.T.; Ugliano, M.; Dieval, J.B.; Moio, L. Oxygen exposure of tannins-rich red wines during bottle aging. Influence on phenolics and color, astringency markers and sensory attributes. Eur. Food Res. Technol. 2017, 243, 669–680. [Google Scholar] [CrossRef]
- Ugliano, M. Oxygen Contribution to Wine Aroma Evolution during Bottle Aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef] [PubMed]
- Echave, J.; Barral, M.; Fraga-Corral, M.; Prieto, M.; Simal-Gandara, J. Bottle Aging and Storage of Wines: A Review. Molecules 2021, 26, 713. [Google Scholar] [CrossRef] [PubMed]
- Danilewicz, J.C. Reaction of Oxygen and Sulfite in Wine. Am. J. Enol. Vitic. 2016, 67, 13–17. [Google Scholar] [CrossRef]
- Ferreira, V.; Carrascón, V.; Bueno, M.; Ugliano, M.; Fernandez-Zurbano, P. Oxygen Consumption by Red Wines. Part I: Consumption Rates, Relationship with Chemical Composition, and Role of SO2. J. Agric. Food Chem. 2015, 63, 10928–10937. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Frost, S.; Ugliano, M.; Cantu, A.R.; Currie, B.L.; Anderson, M.; Chassy, A.W.; Vidal, S.; Diéval, J.-B.; Aagaard, O.; et al. Sulfur Dioxide-Oxygen Consumption Ratio Reveals Differences in Bottled Wine Oxidation. Am. J. Enol. Vitic. 2016, 67, 449–459. [Google Scholar] [CrossRef]
- Oliveira, C.; Ferreira, A.C.S.; Freitas, V.; Silva, A. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Singleton, V.L. Oxygen with Phenols and Related Reactions in Musts, Wines, and Model Systems: Observations and Practical Implications. Am. J. Enol. Vitic. 1987, 38, 9. [Google Scholar]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar]
- Danilewicz, J.C. Review of Reaction Mechanisms of Oxygen and Proposed Intermediate Reduction Products in Wine: Central Role of Iron and Copper. Am. J. Enol. Vitic. 2003, 54, 73–85. [Google Scholar]
- Nikolantonaki, M.; Chichuc, I.; Teissedre, P.-L.; Darriet, P. Reactivity of volatile thiols with polyphenols in a wine-model medium: Impact of oxygen, iron, and sulfur dioxide. Anal. Chim. Acta 2010, 660, 102–109. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Waterhouse, A.L. A Method to Quantify Quinone Reaction Rates with Wine Relevant Nucleophiles: A Key to the Understanding of Oxidative Loss of Varietal Thiols. J. Agric. Food Chem. 2012, 60, 8484–8491. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Magiatis, P.; Waterhouse, A.L. Measuring protection of aromatic wine thiols from oxidation by competitive reactions vs. wine preservatives with ortho-quinones. Food Chem. 2014, 163, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Santos, S.A.; Silvestre, A.J.; Barros, A.S.; Ferreira, A.C.S.; Silva, A.M. Quinones as Strecker degradation reagents in wine oxidation processes. Food Chem. 2017, 228, 618–624. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A Cyclic Voltammetry Method Suitable for Characterizing Antioxidant Properties of Wine and Wine Phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. Correlation of Wine Phenolic Composition versus Cyclic Voltammetry Response. Am. J. Enol. Vitic. 2002, 53, 294–302. [Google Scholar]
