Preventive Effects of Anthraquinones Isolated from an Endophytic Fungus, Colletotrichum sp. JS-0367 in Tumor Necrosis Factor-α-Stimulated Damage of Human Dermal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Determination of ROS
2.3. Determination of Nitric Oxide (NO) Production
2.4. Determination of Protein Secretion
2.5. Determination of Gene Expression
2.6. Determination of Protein Expression
2.7. Statistical Analyses
3. Results and Discussion
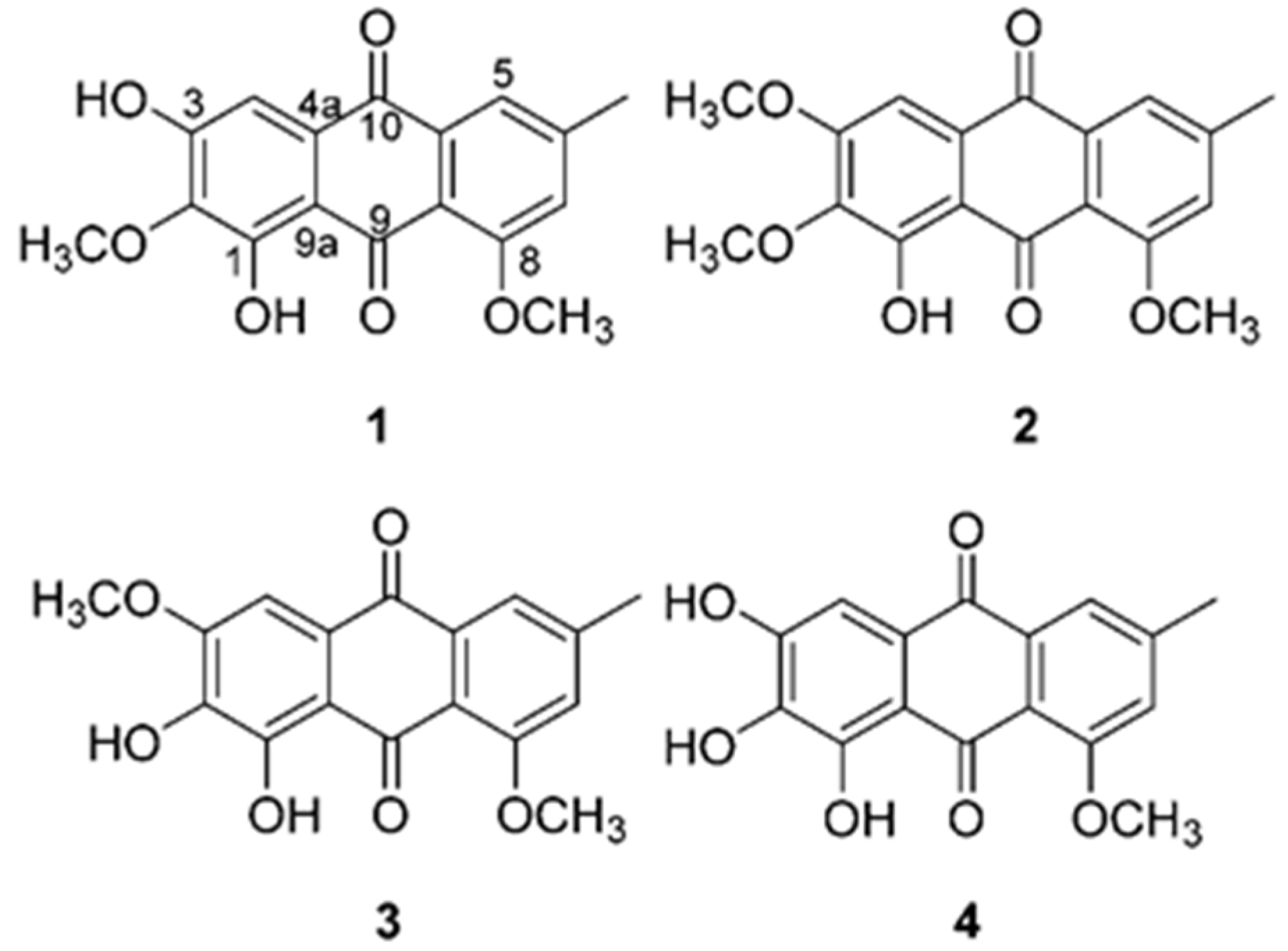
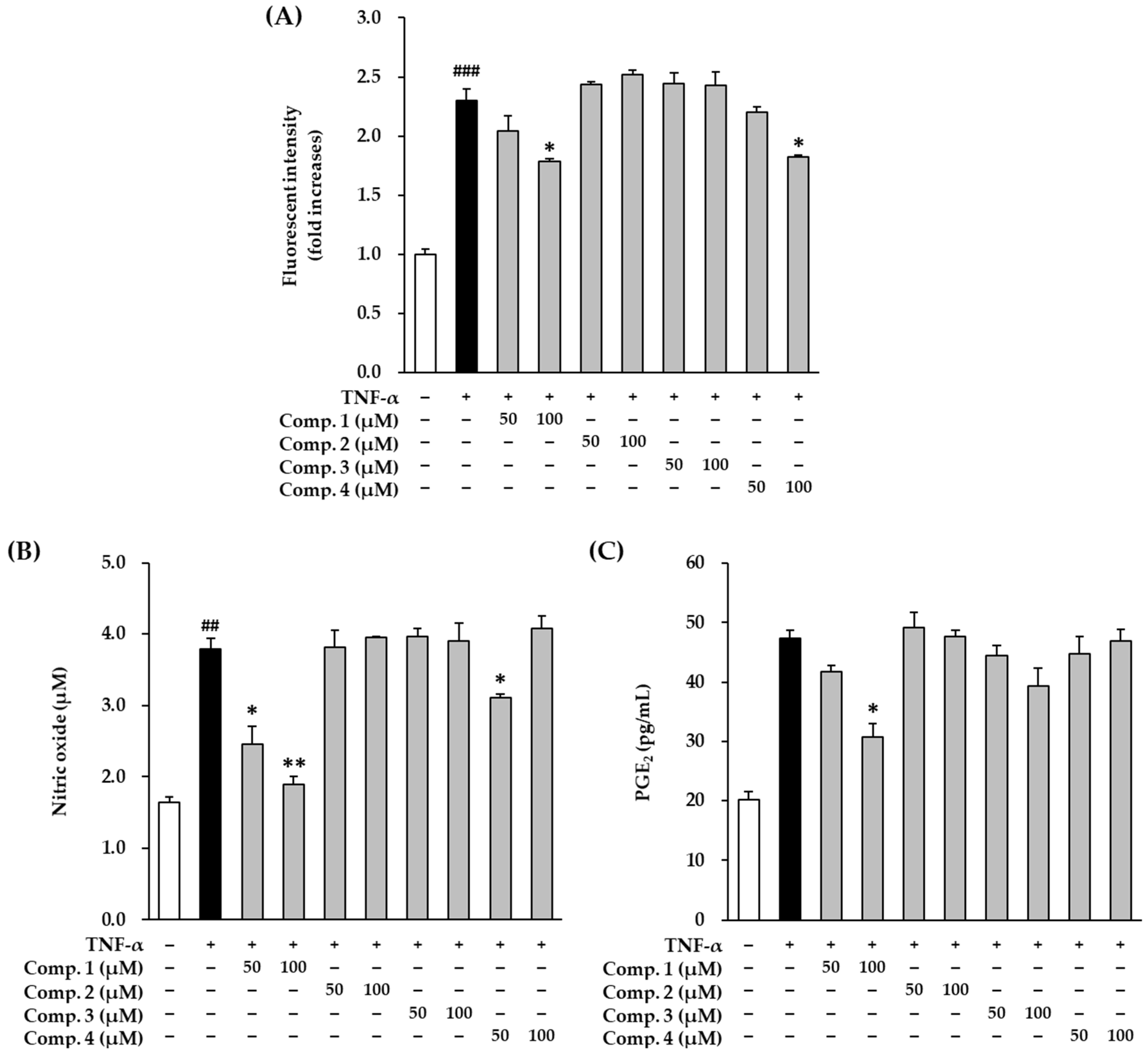
3.1. Effect of Anthraquinones on Production of Intracellular ROS, and Proinflammatory Mediators NO and PGE2 in TNF-α-Stimulated HDFs
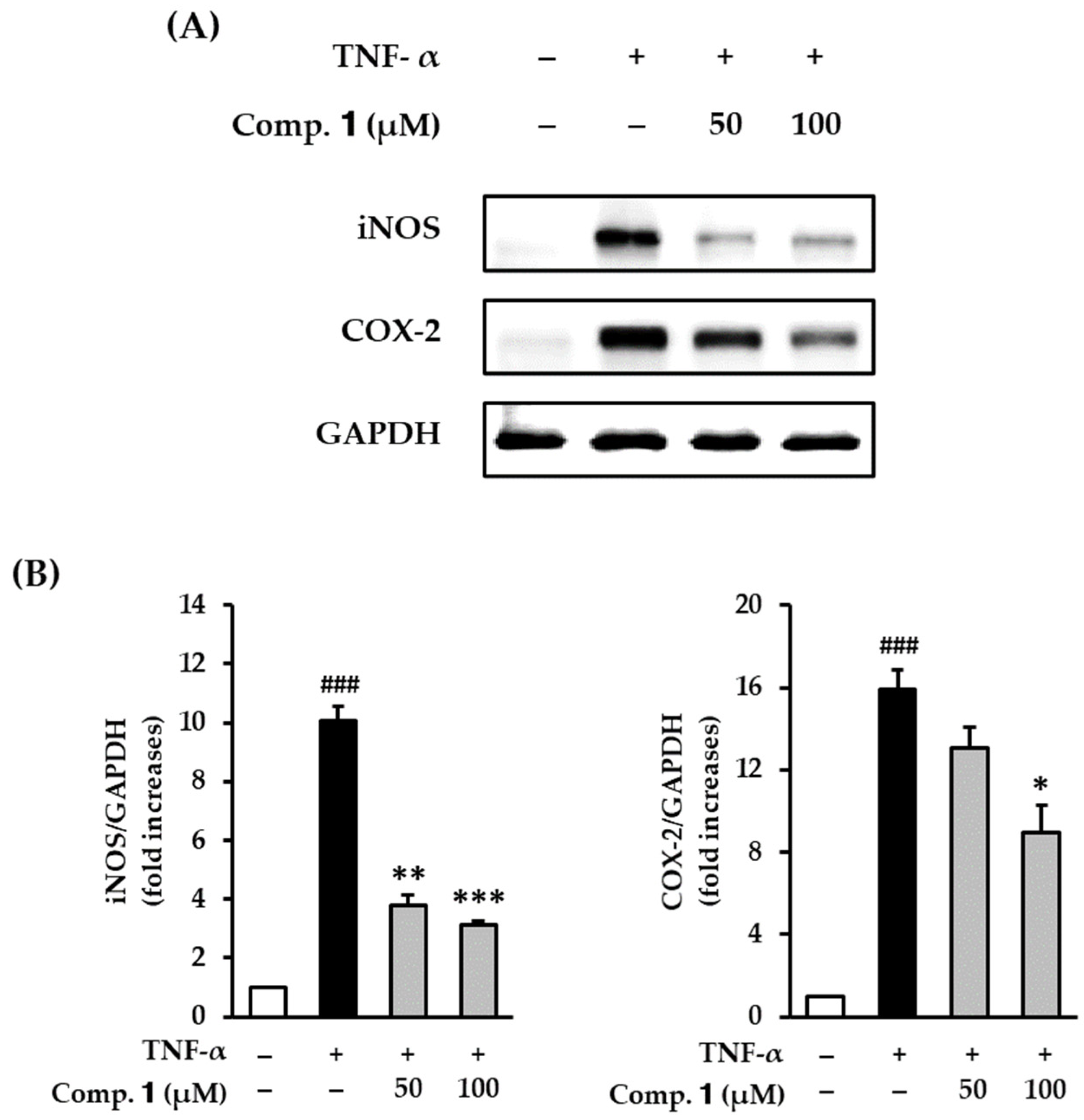
3.2. Effect of Compound 1 on COX-2 and iNOS Expression in TNF-α-Stimulated HDFs
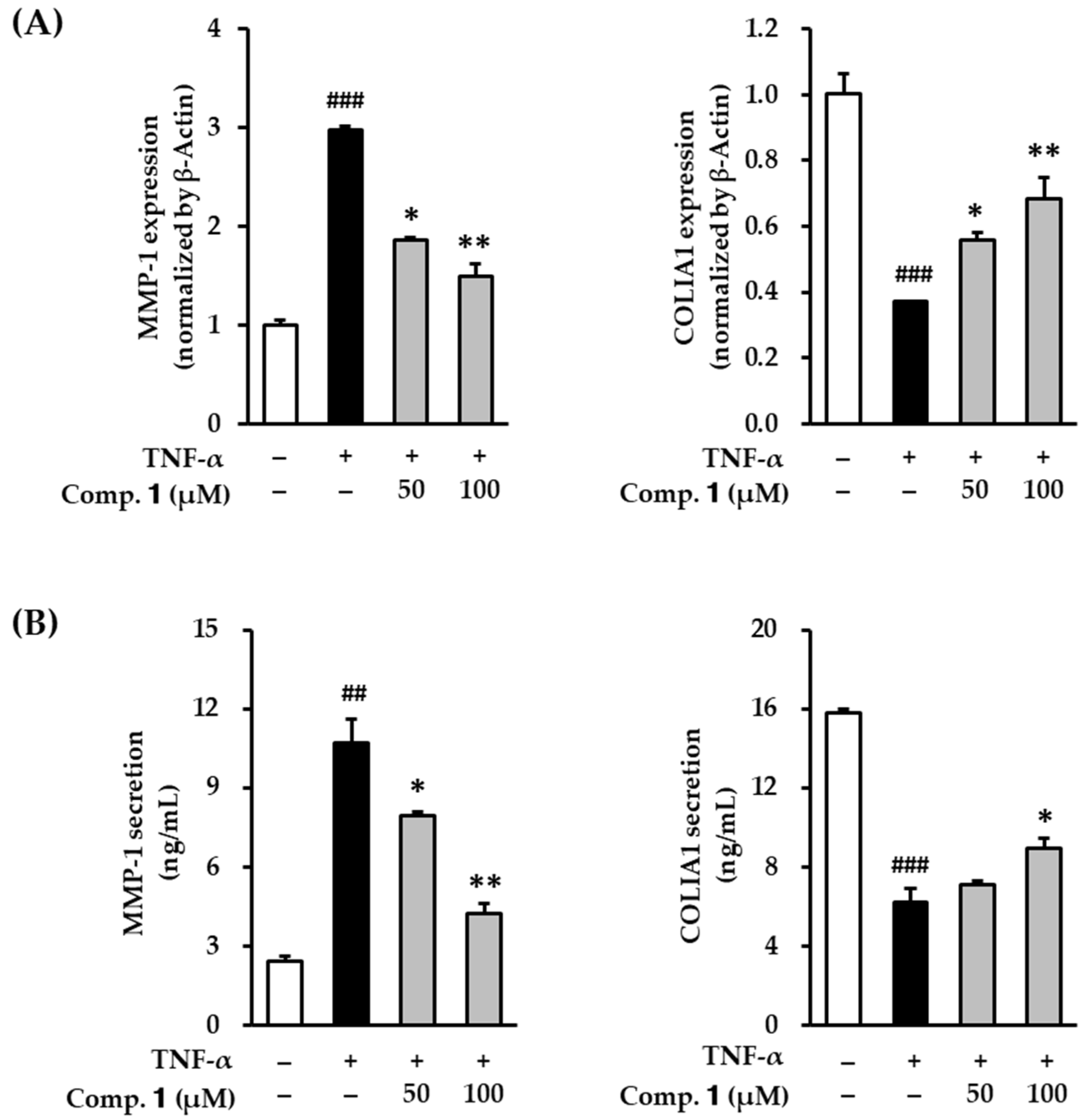
3.3. Effect of Compound 1 on MMP-1 and COLIA1 mRNA in TNF-α-Stimulated HDFs
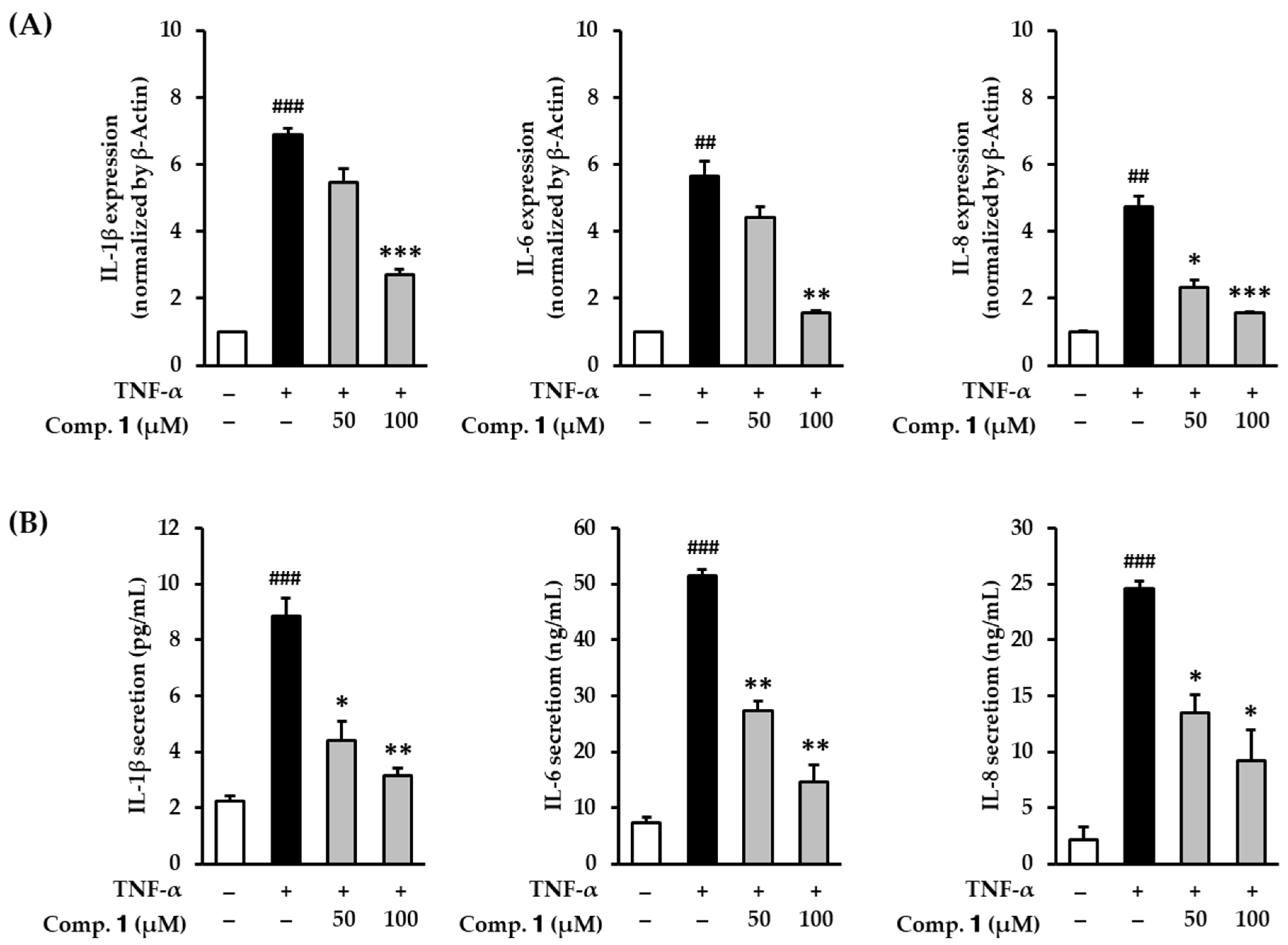
3.4. Effect of Compound 1 on Proinflammatory Cytokines in TNF-α-Stimulated HDFs
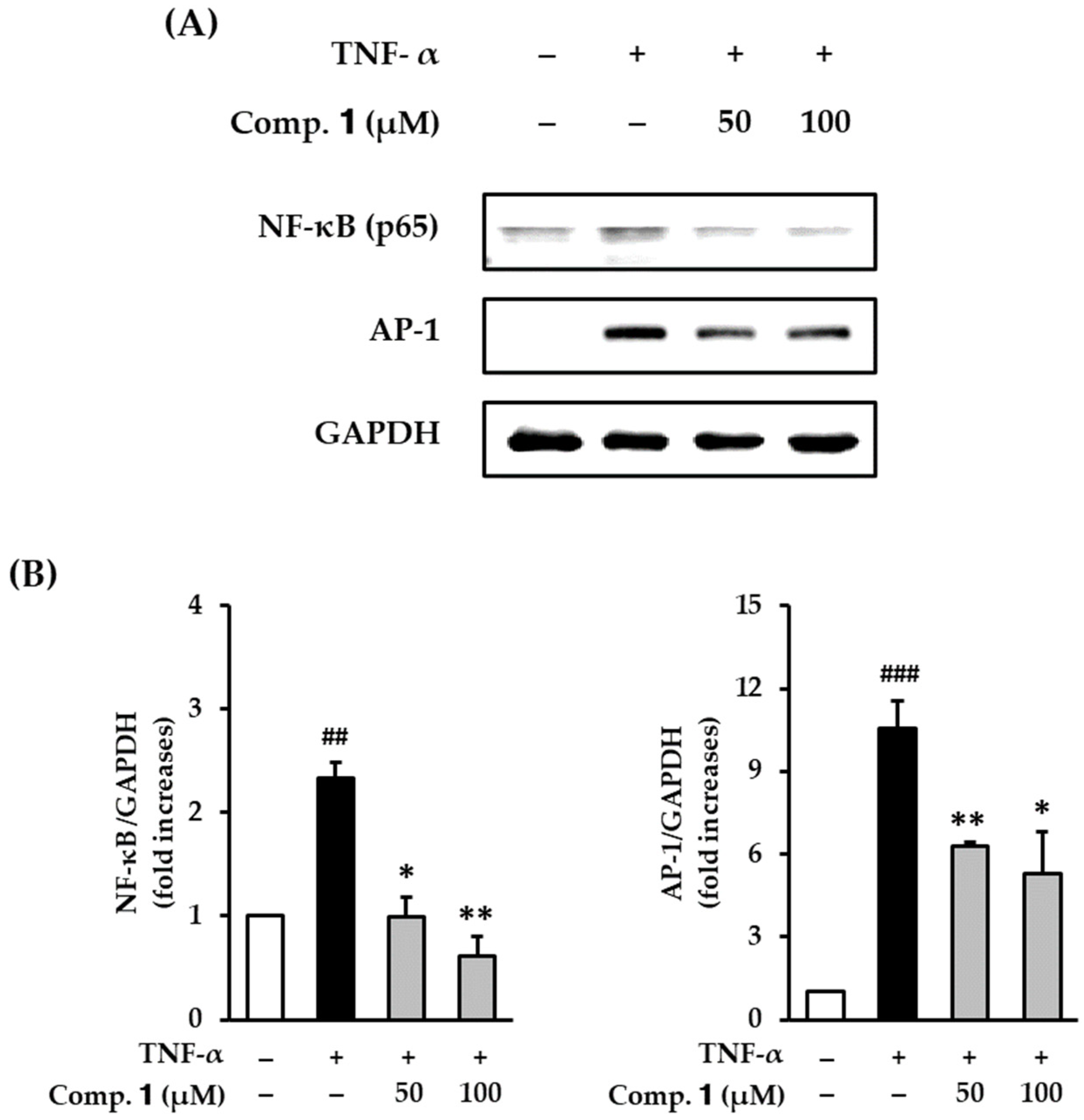
3.5. Effect of Compound 1 on NF-κB and AP-1 Expression in TNF-α-Stimulated HDFs
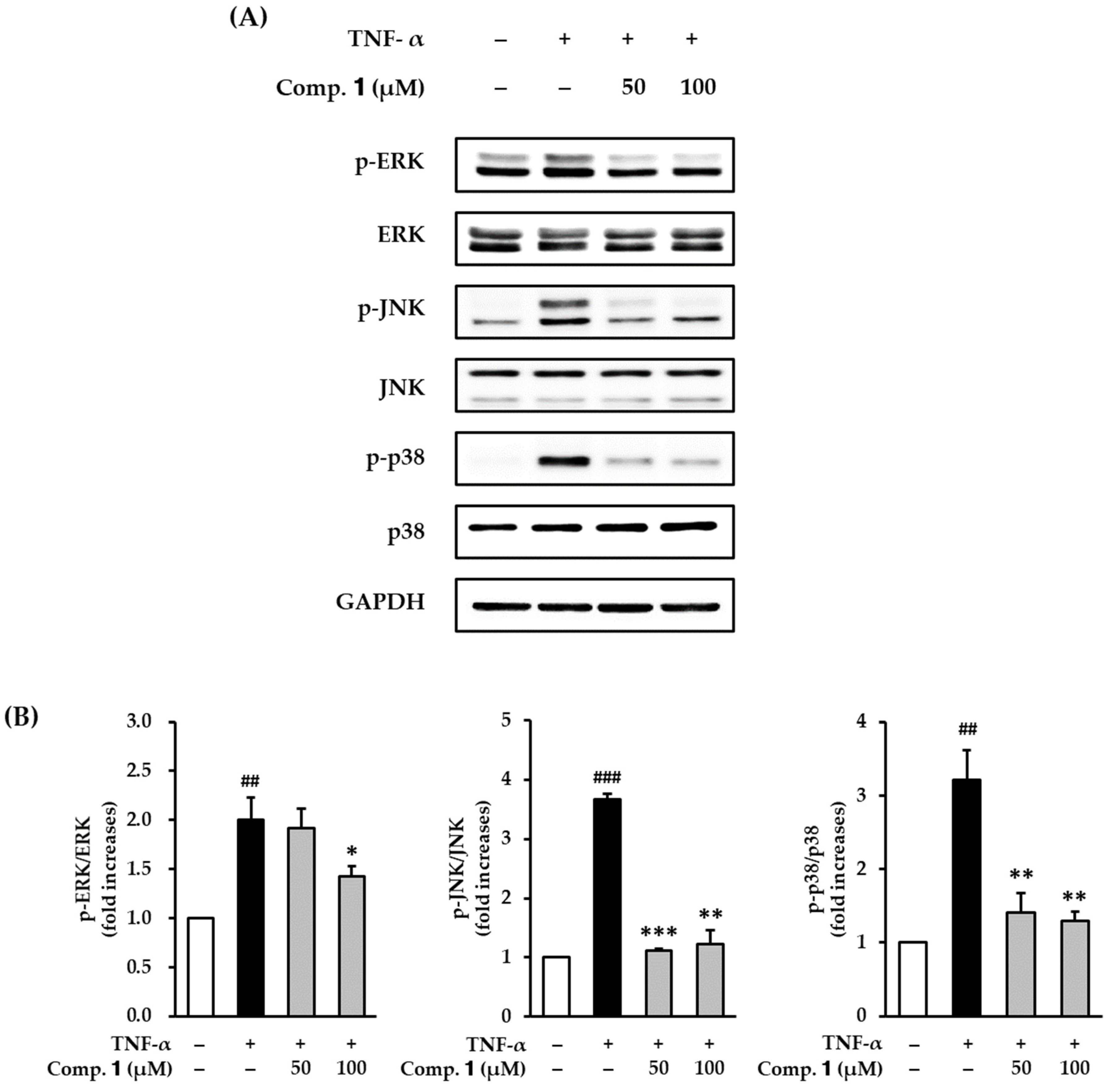
3.6. Effect of Compound 1 on TNF-α-Stimulated Phosphorylation of MAPKs in HDFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adil, M.D.; Kaiser, P.; Satti, N.K.; Zargar, A.M.; Vishwakarma, R.A.; Tasduq, S.A. Effect of Emblica officinalis (fruit) against UVB-induced photo-aging in human skin fibroblasts. J. Ethnopharmacol. 