The Relationship between Ozone and Human Blood in the Course of a Well-Controlled, Mild, and Transitory Oxidative Eustress
Abstract
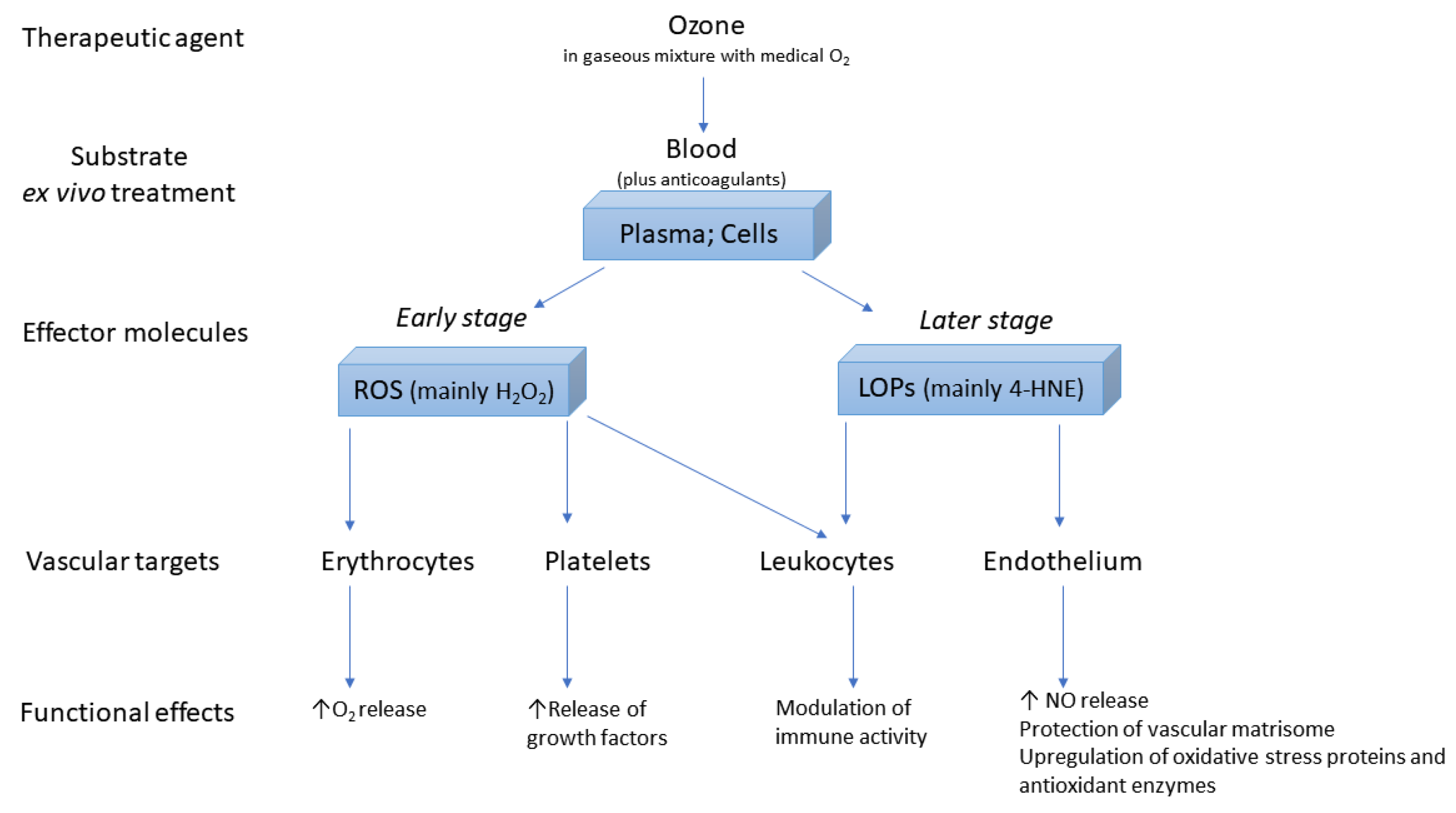
:1. Introduction
2. Background
3. Clinical Applications
3.1. An Appraisal of Recent Literature
- -
- Acute and chronic infectious diseases, particularly due to antibiotic or chemo-resistant bacteria, viruses, and fungi. It must be clear that ozone therapy in itself cannot substitute for antibiotics because both ozone and hydrogen peroxide are exhausted in the glass bottle and are not present in the circulation; however, it is a clinically useful supportive adjunctive therapy [58].