- Makhotkina, O.; Kilmartin, P.A. Uncovering the influence of antioxidants on polyphenol oxidation in wines using an electrochemical method: Cyclic voltammetry. J. Electroanal. Chem. 2009, 633, 165–174. [Google Scholar] [CrossRef]
- Ugliano, M. Rapid fingerprinting of white wine oxidizable fraction and classification of white wines using disposable screen printed sensors and derivative voltammetry. Food Chem. 2016, 212, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Piljac-Žegarac, J.; Valek, L.; Stipčević, T.; Martinez, S. Electrochemical determination of antioxidant capacity of fruit tea infusions. Food Chem. 2010, 121, 820–825. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.-L. Evolution of Analysis of Polyhenols from Grapes, Wines, and Extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Geană, E.-I.; Ciucure, C.T.; Artem, V.; Apetrei, C. Wine varietal discrimination and classification using a voltammetric sensor array based on modified screen-printed electrodes in conjunction with chemometric analysis. Microchem. J. 2020, 159, 105451. [Google Scholar] [CrossRef]
- Gonzalez, A.; Vidal, S.; Ugliano, M. Untargeted voltammetric approaches for characterization of oxidation patterns in white wines. Food Chem. 2018, 269, 1–8. [Google Scholar] [CrossRef]
- Dai, Y.-Q.; Shiu, K.-K. Glucose Biosensor Based on Multi-Walled Carbon Nanotube Modified Glassy Carbon Electrode. Electroanalysis 2004, 16, 1697–1703. [Google Scholar] [CrossRef]
- Liu, M.; Xiang, J.; Zhou, J.; Ding, H. A disposable amperometric sensor for rapid detection of serotonin in the blood and brain of the depressed mice based on Nafion membrane-coated colloidal gold screen-printed electrode. J. Electroanal. Chem. 2010, 640, 1–7. [Google Scholar] [CrossRef]
- Bordonaba, J.G.; Terry, L. Electrochemical behaviour of polyphenol rich fruit juices using disposable screen-printed carbon electrodes: Towards a rapid sensor for antioxidant capacity and individual antioxidants. Talanta 2012, 90, 38–45. [Google Scholar] [CrossRef]
- Pasakon, P.; Mensing, J.P.; Phokaratkul, D.; Karuwan, C.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. A high-performance, disposable screen-printed carbon electrode modified with multi-walled carbon nanotubes/graphene for ultratrace level electrochemical sensors. J. Appl. Electrochem. 2019, 49, 217–227. [Google Scholar] [CrossRef]
- Chowdhry, A.; Kaur, J.; Khatri, M.; Puri, V.; Tuli, R.; Puri, S. Characterization of functionalized multiwalled carbon nanotubes and comparison of their cellular toxicity between HEK 293 cells and zebra fish in vivo. Heliyon 2019, 5, e02605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gokceli, G.; Eksik, O.; Zayim, E.O.; Karatepe, N. A Comparative Study of Single- and Multi-Walled Carbon Nanotube Incorporation to Indium Tin Oxide Electrodes for Solar Cells. Int. J. Mater. Metall. Eng. 2019, 13, 24–27. [Google Scholar]