2010, 132, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Mercado, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahmane, R.G.; Godić, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol. Alp. Pannonica Adriat. 2012, 21, 33–36. [Google Scholar] [PubMed]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-Induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. 2008, 7, s12. [Google Scholar]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Rract. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Wang, X.; Hong, H.; Wu, J. Hen collagen hydrolysate alleviates UVA-induced damage in human dermal fibroblasts. J. Funct. Foods 2019, 63, 103574. [Google Scholar] [CrossRef]
- Jeon, S.; Choi, M. Anti-inflammatory and anti-aging effects of hydroxytyrosol on human dermal fibroblasts (HDFs). Biomed. Dermatol. 2018, 2, 21. [Google Scholar] [CrossRef]
- Anderson, A.; Bowman, A.; Boulton, S.J.; Manning, P.; Birch-Machin, M.A. A role for human mitochondrial complex II in the production of reactive oxygen species in human skin. Redox Biol. 2014, 2, 1016–1022. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, J.J. Collagen synthesis and degradation in the uterine deciduoma: Regulation of collagenase activity by progesterone. Coll. Relat. Res. 1981, 1, 257–268. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef]
- Farage, M.; Miller, K.; Elsner, P.; Maibach, H. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc 2006, 150, 25. [Google Scholar] [CrossRef]
- Athar, M.; An, K.P.; Morel, K.D.; Kim, A.L.; Aszterbaum, M.; Longley, J.; Epstein Jr, E.H.; Bickers, D.R. Ultraviolet B (UVB)-induced cox-2 expression in murine skin: An immunohistochemical study. Biochem. Biophys. Res. Commun. 2001, 280, 1042–1047. [Google Scholar] [CrossRef]