- -
- Ischemic diseases (cerebral and heart ischemia). Anecdotal results seem positive, but they are yet to be validated in randomized clinical trials [59].
- -
- -
- Pulmonary diseases: emphysema, asthma, and acute respiratory distress syndrome. These affections are becoming the fourth cause of death. Ozonated autohemotherapy performed using low concentrations of ozone dosing reduces the chronic oxidative stress and improves oxygenation thus providing an important clinical adjunct for these patients [45,62].
- -
- Terminal nephropathies are progressively aggravated by chronic oxidative stress and as yet mainstream medicine has not developed the therapeutic means to control or modulate these. Ozone therapy could stabilize this serious dysfunction and improve the quality of life of these patients [63].
- -
- Similarly, in the metabolic syndrome ozone therapy is proving to be very useful well exemplified in patients with type-2 diabetes, also suffering from chronic ulcers with no tendency to heal [64].
- -
- Chemoresistant metastatic cancer and therapy of cancer-related fatigue [65].
- -
- Chronic fatigue syndrome and fibromyalgia, where ozone therapy appears to be useful in most patients [66].
- -
- Sickle cell disease, where ozone therapy appears to be very useful because it procures clinical improvement without adverse effects [67].
3.2. Other Modalities of Ozone Administration
4. Molecular Aspects
4.1. Antioxidant System
4.2. Practical Considerations
5. Unpublished Case Reports
6. Critical Evaluation of Unconventional Systemic Ozone Administration
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potter, G.D.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [Green Version]
- Ho, W.C.; Zhang, J. Evolutionary adaptations to new environments generally reverse plastic phenotypic changes. Nat. Commun. 2018, 9, 350. [Google Scholar] [CrossRef]
- Li, G.; He, H. Hormesis, allostatic buffering capacity and physiological mechanism of physical activity: A new theoretic framework. Med. Hypotheses 2009, 72, 527–532. [Google Scholar] [CrossRef]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 41, 101867. [Google Scholar] [CrossRef]
- Mokoena, M.L.; Brink, C.B.; Harvey, B.H.; Oliver, D.W. Appraisal of ozone as biologically active molecule and experimental tool in biomedical sciences. Med. Chem. Res. 2011, 20, 1687–1695. [Google Scholar] [CrossRef]
- Horvath, M.; Bilitzky, L.; Hüttner, J.O. Topics in Inorganic and General Chemistry. Monograph 20; Elsevier: Amsterdam, The Netherlands, 1985; p. 350. ISBN 0444996257. [Google Scholar] [CrossRef]
- Tricarico, G.; Isakovic, J.; Song, M.S.; Rustichelli, F.; Travagli, V.; Mitrecic, D. Ozone influences migration and proliferation of neural stem cells in vitro. Neurosci. Lett. 2020, 739, 135390. [Google Scholar] [CrossRef]
- Zhao, Z.; Ozcan, E.E.; VanArsdale, E.; Li, J.; Kim, E.; Sandler, A.D.; Kelly, D.L.; Bentley, W.E.; Payne, G.F. Mediated electrochemical probing: A systems-level tool for redox biology. ACS Chem. Biol. 2021, 16, 1099–1110. [Google Scholar] [CrossRef]
- Bocci, V. Is it true that ozone is always toxic? The end of a dogma. Toxicol. Appl. Pharmacol. 2006, 216, 493–504. [Google Scholar] [CrossRef]
- Zanardi, I.; Borrelli, E.; Valacchi, G.; Travagli, V.; Bocci, V. Ozone: A multifaceted molecule with unexpected therapeutic activity. Curr. Med. Chem. 2016, 23, 304–314. [Google Scholar] [CrossRef]
- Wolff, H. Das Medizinische Ozon: Theoretische Grundlagen, Therapeutische Anwen-Dungen; Verlag für Medizin, Dr. Fischer: Heidelberg, Germany, 1979. [Google Scholar]
- Dehmlow, R. Infrarot-A-therapie mit wassergefilterter Infrarot-Strahlung nach E. Braun und die sauerstofftherapien. Erfahrungsheilkunde 2000, 49, 721–735. [Google Scholar] [CrossRef]