- Newair, E.F.; Kilmartin, P.A.; Garcia, F. Square wave voltammetric analysis of polyphenol content and antioxidant capacity of red wines using glassy carbon and disposable carbon nanotubes modified screen-printed electrodes. Eur. Food Res. Technol. 2018, 244, 1225–1237. [Google Scholar] [CrossRef]
- Carrascón, V.; Vallverdu-Queralt, A.; Meudec, E.; Sommerer, N.; Fernandez-Zurbano, P.; Ferreira, V. The kinetics of oxygen and SO2 consumption by red wines. What do they tell about oxidation mechanisms and about changes in wine composition? Food Chem. 2018, 241, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshaies, S.; Cazals, G.; Enjalbal, C.; Constantin, T.; Garcia, F.; Mouls, L.; Saucier, C. Red Wine Oxidation: Accelerated Ageing Tests, Possible Reaction Mechanisms and Application to Syrah Red Wines. Antioxidants 2020, 9, 663. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L. Rapid microplate high-throughput methodology for assessment of Folin-Ciocalteu reducing capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Stonestreet, E. Le Dosage Des Tannins Du Vin Rouge et La Détermination de Leur Structure. Chim. Anal. 1966, 48, 188–196. [Google Scholar]
- Labarbe, B. Le Potentiel Polyphenolique de La Grappe de Vitis vinifera var. Gamay Noir et Son Devenir En Vinification Beaujolaise. Ph.D. Thesis, Ecole Nationale Supérieure Agronomique, Montpellier, France, 2000. [Google Scholar]
- Ducasse, M.-A.; Canal-Llauberes, R.-M.; de Lumley, M.; Williams, P.; Souquet, J.-M.; Fulcrand, H.; Doco, T.; Cheynier, V. Effect of macerating enzyme treatment on the polyphenol and polysaccharide composition of red wines. Food Chem. 2010, 118, 369–376. [Google Scholar] [CrossRef]
- Clark, A.C.; Kontoudakis, N.; Barril, C.; Schmidtke, L.; Scollary, G.R. Measurement of labile copper in wine by medium exchange stripping potentiometry utilising screen printed carbon electrodes. Talanta 2016, 154, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Kontoudakis, N.; Smith, M.; Guo, A.; Smith, P.A.; Scollary, G.R.; Wilkes, E.N.; Clark, A.C. The impact of wine components on fractionation of Cu and Fe in model wine systems: Macromolecules, phenolic and sulfur compounds. Food Res. Int. 2017, 98, 95–102. [Google Scholar] [CrossRef]
- Danilewicz, J.C.; Seccombe, J.T.; Whelan, J. Mechanism of Interaction of Polyphenols, Oxygen, and Sulfur Dioxide in Model Wine and Wine. Am. J. Enol. Vitic. 2008, 59, 128–136. [Google Scholar]
- Bradshaw, M.P.; Barril, C.; Clark, A.C.; Prenzler, P.; Scollary, G.R. Ascorbic Acid: A Review of its Chemistry and Reactivity in Relation to a Wine Environment. Crit. Rev. Food Sci. Nutr. 2011, 51, 479–498. [Google Scholar] [CrossRef]
- Arapitsas, P.; Ugliano, M.; Perenzoni, D.; Angeli, A.; Pangrazzi, P.; Mattivi, F. Wine metabolomics reveals new sulfonated products in bottled white wines, promoted by small amounts of oxygen. J. Chromatogr. A 2016, 1429, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Carrascón, V.; Bueno, M.; Fernandez-Zurbano, P.; Ferreira, V. Oxygen and SO2 Consumption Rates in White and Rosé Wines: Relationship with and Effects on Wine Chemical Composition. J. Agric. Food Chem. 2017, 65, 9488–9495. [Google Scholar] [CrossRef] [Green Version]