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef]
- Tanaka, K.; Asamitsu, K.; Uranishi, H.; Iddamalgoda, A.; Ito, K.; Kojima, H.; Okamoto, T. Protecting skin photoaging by NF-κB inhibitor. Curr. Drug Metab. 2010, 11, 431–435. [Google Scholar] [CrossRef]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Ladwig, G.; Wysocki, A. Extracellular matrix: Review of its roles in acute and chronic wounds. World Wide Wounds 2005, 2005, 1–18. [Google Scholar]
- Lee, B.-C.; Lee, S.Y.; Lee, H.J.; Sim, G.-S.; Kim, J.-H.; Kim, J.-H.; Cho, Y.-H.; Lee, D.-H.; Pyo, H.-B.; Choe, T.-B. Anti-oxidative and photo-protective effects of coumarins isolated fromFraxinus chinensis. Arch. Pharm. Res. 2007, 30, 1293. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Gani, S.; Mokhtar, N. Anti-elastase, anti-collagenase and antimicrobial activities of the underutilized red pitaya peel: An in vitro study for anti-aging applications. Asian J. Pharm. Clin. Res. 2017, 10, 251–255. [Google Scholar] [CrossRef]
- SAMEJIMA, H.; PARK, B.-J. Inhibition Activity of Guava (Psidium guajava L.) Leaf Extract against Collagenase, Elastase, Hyaluronidase, and Carbohydrate Digestion Enzymes. Trop. Agric. Dev. 2019, 63, 12–17. [Google Scholar]
- Devi, B.; Sharma, N.; Kumar, D.; Jeet, K. Morus alba Linn: A phytopharmacological review. Int. J. Pharm. Pharm. Sci. 2013, 5, 14–18. [Google Scholar]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of mulberry fruit (Morus alba L.) consumption on health outcomes: A mini-review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef]