- Bocci, V.; Paulesu, L. Studies on the biological effects of ozone 1. Induction of interferon gamma on human leucocytes. Haematologica 1990, 75, 510–515. [Google Scholar]
- Paulesu, L.; Luzzi, E.; Bocci, V. Studies on the biological effects of ozone: 2. Induction of tumor necrosis factor (TNF-alpha) on human leucocytes. Lymphokine Cytokine Res. 1991, 10, 409–412. [Google Scholar] [PubMed]
- Bocci, V.; Luzzi, E.; Corradeschi, F.; Paulesu, L.; Di Stefano, A. Studies on the biological effects of ozone: 3. An attempt to define conditions for optimal induction of cytokines. Lymphokine Cytokine Res. 1993, 12, 121–126. [Google Scholar] [PubMed]
- Bocci, V.; Luzzi, E.; Corradeschi, F.; Paulesu, L.; Rossi, R.; Cardaioli, E.; Di Simplicio, P. Studies on the biological effects of ozone: 4. Cytokine production and glutathione levels in human erythrocytes. J. Biol. Regul. Homeost. Agents 1993, 7, 133–138. [Google Scholar]
- Bocci, V.; Luzzi, E.; Corradeschi, F.; Paulesu, L. Studies on the biological effects of ozone: 5. Evaluation of immunological parameters and tolerability in normal volunteers receiving ambulatory autohaemotherapy. Biotherapy 1993, 7, 83–90. [Google Scholar] [CrossRef]
- Bocci, V.; Luzzi, E.; Corradeschi, F.; Silvestri, S. Studies on the biological effects of ozone: 6. Production of transforming growth factor 1 by human blood after ozone treatment. J. Biol. Regul. Homeost. Agents 1994, 8, 108–112. [Google Scholar]
- Bocci, V.; Valacchi, G.; Corradeschi, F.; Aldinucci, C.; Silvestri, S.; Paccagnini, E.; Gerli, R. Studies on the biological effects of ozone: 7. Generation of reactive oxygen species (ROS) after exposure of human blood to ozone. J. Biol. Regul. Homeost. Agents 1998, 12, 67–75. [Google Scholar]
- Bocci, V.; Valacchi, G.; Corradeschi, F.; Fanetti, G. Studies on the biological effects of ozone: 8. Effects on the total antioxidant status and on interleukin-8 production. Mediat. Inflamm. 1998, 7, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Bocci, V.; Valacchi, G.; Rossi, R.; Giustarini, D.; Paccagnini, E.; Pucci, A.M.; Di Simplicio, P. Studies on the biological effects of ozone: 9. Effects of ozone on human platelets. Platelets 1999, 10, 110–116. [Google Scholar] [CrossRef]
- Valacchi, G.; Bocci, V. Studies on the Biological Effects of Ozone: 10. Release of factors from ozonated human platelets. Mediat. Inflamm. 1999, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Valacchi, G.; Bocci, V. Studies on the biological effects of ozone: 11. Release of factors from human endothelial cells. Mediat. Inflamm. 2000, 9, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Travagli, V.; Zanardi, I.; Bernini, P.; Nepi, S.; Tenori, L.; Bocci, V. Effects of ozone blood treatment on the metabolite profile of human blood. Int. J. Toxicol. 2010, 29, 165–174. [Google Scholar] [CrossRef]
- Bocci, V.; Zanardi, I.; Borrelli, E.; Travagli, V. Reliable and effective oxygen-ozone therapy at a crossroads with ozonated saline infusion and ozone rectal insufflation. J. Pharm. Pharmacol. 2012, 64, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Scassellati, C.; Costanzo, M.; Cisterna, B.; Nodari, A.; Galiè, M.; Cattaneo, A.; Covi, V.; Tabaracci, G.; Bonvicini, C.; Malatesta, M. Effects of mild ozonisation on gene expression and nuclear domains organization in vitro. Toxicol. Vitro 2017, 44, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.A.; Tao, X.; Zheng, L.J.; Huang, L.; Peng, Y.; Liao, R.Y.; Zhu, Y.M. Ozone alleviates ischemia/reperfusion injury by inhibiting mitochondrion-mediated apoptosis pathway in SH-SY5Y cells. Cell Biol. Int. 2020, 44, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.; Tahmasebi, S.; Pustokhina, I.; Yumashev, A.V.; Lakzaei, T.; Alvanegh, A.G.; Roshangar, L.; Dadashpour, M.; Yousefi, M.; Ahmadi, M. Changes in Th17 cells frequency and function after ozone therapy used to treat multiple sclerosis patients. Mult. Scler. Relat. Disord. 2020, 46, 102466. [Google Scholar] [CrossRef]