- Souza, L.P.; Calegari, F.; Zarbin, A.J.G.; Marcolino-Júnior, L.H.; Bergamini, M.F. Voltammetric Determination of the Antioxidant Capacity in Wine Samples Using a Carbon Nanotube Modified Electrode. J. Agric. Food Chem. 2011, 59, 7620–7625. [Google Scholar] [CrossRef]
- Petrovic, S.C. Correlation of Perceived Wine Astringency to Cyclic Voltammetric Response. Am. J. Enol. Vitic. 2009, 60, 373–378. [Google Scholar]
- Rebelo, M.; Rego, R.; Ferreira, M.; Oliveira, M. Comparative study of the antioxidant capacity and polyphenol content of Douro wines by chemical and electrochemical methods. Food Chem. 2013, 141, 566–573. [Google Scholar] [CrossRef]
- Novak, I.; Šeruga, M.; Komorsky-Lovrić, Š. Square-wave and cyclic voltammetry of epicatechin gallate on glassy carbon electrode. J. Electroanal. Chem. 2009, 631, 71–75. [Google Scholar] [CrossRef]
- Martins, R.C.; Oliveira, R.; Bento, F.; Geraldo, D.; Lopes, V.; de Pinho, P.G.; Oliveira, C.; Ferreira, A.C.S. Oxidation Management of White Wines Using Cyclic Voltammetry and Multivariate Process Monitoring. J. Agric. Food Chem. 2008, 56, 12092–12098. [Google Scholar] [CrossRef]
- Rodrigues, A.; Ferreira, A.C.S.; de Pinho, P.G.; Bento, F.; Geraldo, D. Resistance to Oxidation of White Wines Assessed by Voltammetric Means. J. Agric. Food Chem. 2007, 55, 10557–10562. [Google Scholar] [CrossRef] [PubMed]
- Benbouguerra, N.; Richard, T.; Saucier, C.; Garcia, F. Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis vinifera var. Merlot, Tannat, and Syrah). Antioxidants 2020, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, Â.; Valderrama, P.; Fontes, N.; Geraldo, D.; Bento, F. Evaluation of total polyphenol content of wines by means of voltammetric techniques: Cyclic voltammetry vs. differential pulse voltammetry. Food Chem. 2019, 276, 719–725. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. The phenolic composition of Sauvignon blanc juice profiled by cyclic voltammetry. Electrochim. Acta 2012, 83, 188–195. [Google Scholar] [CrossRef]
- Singleton, V.L.; Salgues, M.; Zaya, J.; Trousdale, E. Caftaric Acid Disappearance and Conversion to Products of En-zymic Oxidation in Grape Must and Wine. Am. J. Enol. Vitic. 1985, 36, 50–56. [Google Scholar]
- Lopes, P.; Richard, T.; Saucier, C.; Teissedre, P.-L.; Monti, J.-P.; Glories, Y. Anthocyanone A: A Quinone Methide Derivative Resulting from Malvidin 3-O-Glucoside Degradation. J. Agric. Food Chem. 2007, 55, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Picariello, L.; Forino, M.; Moio, L.; Gambuti, A. Comparison of Three Accelerated Oxidation Tests Applied to Red Wines with Different Chemical Composition. Molecules 2021, 26, 815. [Google Scholar] [CrossRef] [PubMed]


| Wine Sample | Total Phenolic Content (g GAE/L) | Total Tannins Content (g/L) | Total Pigments Index | SO2 Bleaching Resistant Pigments Index |