- Memon, A.A.; Memon, N.; Luthria, D.L.; Bhanger, M.I.; Pitafi, A.A. Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol. J. Food Nutr. Sci. 2010, 60, 25–32. [Google Scholar]
- Singab, A.N.B.; El-Beshbishy, H.A.; Yonekawa, M.; Nomura, T.; Fukai, T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 333–338. [Google Scholar] [CrossRef]
- Chen, F.; Nakashima, N.; Kimura, I.; Kimura, M. Hypoglycemic activity and mechanisms of extracts from mulberry leaves (folium mori) and cortex mori radicis in streptozotocin-induced diabetic mice. Yakugaku Zasshi 1995, 115, 476–482. [Google Scholar] [CrossRef]
- Hunyadi, A.; Martins, A.; Hsieh, T.-J.; Seres, A.; Zupkó, I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef] [PubMed]
- Ann, J.-Y.; Eo, H.; Lim, Y. Mulberry leaves (Morus alba L.) ameliorate obesity-induced hepatic lipogenesis, fibrosis, and oxidative stress in high-fat diet-fed mice. Genes Nutr. 2015, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Verma, R.; David, S.R.; Chellappan, D.K.; Anwar, F.; Dua, K. Hepatoprotective activity of moralbosteroid, a steroidal glycoside isolated from Morus alba. Orient. Pharm. Exp. Med. 2014, 14, 285–289. [Google Scholar] [CrossRef]
- Stone, J.K.; Polishook, J.D.; White, J.F. Endophytic fungi. In Biodiversity of Fungi; Elsevier Academic Press: Burlington, NJ, USA, 2004; pp. 241–270. [Google Scholar]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.-K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.L.; Hughes, A.F.; Gil, V.B.; Vaz, A.B.; Alves, T.M.; Zani, C.L.; Rosa, C.A.; Rosa, L.H. Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell.(Solanaceae). Can. J. Microbiol. 2012, 58, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Lee, C.; Lee, D.; Kim, S.; Bang, S.; Shin, M.-S.; Lee, J.; Kang, K.S.; Shim, S.H. Neuroprotective compound from an endophytic fungus, Colletotrichum sp. JS-0367. J. Nat. Prod. 2018, 81, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.M.; Lee, S.; Kang, K.S. Protective Effects of Active Compounds from Salviae miltiorrhizae Radix against Glutamate-Induced HT-22 Hippocampal Neuronal Cell Death. Processes 2020, 8, 914. [Google Scholar] [CrossRef]
- Lee, A.Y.; Lee, S.; Kim, H.Y.; Lee, S.; Cho, E.J. Anti-inflammatory effects of luteolin and luteoloside from Taraxacum coreanum in RAW264. 7 macrophage cells. Appl. Biol. Chem. 2016, 59, 747–754. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-α-Stimulated Normal Human Dermal Fibroblasts. Antioxidants 2020, 9, 981. [Google Scholar] [CrossRef]
- Phung, H.M.; Lee, S.; Hwang, J.H.; Kang, K.S. Preventive Effect of Muscone against Cisplatin Nephrotoxicity in LLC-PK1 Cells. Biomolecules 2020, 10, 1444. [Google Scholar] [CrossRef] [PubMed]
- Ben Ammar, R.; Miyamoto, T.; Chekir-Ghedira, L.; Ghedira, K.; Lacaille-Dubois, M.-A. Isolation and identification of new anthraquinones from Rhamnus alaternus L and evaluation of their free radical scavenging activity. Nat. Prod. Res. 2019, 33, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, S.; Murthy, Y.; Rao, K.R.M. Studies on synthesis and antioxidant property of anthraquinone analogues. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Eom, T.; Kim, E.; Kim, J.-S. In vitro antioxidant, antiinflammation, and anticancer activities and anthraquinone content from Rumex crispus root extract and fractions. Antioxidants 2020, 9, 726. [Google Scholar] [CrossRef]
- Li, Y.; Guo, F.; Guan, Y.; Chen, T.; Ma, K.; Zhang, L.; Wang, Z.; Su, Q.; Feng, L.; Liu, Y. Novel Anthraquinone Compounds Inhibit Colon Cancer Cell Proliferation via the Reactive Oxygen Species/JNK Pathway. Molecules 2020, 25, 1672. [Google Scholar] [CrossRef]
- Lu, Y.; Suh, S.-J.; Li, X.; Hwang, S.-L.; Li, Y.; Hwangbo, K.; Park, S.J.; Murakami, M.; Lee, S.H.; Jahng, Y. Citreorosein, a naturally occurring anthraquinone derivative isolated from Polygoni cuspidati radix, attenuates cyclooxygenase-2-dependent prostaglandin D2 generation by blocking Akt and JNK pathways in mouse bone marrow-derived mast cells. Food Chem. Toxicol. 2012, 50, 913–919. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yang, L.-L.; Lee, T.J. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-κB activation. Biochem. Pharmacol. 2000, 59, 1445–1457. [Google Scholar] [CrossRef]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann. N. Y. Acad. Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef]
- Langton, A.; Sherratt, M.; Griffiths, C.; Watson, R. A new wrinkle on old skin: The role of elastic fibres in skin ageing. Int. J. Cosmet. Sci. 2010, 32, 330–339. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative stress and human skin connective tissue aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Chen, Y.; Lyga, J. Brain-skin connection: Stress, inflammation and skin aging. Inflamm. Allergy Drug Targets Former. Curr. Drug Targets Inflamm. Allergy 2014, 13, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Fukuda, K.; Sato, T.; Kawaguchi, H.; Suematsu, M.; Matsuda, S.; Koyasu, S.; Matsui, H.; Yamauchi-Takihara, K.; Harada, M. ERK and p38 MAPK, but not NF-κB, are critically involved in reactive oxygen species–mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ. Res. 2001, 89, 661–669. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef]
- Kim, H.H.; Shin, C.M.; Park, C.-H.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J. Lipid Res. 2005, 46, 1712–1720. [Google Scholar] [CrossRef]
- Kim, H.-I.; Jeong, Y.-U.; Kim, J.-H.; Park, Y.-J. 3, 5, 6, 7, 8, 3′, 4′-Heptamethoxyflavone, a citrus flavonoid, inhibits collagenase activity and induces type I procollagen synthesis in HDFn cells. Int. J. Mol. Sci. 2018, 19, 620. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. Ther. 2002, 4, 157. [Google Scholar] [CrossRef]
| Genes | Sequences | |
|---|---|---|
| Matrix metalloproteinase-1 (AF158733) | Sense | 5′- ATTCTACTGATATCGGGGCTTT -3′ |
| Antisense | 5′- ATGTCCTTGGGGTATCCGTGTA -3′ | |
| Procollagen I α1 (X07884) | Sense | 5′-CTCGAGGTGGACACCACCCT-3′ |
| Antisense | 5′-CAGCTGGATGGCCACATCGG-3′ | |
| Interleukin-1β (NM_000576) | Sense | 5′-CTGTCCTGCGTGTTGAAAGA-3′ |
| Antisense | 5′-TTCTGCTTGAGAGGTGCTGA-3′ | |
| Interleukin-6 (HUMIL6CSF) | Sense | 5′-CAGGAATTGAATGGGTTTGC-3′ |
| Antisense | 5′-AAACCAAGGCACAGTGGAAC-3′ | |