- Tartari, A.; Moreira, F.F.; Pereira, M.; Carraro, E.; Cidral-Filho, F.J.; Salgado, A.I.; Kerppers, I.I. Anti-inflammatory effect of ozone therapy in an experimental model of rheumatoid arthritis. Inflammation 2020, 43, 985–993. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Zhang, L.; Sun, T.; Fu, Z. Intrathecal injection of ozone alleviates CCI-induced neuropathic pain via the GluR6-NF-κB/p65 signalling pathway in rats. Mol. Med. Rep. 2021, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Cisterna, B.; Costanzo, M.; Nodari, A.; Galiè, M.; Zanzoni, S.; Bernardi, P.; Covi, V.; Tabaracci, G.; Malatesta, M. Ozone Activates the Nrf2 pathway and improves preservation of explanted adipose tissue in vitro. Antioxidants 2020, 9, 989. [Google Scholar] [CrossRef]
- Re, L.; Noci, J.B.; Gadelha Serra, M.E.; Mollica, P.; Bonetti, M.; Travagli, V. Safety, pitfalls, and misunderstandings about the use of ozone therapy as a regenerative medicine tool. A narrative review. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. 1), 1–13. [Google Scholar]
- Di Mauro, R.; Cantarella, G.; Bernardini, R.; Di Rosa, M.; Barbagallo, I.; Distefano, A.; Longhitano, L.; Vicario, N.; Nicolosi, D.; Lazzarino, G.; et al. The biochemical and pharmacological properties of ozone: The smell of protection in acute and chronic diseases. Int. J. Mol. Sci. 2019, 20, 634. [Google Scholar] [CrossRef] [Green Version]
- Baeza-Noci, J.; Pinto-Bonilla, R.; Contreras-Velasco, L.; Gomez-Moraleda, M. Scientific approach for ozone absorption in blood during systemic indirect endovenous ozonetherapy. J. Ozone Ther. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Aldini, G.; Vistoli, G.; Regazzoni, L.; Gamberoni, L.; Facino, R.M.; Yamaguchi, S.; Uchida, K.; Carini, M. Albumin is the main nucleophilic target of human plasma: A protective role against pro-atherogenic electrophilic reactive carbonyl species? Chem. Res. Toxicol. 2008, 21, 824–835. [Google Scholar] [CrossRef]
- Sagai, M.; Bocci, V. Mechanisms of action involved in ozone therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocci, V.; Borrelli, E.; Travagli, V.; Zanardi, I. The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med. Res. Rev. 2009, 29, 646–682. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Bocci, V.A.; Zanardi, I.; Travagli, V. Ozone acting on human blood yields a hormetic dose-response relationship. J. Transl. Med. 2011, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Olson, K.R. Are reactive sulfur species the new reactive oxygen species? Antioxid. Redox Signal. 2020, 33, 1125–1142. [Google Scholar] [CrossRef] [PubMed]
- Scassellati, C.; Ciani, M.; Galoforo, A.C.; Zanardini, R.; Bonvicini, C.; Geroldi, C. Molecular mechanisms in cognitive frailty: Potential therapeutic targets for oxygen-ozone treatment. Mech. Ageing Dev. 2020, 186, 111210. [Google Scholar] [CrossRef]
- Togi, S.; Togi, M.; Nagashima, S.; Kitay, Y.; Muromoto, R.; Kashiwakura, J.; Miura, T.; Matsuda, T. Implication of NF-kB activation on ozone-induced HO-1 activation. BPB Rep. 2021, 4, 59–63. [Google Scholar] [CrossRef]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Sabbioni, G.; Turesky, R.J. Biomonitoring human albumin adducts: The past, the present, and the future. Chem. Res. Toxicol. 2017, 30, 332–366. [Google Scholar] [CrossRef]
- Pecorelli, A.; Bocci, V.; Acquaviva, A.; Belmonte, G.; Gardi, C.; Virgili, F.; Ciccoli, L.; Valacchi, G. NRF2 activation is involved in ozonated human serum upregulation of HO-1 in endothelial cells. Toxicol. Appl. Pharmacol. 2013, 267, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, M.; Zhang, X.; Li, J.; Wang, Y.; Fan, Y.; Shi, R. Limb ischemic preconditioning protects endothelium from oxidative stress by enhancing nrf2 translocation and upregulating expression of antioxidases. PLoS ONE 2015, 10, e0128455. [Google Scholar] [CrossRef] [Green Version]