|---|---|---|---|---|
| R1 | 1.9 ± 0.1 d | 2.8 ± 0.2 d | 19.2 ± 0.9 a | 5.4 ± 0.4 b |
| R2 | 1.9 ± 0.1 d | 3.2 ± 0.1 c,d | 4.9 ± 0.1 d | 4.3 ± 0.1 c,d |
| R3 | 2.5 ± 0.1 b | 4.4 ± 0.1 a,b | 6.0 ± 0.5 d | 4.8 ± 0.1 b,c |
| R4 | 2.4 ± 0.1 b | 4.2 ± 0.1 a,b | 8.35 ± 1.1 c | 4.6 ± 0.3 b,c |
| R5 | 2.6 ± 0.1 b,c | 3.4 ± 0.1 c | 9.1 ± 0.1 c | 3.5 ± 0.2 d |
| R6 | 2.7 ± 0.2 b | 2.8 ± 0.1 d | 4.8 ± 0.3 d | 1.7 ± 0.1 e |
| R7 | 1.4 ± 0.1 e | 2.1 ± 0.1 e | 14.7 ± 0.5 b | 3.5 ± 0.4 d |
| R8 | 3.1 ± 0.05 a | 4.6 ± 0.1 a | 16.2 ± 1.3 b | 6.8 ± 0.2 a |
| R9 | 2.5 ± 0.1 b | 4.1 ± 0.2 b | 21.2 ± 1.9 a | 7.2 ± 0.1 a |
| Initial O2 Rate iOCR | Average O2 Rate aOCR | Initial O2 Rate iOCR | Average O2 Rate aOCR | Initial O2 Rate iOCR | Average O2 Rate aOCR | |||
|---|---|---|---|---|---|---|---|---|
| R1 60 °C | 2.40 ± 0.06 a,b | 1.27 ± 0.05 a | R1 lac | 3.33 ± 0.05 b,c | 1.35 ± 0.21 a,b | R1 H2O2 | 2.86 ± 0.28 c,d | 1.44 ± 0.21 c |
| R2 60 °C | 1.83 ± 0.52 a,b,c | 0.71 ± 0.30 b,c,d | R2 lac | 2.58 ± 0.40 c,d | 1.19 ± 0.13 b,c | R2 H2O2 | 5.1 ± 0.19 b | 2.65 ± 0.74 b |
| R3 60 °C | 2.59 ± 0.32 a | 1.02 ± 0.10 a,b | R3 lac | 5.47 ± 0.82 a | 1.9 ± 0.50 a | R3 H2O2 | 3.74 ± 0.05 b,c | 2.35 ± 0.17 b,c |
| R4 60 °C | 1.07 ± 0.09 c,d | 0.54 ± 0.11 c,d | R4 lac | 1.2 ± 0.04 e,f | 0.77 ± 0.09 b,c,d | R4 H2O2 | 5.33 ± 0.77 b | 2.9 ± 0.12 b |
| R5 60 °C | 2.14 ± 0.10 a,b | 0.93 ± 0.11 a,b,c | R5 lac | 1.4 ± 0.05 e,f | 0.8 ± 0.05 b,c,d | R5 H2O2 | 4.89 ± 0.89 b | 2.4 ± 0.14 b,c |
| R6 60 °C | 0.87 ± 0.21 c,d | 0.33 ± 0.12 d | R6 lac | 1.85 ± 0.11 d,e | 0.57 ± 0.13 c,d | R6 H2O2 | 1.69 ± 0.58 d | 0.25 ± 0.08 d |
| R7 60 °C | 2.4 ± 0.24 a,b | 0.86 ± 0.02 a,b,c | R7 lac | 4.2 ± 0.09 b | 2 ± 0.08 a | R7 H2O2 | 8.55 ± 0.19 a | 2.72 ± 0.09 b |
| R8 60 °C | 0.7 ± 0.11 d | 0.37 ± 0.06 d | R8 lac | 0.38 ± 0.10 f | 0.17 ± 0.03 d | R8 H2O2 | 9.32 ± 0.25 a | 4 ± 0.15 a |
| R9 60 °C | 1.61 ± 0.43 b,c,d | 0.89 ± 0.01 a,b,c | R9 lac | 1 ± 0.05 e,f | 0.54 ± 0.01 c,d | R9 H2O2 | 1.69 ± 0.05 d | 0.22 ± 0.06 d |
| Standards | Potential (mV) SWCNT-SPCE (vs. Ag(s)) | |
|---|---|---|
| Ep,a1 | Ep,a2 | |
| Catechin | 151 | 476 |
| Caffeic acid | 166 | / |
| Gallic acid | 154 | 476 |
| Oenin chloride | 398 | / |
| Quercetin | 151 | / |
| A | Q240mV | Q520mV | Q520mV–Q240mV | Q240mV/Q800mV | Q800mV | |
| R1 | 7.64 ± 0.76 d | 20.49 ± 1.74 d | 12.83 ± 1.12 e | 0.21 ± 0.03 b,c | 37.30 ± 1.98 e | |
| R2 | 7.42 ± 1.26 d | 26.11 ± 2.23 d | 18.54 ± 1.01 d,e | 0.15 ± 0.02 c,d | 50.25 ± 3.21 c,d,e | |
| R3 | 9.67 ± 1.55 c,d | 27.62 ± 3.32 d | 17.94 ± 1.67 d,e | 0.23 ± 0.06 b,c | 42.57 ± 3.87 e | |
| R4 | 19.00 ± 5.41 b | 46.00 ± 3.02 b,c | 27.96 ± 2.21 b,c | 0.29 ± 0.03 a,b | 65.11 ± 12.92 b,c,d | |
| R5 | 16.21 ± 1.93 b,c | 38.25 ± 0.29 c | 22.04 ± 1.42 c,d | 0.38 ± 0.01 a | 43.29 ± 6.78 d,e | |
| R6 | 20.73 ± 0.46 b | 51.30 ± 4.36 b | 30.55 ± 3.62 b | 0.31 ± 0.01 a,b | 67.75 ± 0.96 b,c | |
| R7 | 3.60 ± 0.74 d | 17.93 ± 1.82 d | 14.33 ± 1.07 e | 0.09 ± 0.01 d | 37.75 ± 6.28 e | |