| Interleukin-8 (HUMIL8A) | Sense | 5′-CTCCTTCTCCACAAGCGCC-3′ |
| Antisense | 5′-GCCGAAGAGCCCTCAGGC-3′ | |
| β-Actin (DQ407611) | Sense | 5′-AGAGATGGCCACGGCTGCTT-3′ |
| Antisense | 5′-ATTTGCGGTGGACGATGGAG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Nguyen, Q.N.; Phung, H.M.; Shim, S.H.; Kim, D.; Hwang, G.S.; Kang, K.S. Preventive Effects of Anthraquinones Isolated from an Endophytic Fungus, Colletotrichum sp. JS-0367 in Tumor Necrosis Factor-α-Stimulated Damage of Human Dermal Fibroblasts. Antioxidants 2021, 10, 200. https://doi.org/10.3390/antiox10020200
Lee S, Nguyen QN, Phung HM, Shim SH, Kim D, Hwang GS, Kang KS. Preventive Effects of Anthraquinones Isolated from an Endophytic Fungus, Colletotrichum sp. JS-0367 in Tumor Necrosis Factor-α-Stimulated Damage of Human Dermal Fibroblasts. Antioxidants. 2021; 10(2):200. https://doi.org/10.3390/antiox10020200
Chicago/Turabian StyleLee, Sullim, Quynh Nhu Nguyen, Hung Manh Phung, Sang Hee Shim, Daeyoung Kim, Gwi Seo Hwang, and Ki Sung Kang. 2021. "Preventive Effects of Anthraquinones Isolated from an Endophytic Fungus, Colletotrichum sp. JS-0367 in Tumor Necrosis Factor-α-Stimulated Damage of Human Dermal Fibroblasts" Antioxidants 10, no. 2: 200. https://doi.org/10.3390/antiox10020200
APA StyleLee, S., Nguyen, Q. N., Phung, H. M., Shim, S. H., Kim, D., Hwang, G. S., & Kang, K. S. (2021). Preventive Effects of Anthraquinones Isolated from an Endophytic Fungus, Colletotrichum sp. JS-0367 in Tumor Necrosis Factor-α-Stimulated Damage of Human Dermal Fibroblasts. Antioxidants, 10(2), 200. https://doi.org/10.3390/antiox10020200