- Clavo, B.; Martínez-Sánchez, G.; Rodríguez-Esparragón, F.; Rodríguez-Abreu, D.; Galván, S.; Aguiar-Bujanda, D.; Díaz-Garrido, J.A.; Cañas, S.; Torres-Mata, L.B.; Fabelo, H.; et al. Modulation by ozone therapy of oxidative stress in chemotherapy-induced peripheral neuropathy: The background for a randomized clinical trial. Int. J. Mol. Sci. 2021, 22, 2802. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Schramm, M. Reactive oxygen species: Not omnipresent but important in many locations. Front. Cell Dev. Biol. 2021, 9, 716406. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Tang, M.X.; Balestra, C.; Eckersley, R.J.; Karapantsios, T.D. Circulatory bubble dynamics: From physical to biological aspects. Adv. Colloid Interface Sci. 2014, 206, 239–249. [Google Scholar] [CrossRef]
- Yu, L.; Buttgereit, T.; Stahl Skov, P.; Schmetzer, O.; Scheffel, J.; Kocatürk, E.; Zawar, V.; Magerl, M.; Maurer, M. Immunological effects and potential mechanisms of action of autologous serum therapy in chronic spontaneous urticaria. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1747–1754. [Google Scholar] [CrossRef]
- Sozio, E.; De Monte, A.; Sermann, G.; Bassi, F.; Sacchet, D.; Sbrana, F.; Ripoli, A.; Curcio, F.; Fabris, M.; Marengo, S.; et al. CORonavirus-19 mild to moderate pneumonia Management with blood Ozonization in patients with Respiratory failure (CORMOR) multicentric prospective randomized clinical trial. Int. Immunopharmacol. 2021, 98, 107874. [Google Scholar] [CrossRef] [PubMed]
- Araimo, F.; Imperiale, C.; Tordiglione, P.; Ceccarelli, G.; Borrazzo, C.; Alessandri, F.; Santinelli, L.; Innocenti, G.P.; Pinacchio, C.; Mauro, V.; et al. Ozone as adjuvant support in the treatment of COVID-19: A preliminary report of probiozovid trial. J. Med. Virol. 2021, 93, 2210–2220. [Google Scholar] [CrossRef]
- Morrison, C.; Atkinson, A.; Zamyadi, A.; Kibuye, F.; McKie, M.; Hogard, S.; Mollica, P.; Jasim, S.; Wert, E.C. Critical review and research needs of ozone applications related to virus inactivation: Potential implications for SARS-CoV-2. Ozone Sci. Eng. 2021, 43, 2–20. [Google Scholar] [CrossRef]
- Tylicki, L.; Niew Głowski, T.; Biedunkiewicz, B.; Burakowski, S.; Rutkowski, B. Beneficial clinical effects of ozonated autohemotherapy in chronically dialysed patients with atherosclerotic ischemia of the lower limbs—Pilot study. Int. J. Artif. Organs 2001, 24, 79–82. [Google Scholar] [CrossRef] [PubMed]
- De Monte, A.; van der Zee, H.; Bocci, V. Major ozonated autohemotherapy in chronic limb ischemia with ulcerations. J. Altern. Complementary Med. 2005, 11, 363–367. [Google Scholar] [CrossRef]
- Borrelli, E.; Diadori, A.; Zalaffi, A.; Bocci, V. Effects of major ozonated autohemotherapy in the treatment of dry age related macular degeneration: A randomized controlled clinical study. Int. J. Ophthalmol. 2012, 5, 708–713. [Google Scholar] [CrossRef]
- Borrelli, E.; Bocci, V. Oxygen ozone therapy in the treatment of chronic obstructive pulmonary disease: An integrative approach. Am. J. Clin. Exp. Med. 2014, 2, 9–13. [Google Scholar] [CrossRef]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, S.A. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas Res. 2017, 7, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocci, V.; Zanardi, I.; Travagli, V. Ozone: A new therapeutic agent in vascular diseases. Am. J. Cardiovasc. Drugs 2011, 11, 73–82. [Google Scholar] [CrossRef]