| R8 | 29.28 ± 2.77 a | 96.52 ± 1.12 a | 69.71 ± 0.87 a | 0.30 ± 0.03 a,b | 99.00 ± 1.88 a | |
| R9 | 10.67 ± 2.13 c,d | 38.98 ± 4.40 c | 28.31 ± 2.25 b,c | 0.14 ± 0.04 c,d | 75.98 ± 8.51 a,b | |
| B | ΔQ240mV | ΔQ520mV | Δ(Q520mV–Q240mV) | Δ(Q240mV/Q800mV) | ΔQ800mV | |
| R1 | 60 °C | 0 f | 0 d | 0 f | 0 a | 0 d |
| R2 | 60 °C | 8.20 ± 0.86 b,c | 17.30 ± 0.87 c | 9.11 ± 0.01 d | 0.34 ± 0.03 a | 24.11 ± 0.10 b |
| R3 | 60 °C | 0.56 ± 0.53 e,f | 3.88 ± 0.46 d | 3.35 ± 0.07 e | 0.05 ± 0.05 a | 10.62 ± 0.8 c,d |
| R4 | 60 °C | 10.70 ± 0.37 b | 70.20 ± 0.56 a | 59.53 ± 0.19 a | 0.24 ± 0.01 a | 44.32 ± 0.50 a |
| R5 | 60 °C | 3.36 ± 2.05 d,e | 5.50 ± 4.91 d | 2.13 ± 2.87 e,f | 0.52 ± 0.60 a | 11.70 ± 7.22 c |
| R6 | 60 °C | 10.56 ± 1.06 b | 23.80 ± 1.64 b | 13.24 ± 0.58 c | 0.40 ± 0.03 a | 26.42 ± 4.41 b |
| R7 | 60 °C | 0 f | 2.35 ± 1.09 d | 2.34 ± 1.02 e,f | 0 a | 4.61 ± 1.12 c,d |
| R8 | 60 °C | 23.22 ± 1.66 a | 49.04 ± 6.16 a | 50.23 ± 0.82 b | 0.48 ± 0.03 a | 73.45 ± 2.48 a |
| R9 | 60 °C | 5.64 ± 1.06 c,d | 16.27 ± 2.15 c | 10.64 ± 1.09 c,d | 0.17 ± 0.02 a | 31.98 ± 5.26 b |
| C | ΔQ240mV | ΔQ520mV | Δ(Q520mV–Q240mV) | Δ(Q240mV/Q800mV) | ΔQ800mV | |
| R1 | Lac. | 3.37 ± 0.60 c,d,e | 5.74 ± 1.59 d,e | 2.36 ± 1.00 d,e | 0.30 ± 0.09 a,b | 11.37 ± 1.48 c |
| R2 | Lac. | 0.22 ± 0.17 e | 0.61 ± 0.24 e,f | 0.40 ± 0.08 e | 0.02 ± 0.01 c | 9.49 ± 2.47 c |
| R3 | Lac. | 0 e | 0 f | 0 e | 0 c | 1.61 ± 0.53 c |
| R4 | Lac. | 2.05 ± 0.13 d,e | 1.48 ± 0.52 e,f | 0 e | 0.47 ± 0.19 a | 4.93 ± 2.11 c |
| R5 | Lac. | 5.88 ± 1.69 c | 8.92 ± 2.63 d | 3.04 ± 0.94 d,e | 0.41 ± 0.03 a | 14.15 ± 3.04 c |
| R6 | Lac. | 11.80 ± 1.29 b | 32.31 ± 1.48 b | 20.50 ± 0.22 b | 0.40 ± 0.02 a | 29.13 ± 1.43 b |
| R7 | Lac. | 0.63 ± 0.51 e | 4.68 ± 1.63 d,e,f | 4.03 ± 1.12 d | 0.05 ± 0.03 c | 12.18 ± 4.83 c |
| R8 | Lac. | 22.80 ± 2.31 a | 46.31 ± 8.55 a | 48.78 ± 1.59 a | 0.50 ± 0.04 a | 71.58 ± 0.72 a |
| R9 | Lac. | 5.70 ± 2.09 c,d | 16.43 ± 4.40 c | 10.73 ± 2.31 c | 0.18 ± 0.02 b,c | 31.49 ± 8.20 b |
| D | ΔQ240mV | ΔQ520mV | Δ(Q520mV–Q240mV) | Δ(Q240mV/Q800mV) | ΔQ800mV | |
| R1 | H2O2 | 3.29 ± 0.13 c,d | 5.73 ± 0.08 c,d,e | 2.45 ± 0.05 b,c | 0.07 ± 0.01 a | 45.39 ± 2.64 a |
| R2 | H2O2 | 1.45 ± 0.04 d,e | 4.05 ± 1.08 d,e,f | 2.7 ± 0.88 b | 0.55 ± 0.58 a | 5.28 ± 4.12 c,d |
| R3 | H2O2 | 3.66 ± 0.88 b,c | 9.56 ± 1.13 b,c | 5.87 ± 0.25 a | 0.18 ± 0.03 a | 19.77 ± 1.81 b |
| R4 | H2O2 | 2.22 ± 0.32 c,d | 8.13 ± 0.56 b,c,d | 5.91 ± 0.23 a | 0.16 ± 0.08 a | 17.22 ± 9.48 b,c |
| R5 | H2O2 | 2.68 ± 0.89 c,d | 5.36 ± 2.49 c,d,e | 2.68 ± 1.57 b | 0.11 ± 0.03 a | 23.75 ± 0.53 b |
| R6 | H2O2 | 13.11 ± 1.46 a | 15.50 ± 3.31 a | 2.39 ± 1.85 b,c | 0.30 ± 0.01 a | 43.27 ± 6.75 a |
| R7 | H2O2 | 0 e | 1.35 ± 0.19 e,f | 1.35 ± 0.19 b,c | 0 a | 3.77 ± 1.45 c,d |
| R8 | H2O2 | 0 e | 0 f | 0 c | 0 a | 0 d |
| R9 | H2O2 | 5.72 ± 1.03 b | 11.92 ± 1.90 a,b | 6.20 ± 0.87 a | 0.39 ± 0.09 a | 15.59 ± 5.98 b,c |
| Folin TPC | TC | TP | RP | Q240mV ref | Q520mV ref | Q520mV–Q240mV ref | Q240/800mV ref | Q800mV ref | ΔQ240mV H2O2 | ΔQ520mV H2O2 | Δ(Q520mV–Q240mV)H2O2 | Q240/800mV H2O2 | ΔQ800mV H2O2 | |||
| Folin TPC | 1.00 | 0.91 ** | −0.08 | 0.26 | 0.86 ** | 0.80 ** | 0.74 * | 0.53 | 0.74 * | 0.31 | 0.29 | 0.09 | 0.00 | 0.06 | ||
| TC | 1.00 | −0.15 | 0.36 | 0.72 * | 0.69 * | 0.65 | 0.33 | 0.73 * | 0.25 | 0.36 | 0.37 | 0.14 | −0.07 | |||
| TP | 1.00 | 0.72 * | −0.10 | 0.09 | 0.18 | −0.49 | 0.21 | −0.26 | −0.25 | −0.09 | −0.35 | −0.06 | ||||
| RP | 1.00 | 0.10 | 0.32 | 0.41 | −0.40 | 0.45 | −0.45 | −0.29 | 0.18 | −0.06 | −0.38 | |||||
| Q240mV ref | 1.00 | 0.94 ** | 0.87 ** | 0.66 | 0.80 ** | 0.18 | 0.03 | −0.25 | −0.23 | −0.03 | ||||||
| Q520mV ref | 1.00 | 0.99 ** | 0.43 | 0.90 ** | 0.00 | −0.16 | −0.35 | −0.23 | −0.27 | |||||||
| Q520mV–Q240mV ref | 1.00 | 0.31 | 0.90 ** | −0.08 | −0.24 | −0.39 | −0.23 | −0.37 | ||||||||
| Q240/800mV ref | 1.00 | 0.09 | 0.14 | 0.00 | −0.25 | −0.35 | 0.31 | |||||||||
| Q800mV ref | 1.00 | 0.14 | 0.07 | −0.09 | 0.07 | −0.30 | ||||||||||
| ΔQ240mV H2O2 | 1.00 | 0.90 ** | 0.21 | 0.37 | 0.70 * | |||||||||||
| ΔQ520mV H2O2 | 1.00 | 0.62 | 0.47 | 0.63 | ||||||||||||
| Δ(Q520mV–Q240mV)H2O2 | 1.00 | 0.39 | 0.14 | |||||||||||||
| ΔQ240/800mV H2O2 | 1.00 | 0.00 | ||||||||||||||
| ΔQ800mV H2O2 | 1.00 | |||||||||||||||
| ΔQ240mV lac | ΔQ520mV lac | Δ(Q520mV-Q240mV)lac | ΔQ240/800mV lac | ΔQ800mV lac | ΔQ240mV 60°C | ΔQ520mV 60°C | Δ(Q520mV–Q240mV)60°C | ΔQ240/800mV 60°C | ΔQ800mV 60°C | aOCR H2O2 | aOCR lac | aOCR 60 °C | iOCR H2O2 | iOCR lac | iOCR 60 °C | |
| Folin TPC | 0.71 * | 0.65 | 0.53 | 0.62 | 0.58 | 0.67 * | 0.55 | 0.49 | 0.66 | 0.65 | 0.02 | −0.77 * | −0.49 | −0.12 | −0.58 | −0.63 |
| TC | 0.49 | 0.48 | 0.33 | 0.40 | 0.39 | 0.63 | 0.63 | 0.60 | 0.43 | 0.74 * | 0.06 | −0.61 | −0.48 | −0.15 | −0.43 | −0.60 |
| TP | 0.23 | 0.26 | −0.49 | 0.10 | 0.43 | −0.03 | −0.03 | −0.02 | −0.35 | −0.03 | −0.10 | −0.15 | 0.36 | 0.08 | −0.22 | 0.07 |
| RP | 0.24 | 0.29 | −0.40 | 0.02 | 0.36 | 0.26 | 0.29 | 0.28 | −0.15 | 0.35 | 0.23 | −0.27 | 0.21 | 0.14 | −0.27 | −0.06 |
| Q240mV ref | 0.86 ** | 0.81 ** | 0.66 | 0.83 ** | 0.65 | 0.87 ** | 0.80 ** | 0.73 * | 0.74 * | 0.76 * | 0.25 | −0.82 ** | −0.75 * | 0.21 | −0.71 * | −0.84 ** |
| Q520mV ref | 0,93 ** | 0.92 ** | 0.43 | 0.69 * | 0.79 * | 0.94 ** | 0.81 ** | 0.72 * | 0.66 | 0.80 ** | 0.36 | −0.79 * | −0.72 * | 0.38 | −0.69 * | −0.82 ** |
| Q520mV–Q240mV ref | 0.93 ** | 0.94 ** | 0.31 | 0.59 | 0.83 ** | 0.93 ** | 0.78 * | 0.68 * | 0.60 | 0.78 * | 0.40 | −0.74 * | −0.68 * | 0.44 | −0.65 | −0.77 * |
| Q240/800mV ref | 0.39 | 0.28 | 1.00 ** | 0.68 * | 0.02 | 0.34 | 0.34 | 0.32 | 0.62 | 0.16 | 0.28 | −0.33 | −0.33 | 0.11 | −0.25 | −0.31 |
| Q800mV ref | 0.81 ** | 0.82 ** | 0.09 | 0.54 | 0.83 ** | 0.90 ** | 0.79 * | 0.71 * | 0.53 | 0.91 ** | 0.10 | −0.84 ** | −0.74 * | 0.16 | −0.76 * | −0.88 ** |
| ΔQ240mV H2O2 | 0.13 | 0.08 | 0.14 | 0.17 | 0.20 | −0.02 | −0.16 | −0.20 | 0.15 | 0.02 | −0.84 ** | −0.28 | −0.27 | −0.77 * | −0.10 | −0.31 |
| ΔQ520mV H2O2 | −0.14 | −0.19 | 0.00 | 0.04 | −0.04 | −0.18 | −0.16 | −0.15 | −0.03 | 0.05 | −0.84 ** | −0.17 | −0.10 | −0.88 ** | −0.02 | −0.17 |