- Scassellati, C.; Galoforo, A.C.; Bonvicini, C.; Esposito, C.; Ricevuti, G. Ozone: A natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res. Rev. 2020, 63, 101138. [Google Scholar] [CrossRef]
- Ameli, J.; Banki, A.; Khorvash, F.; Simonetti, V.; Jafari, N.J.; Izadi, M. Mechanisms of pathophysiology of blood vessels in patients with multiple sclerosis treated with ozone therapy: A systematic review. Acta Biomed. Atenei Parm. 2019, 90, 213–217. [Google Scholar] [CrossRef]
- Borrelli, E. The use of ozone as redox modulator in the treatment of the chronic obstructive pulmonary disease (COPD). In Oxidative Stress in Lung Diseases; Chakraborti, S., Parinandi, N., Ghosh, R., Ganguly, N., Chakraborti, T., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Sancak, E.B.; Turkön, H.; Çukur, S.; Erimsah, S.; Akbas, A.; Gulpinar, M.T.; Toman, H.; Sahin, H.; Uzun, M. Major ozonated autohemotherapy preconditioning ameliorates kidney ischemia-reperfusion injury. Inflammation 2016, 39, 209–217. [Google Scholar] [CrossRef]
- Bocci, V.; Zanardi, I.; Huijberts, M.S.; Travagli, V. An integrated medical treatment for type-2 diabetes. Diabetes Metab. Syndr. 2014, 8, 57–61. [Google Scholar] [CrossRef]
- Clavo, B.; Rodríguez-Esparragón, F.; Rodríguez-Abreu, D.; Martínez-Sánchez, G.; Llontop, P.; Aguiar-Bujanda, D.; Fernández-Pérez, L.; Santana-Rodríguez, N. Modulation of oxidative stress by ozone therapy in the prevention and treatment of chemotherapy-induced toxicity: Review and prospects. Antioxidants 2019, 8, 588. [Google Scholar] [CrossRef] [Green Version]
- Tirelli, U.; Cirrito, C.; Pavanello, M.; Piasentin, C.; Lleshi, A.; Taibi, R. Ozone therapy in 65 patients with fibromyalgia: An effective therapy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Akbudak, I.H.; Kucukatay, V.; Kilic-Erkek, O.; Ozdemir, Y.; Bor-Kucukatay, M. Investigation of the effects of major ozone autohemotherapy application on erythrocyte deformability and aggregation. Clin. Hemorheol. Microcirc. 2019, 71, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Olwin, J.H.; Ratajczak, H.V.; House, R.V. Successful treatment of herpetic infections by autohemotherapy. J. Altern. Complementary Med. 1997, 3, 155–158. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Agostini, F.; Lippi, L.; Mangone, M.; Marchese, S.; Cisari, C.; Bernetti, A.; Invernizzi, M. Oxygen–ozone therapy in the rehabilitation field: State of the art on mechanisms of action, safety and effectiveness in patients with musculoskeletal disorders. Biomolecules 2021, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, G.; Rodrigues Orlandin, J.; Rocchetti, V.; Ambrosio, C.E.; Travagli, V. A critical evaluation of the use of ozone and its derivatives in dentistry. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9071–9093. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, A.; Kalim, U.; Rabha, A.K. Ozone therapy—A boon to dental sciences. Indian J. Forensic Med. Toxicol. 2021, 15, 47–51. [Google Scholar] [CrossRef]
- Orlandin, J.R.; Machado, L.C.; Ambrósio, C.E.; Travagli, V. Ozone and its derivatives in veterinary medicine: A careful appraisal. Vet. Anim. Sci. 2021, 13, 100191. [Google Scholar] [CrossRef]
- Bocci, V. The Still Uncertain Future of Ozone Therapy in Medicine. In Ozone: A New Medical Drug; Springer: Dordrecht, The Netherlands, 2010; pp. 237–240. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Jansen, E.; Ruskovska, T. Comparative analysis of serum (anti)oxidative status parameters in healthy persons. Int. J. Mol. Sci. 2013, 14, 6106–6115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total antioxidant capacity-Relevance, methods and clinical implications. Andrologia 2021, 53, e13624. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994, 234, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Habdous, M.; Herbeth, B.; Vincent-Viry, M.; Lamont, J.V.; Fitzgerald, P.S.; Visvikis, S.; Siest, G. Serum total antioxidant status, erythrocyte superoxide dismutase and whole-blood glutathione peroxidase activities in the Stanislas cohort: Influencing factors and reference intervals. Clin. Chem. Lab. Med. 2003, 41, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Prenesti, E.; Berto, S.; Gosmaro, F.; Bagnati, M.; Bellomo, G. Biomolecules responsible for the total antioxidant capacity (TAC) of human plasma in healthy and cardiopathic individuals: A chemical speciation model. Antioxidants 2021, 10, 656. [Google Scholar] [CrossRef]
- Bocci, V.; Aldinucci, C. Biochemical modifications induced in human blood by oxygenation-ozonation. J. Biochem. Mol. Toxicol. 2006, 20, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef]
- Ukinc, K.; Eminagaoglu, S.; Ersoz HOErem, C.; Karahan, C.; Hacihasanoglu, A.B.; Kocak, M. A novel indicator of widespread endothelial damage and ischemia in diabetic patients: Ischemia-modified albumin. Endocrine 2009, 36, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Alay, H.; Laloglu, E.; Kesmez Can, F. An evaluation of ischaemia-modified albumin levels in the development of diabetic foot ulcer. Int. J. Clin. Pract. 2021, 75, e14589. [Google Scholar] [CrossRef]
- Wang, Y.; Chun, O.; Song, W. Plasma and dietary antioxidant status as cardiovascular disease risk factors: A review of human studies. Nutrients 2013, 5, 2969–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metherel, A.H.; Aristizabal Henao, J.J.; Stark, K.D. EPA and DHA levels in whole blood decrease more rapidly when stored at −20 °C as compared with room temperature, 4 and −75 °C. Lipids 2013, 48, 1079–1091. [Google Scholar] [CrossRef]
- Schirrmacher, V. Less Can Be More: The hormesis theory of stress adaptation in the global biosphere and its implications. Biomedicines 2021, 9, 293. [Google Scholar] [CrossRef]
- Viebahn-Haensler, R.; León Fernández, O.S. Ozone in medicine. the low-dose ozone concept and its basic biochemical mechanisms of action in chronic inflammatory diseases. Int. J. Mol. Sci. 2021, 22, 7890. [Google Scholar] [CrossRef]
- Thorp, K.E.; Thorp, J.A. Ozone preconditioning: Waking up the dragon. Gaz. Med. Sci. 2021, 2, 10–39. [Google Scholar] [CrossRef]
- Yamaoka-Tojo, M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed. J. 2020, 43, 399–413. [Google Scholar] [CrossRef]
- Tricarico, G.; Travagli, V. COVID-19 fatal outcomes: Role of the endothelial glycocalyx in both cell adhesion and migration. Biomed. J. 2021, 44, 512–513. [Google Scholar] [CrossRef]
- Ma, L.; Wen, S.; Yuan, J.; Zhang, D.; Lu, Y.L.; Zhang, Y.; Li, Y.; Cao, S. Detection of chlorite, chlorate and perchlorate in ozonated saline. Exp. Ther. Med. 2020, 20, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Cui, H.; Li, S.; Niu, S. Simultaneous determination of chlorite, chlorate, perchlorate and bromate in ozonated saline by using IC-MS. Anal. Methods 2020, 12, 5916–5921. [Google Scholar] [CrossRef] [PubMed]
- Faraji, N.; Goli, R.; Choobianzali, B.; Bahrami, S.; Sadeghian, A.; Sepehrnia, N.; Ghalandari, M. Ozone therapy as an alternative method for the treatment of diabetic foot ulcer: A case report. J. Med. Case Rep. 2021, 15, 234. [Google Scholar] [CrossRef] [PubMed]

| Title | Identifier (Location) |
|---|---|
| Blood Ozonization in Patients With SARS-CoV-2 Respiratory Failure (CORMOR) | NCT04388514 (Italy) |
| Oxygen–Ozone as Adjuvant Treatment in Early Control of COVID-19 Progression and Modulation of the Gut Microbial Flora (PROBIOZOVID) | NCT04366089 (Italy) |
| Indirect Endovenous Systemic Ozone for New Coronavirus Disease (COVID-19) in Non-intubated Patients (Ozono COVID-19) | NCT04359303 (Spain) |
| Ozone Autohemotherapy for COVID-19 Pneumonia (COVID-19 OZONE) | NCT04370223 (Spain) |