| Δ(Q520mV–Q240mV)H2O2 | −0.55 | −0.57 | −0.25 | −0.23 | −0.45 | −0.35 | −0.08 | 0.02 | −0.34 | 0.07 | −0.36 | 0.12 | 0.27 | −0.59 | 0.14 | 0.17 |
| ΔQ240/800mV H2O2 | −0.28 | −0.28 | −0.35 | −0.38 | −0.08 | −0.01 | −0.14 | −0.18 | 0.16 | 0.17 | −0.44 | −0.14 | −0.12 | −0.55 | −0.10 | −0.11 |
| ΔQ800mV H2O2 | −0.09 | −0.19 | 0.31 | 0.26 | −0.15 | −0.35 | −0.36 | −0.34 | −0.13 | −0.40 | −0.70 * | −0.02 | 0.26 | −0.75 * | 0.11 | 0.10 |
| ΔQ240mV lac | ΔQ520mV lac | Δ(Q520mV-Q240mV)lac | ΔQ240/800mV lac | ΔQ800mV lac | ΔQ240mV 60°C | ΔQ520mV 60°C | Δ(Q520mV–Q240mV)60°C | ΔQ240/800mV 60°C | ΔQ800mV 60°C | aOCR H2O2 | aOCR lac | aOCR 60 °C | iOCR H2O2 | iOCR lac | iOCR 60 °C | |
| ΔQ240mV lac | 1.00 | 0.99 ** | 0.39 | 0.68 * | 0.91 ** | 0.83 ** | 0.59 | 0.48 | 0.63 | 0.59 | 0.17 | −0.77 * | −0.62 | 0.29 | −0.66 | −0.74 * |
| ΔQ520mV lac | 1.00 | 0.28 | 0.58 | 0.92 ** | 0.84 ** | 0.59 | 0.47 | 0.55 | 0.60 | 0.21 | −0.70 * | −0.63 | 0.36 | −0.59 | −0.72 * | |
| ΔQ(520mV–Q240mV)lac | 1.00 | 0.68 * | 0.02 | 0.34 | 0.34 | 0.32 | 0.62 | 0.16 | 0.28 | −0.33 | −0.33 | 0.11 | −0.25 | −0.31 | ||
| ΔQ240/800mV lac | 1.00 | 0.48 | 0.61 | 0.65 | 0.63 | 0.60 | 0.53 | 0.10 | −0.77 * | −0.48 | 0.08 | −0.76 * | −0.70 * | |||
| ΔQ800mV lac | 1.00 | 0.72 * | 0.43 | 0.31 | 0.50 | 0.55 | −0.06 | −0.75 * | −0.54 | 0.16 | −0.67 * | −0.68 * | ||||
| ΔQ240mV 60°C | 1.00 | 0.88 ** | 0.79 * | 0.68 * | 0.89 ** | 0.39 | −0.79 * | −0.83 ** | 0.39 | −0.72 * | −0.90 ** | |||||
| ΔQ520mV 60°C | 1.00 | 0.99 ** | 0.46 | 0.92 ** | 0.48 | −0.66 | −0.75 * | 0.41 | −0.66 | −0.84 ** | ||||||
| Δ(Q520mV–Q240mV)60°C | 1.00 | 0.36 | 0.88 ** | 0.49 | −0.58 | −0.68 * | 0.40 | −0.60 | −0.78 * | |||||||
| ΔQ240/800mV 60°C | 1.00 | 0.55 | 0.19 | −0.76 * | −0.65 | 0.14 | −0.73 * | −0.64 | ||||||||
| ΔQ800mV 60°C | 1.00 | 0.26 | −0.78 * | −0.79 * | 0.21 | −0.75 * | −0.89 ** | |||||||||
| aOCR H2O2 | 1.00 | 0.07 | −0.17 | 0.89 ** | −0.03 | −0.05 | ||||||||||
| aOCR lac | 1.00 | 0.62 | 0.08 | 0.95 ** | 0.85 ** | |||||||||||
| aOCR 60 °C | 1.00 | −0.27 | 0.57 | 0.90 ** | ||||||||||||
| iOCR H2O2 | 1.00 | −0.06 | −0.10 | |||||||||||||
| iOCR lac | 1.00 | 0.80 ** | ||||||||||||||
| iOCR 60 °C | 1.00 | |||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshaies, S.; Garcia, L.; Veran, F.; Mouls, L.; Saucier, C.; Garcia, F. Red Wine Oxidation Characterization by Accelerated Ageing Tests and Cyclic Voltammetry. Antioxidants 2021, 10, 1943. https://doi.org/10.3390/antiox10121943
Deshaies S, Garcia L, Veran F, Mouls L, Saucier C, Garcia F. Red Wine Oxidation Characterization by Accelerated Ageing Tests and Cyclic Voltammetry. Antioxidants. 2021; 10(12):1943. https://doi.org/10.3390/antiox10121943
Chicago/Turabian StyleDeshaies, Stacy, Luca Garcia, Frédéric Veran, Laetitia Mouls, Cédric Saucier, and François Garcia. 2021. "Red Wine Oxidation Characterization by Accelerated Ageing Tests and Cyclic Voltammetry" Antioxidants 10, no. 12: 1943. https://doi.org/10.3390/antiox10121943