| Ozone Therapy in the Prevention of COVID-19 Infection | NCT04400006 (Turkey) |
| Efficacy of Medical Ozone Therapy in Patients With Chronic Hepatitis B (EMOTCHB) | NCT01342185 (China) |
| Oxygen-ozone Therapy Plus Antibiotic Therapy in the Treatment of Infections Secondary to Implant of Orthopaedic Devices | NCT04787575 (Italy) |
| Clinical study for ozonated autohemotherapy in the treatment of Novel Coronavirus Pneumonia (COVID-19) | ChiCTR2000030165 (China) |
| A randomized controlled trial for the efficacy of ozonated autohemotherapy in the treatment of Novel Coronavirus Pneumonia (COVID-19) | ChiCTR2000030006 (China) |
| Study for improvement of myocardial ischemia-reperfusion injury after perioperative cardiopulmonary bypass by ozone autotransfusion combined with electroacupuncture point therapy | ChiCTR2000029612 (China) |
| Synergy effects and health regulation effect of oxygen-ozone therapy on systemic lupus erythematosus (SLE) | ChiCTR-IOR-17012802 (China) |
| Ozone treatment for Acute Ischemic Stroke within 1 w of Symptom Onset: A Prospective, Randomized, Multi-center, Open-label, Parallel control, Comparative Study | ChiCTR-ICR-15007093 (China) |
| A multicenter randomized controlled trial for ozone autohemotherapy in the treatment of novel coronavirus pneumonia (COVID-19) | ChiCTR2000030102 (China) |
| The effect of ozone treatment on serum markers in patients with fatty liver disease | ChiCTR-TNRC-11001273 (China) |
| Effect of Ozone therapy in the treatment of COVID-19 | IRCT20191125045492N2 (Iran) |
| A study of therapeutic effect of blood ozone therapy of severe COVID-19 patients | IRCT20200616047792N1 (Iran) |
| Investigation of the effects of medical Ozone Autohemotherapy on clinical and paraclinical features of patients with COVID-19 | IRCT20190618043923N4 (Iran) |
| Comparison of the effectiveness of Ozone therapy with conventional therapy in the improvement of visual pathways function in diabetic patients | IRCT20191125045492N1 (Iran) |
| Ozone therapy and routine medical treatment efficacy on serum level changes of TNF-α and CRP as well as neurological improvement | IRCT20200202046342N1 (Iran) |
| Ozone Therapy Effect on Multiple sclerosis | IRCT20171105037262N3 (Iran) |
| Non-Enzymatic Antioxidants and Polyunsaturated Fatty Acids (PUFA) Levels, in Human Plasma (Lower Reference Range, Adult Men) | |||||||
|---|---|---|---|---|---|---|---|
| Uric acid ~1.1019 molecules/mL | |||||||
| Ascorbic acid ~1.7 × 1018 molecules/mL | |||||||
| Glutathione ~1.1 × 1017 molecules/mL | |||||||
| Albumin ~2.6 × 1019 molecules/mL | |||||||
| Available PUFA ~2.4 × 1019 molecules/mL | |||||||
| [O3, μg/mL] | 10 | 15 | 20 | 25 | 30 | 35 | 40 |
| No. O3 molecules/mL (×1019) | 1.3 | 2.0 | 2.6 | 3.3 | 3.9 | 4.6 | 5.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tricarico, G.; Travagli, V. The Relationship between Ozone and Human Blood in the Course of a Well-Controlled, Mild, and Transitory Oxidative Eustress. Antioxidants 2021, 10, 1946. https://doi.org/10.3390/antiox10121946
Tricarico G, Travagli V. The Relationship between Ozone and Human Blood in the Course of a Well-Controlled, Mild, and Transitory Oxidative Eustress. Antioxidants. 2021; 10(12):1946. https://doi.org/10.3390/antiox10121946
Chicago/Turabian StyleTricarico, Gerardo, and Valter Travagli. 2021. "The Relationship between Ozone and Human Blood in the Course of a Well-Controlled, Mild, and Transitory Oxidative Eustress" Antioxidants 10, no. 12: 1946. https://doi.org/10.3390/antiox10121946
APA StyleTricarico, G., & Travagli, V. (2021). The Relationship between Ozone and Human Blood in the Course of a Well-Controlled, Mild, and Transitory Oxidative Eustress. Antioxidants, 10(12), 1946. https://doi.org/10.3390/antiox10121946







