The Influence of Flaxseed Oil Cake Extract on Oxidative Stability of Microencapsulated Flaxseed Oil in Spray-Dried Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of Emulsion and Spray Drying Process
2.3. Powder Characterization
2.3.1. Water Activity, Hygroscopicity and Particle Size of Powders
2.3.2. Encapsulation Efficiency and Entrapment Oil
2.4. pH Measurements and Titratable Acidity
2.5. Color Measurements
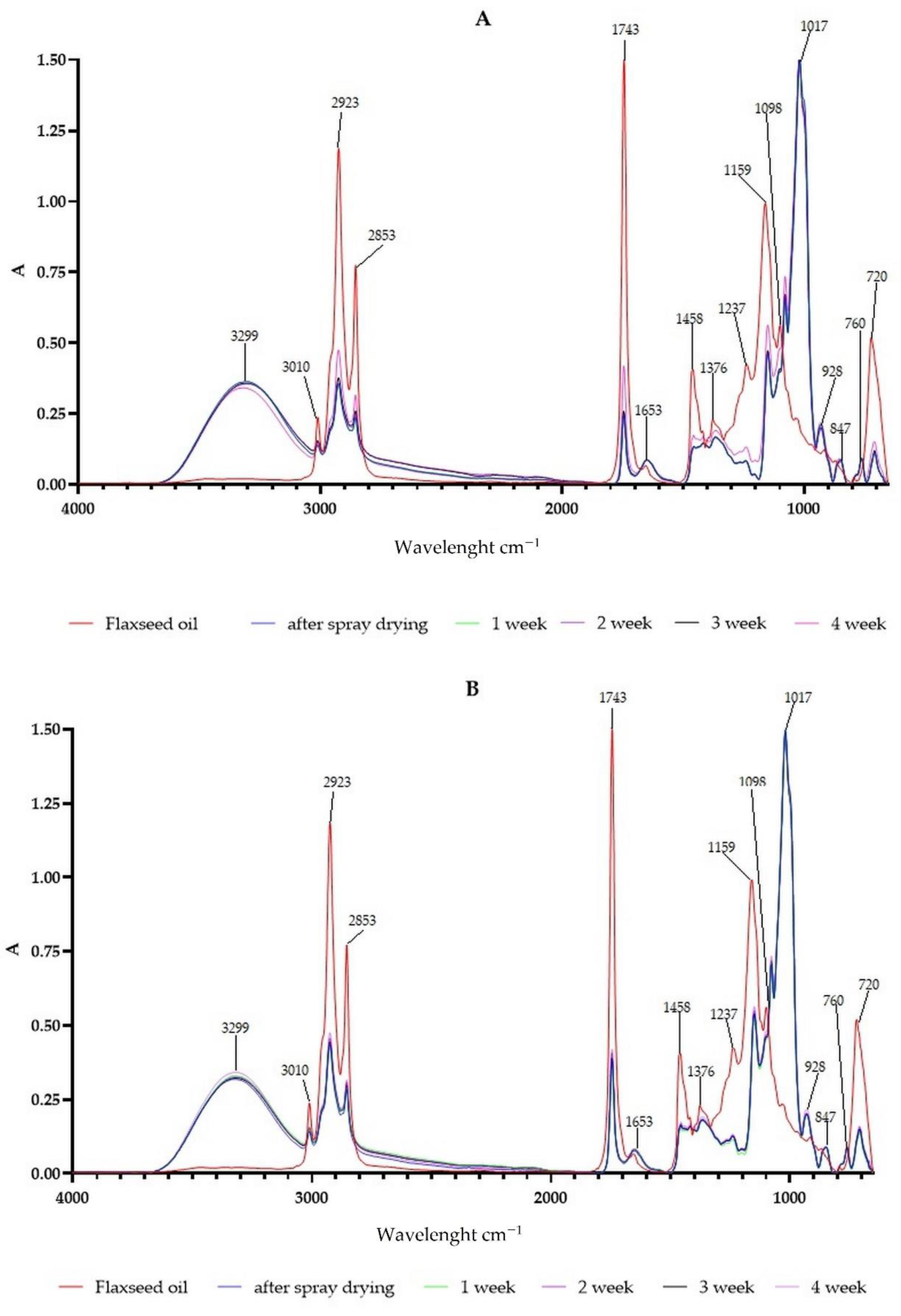
2.6. FTIR Analyses
2.7. Antioxidant Activity, Total Free Amino Acids, and Total Polyphenolic Content of Powders
2.8. Oxidative Stability of Flaxseed Oil during Storage
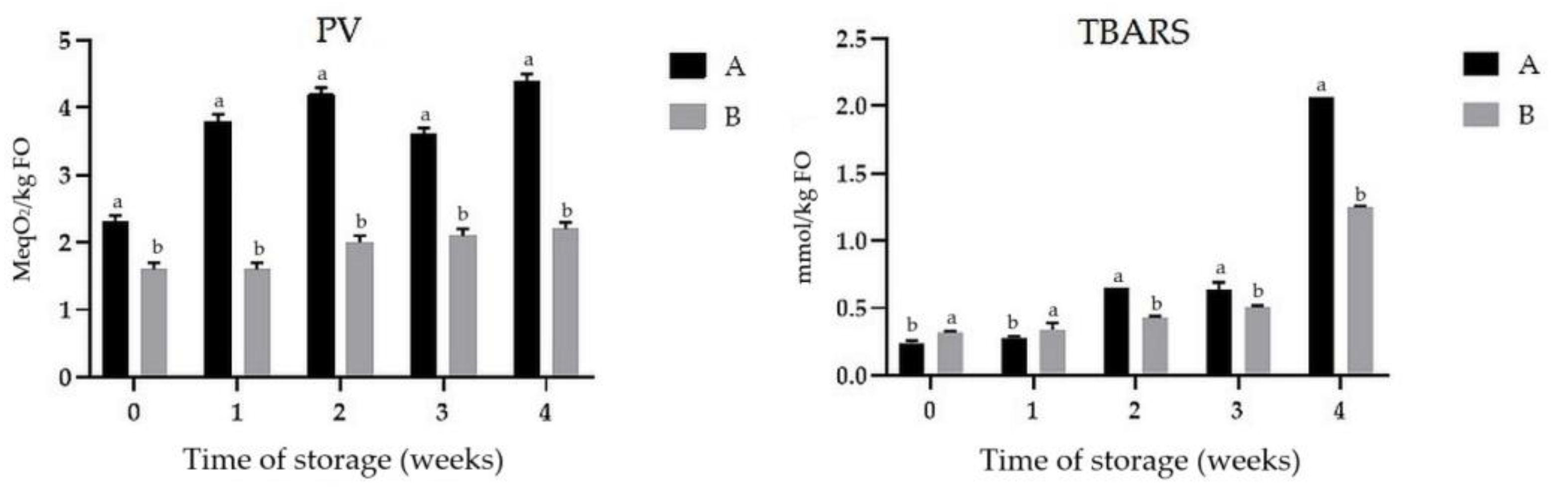
2.8.1. Secondary Oxidation Compounds
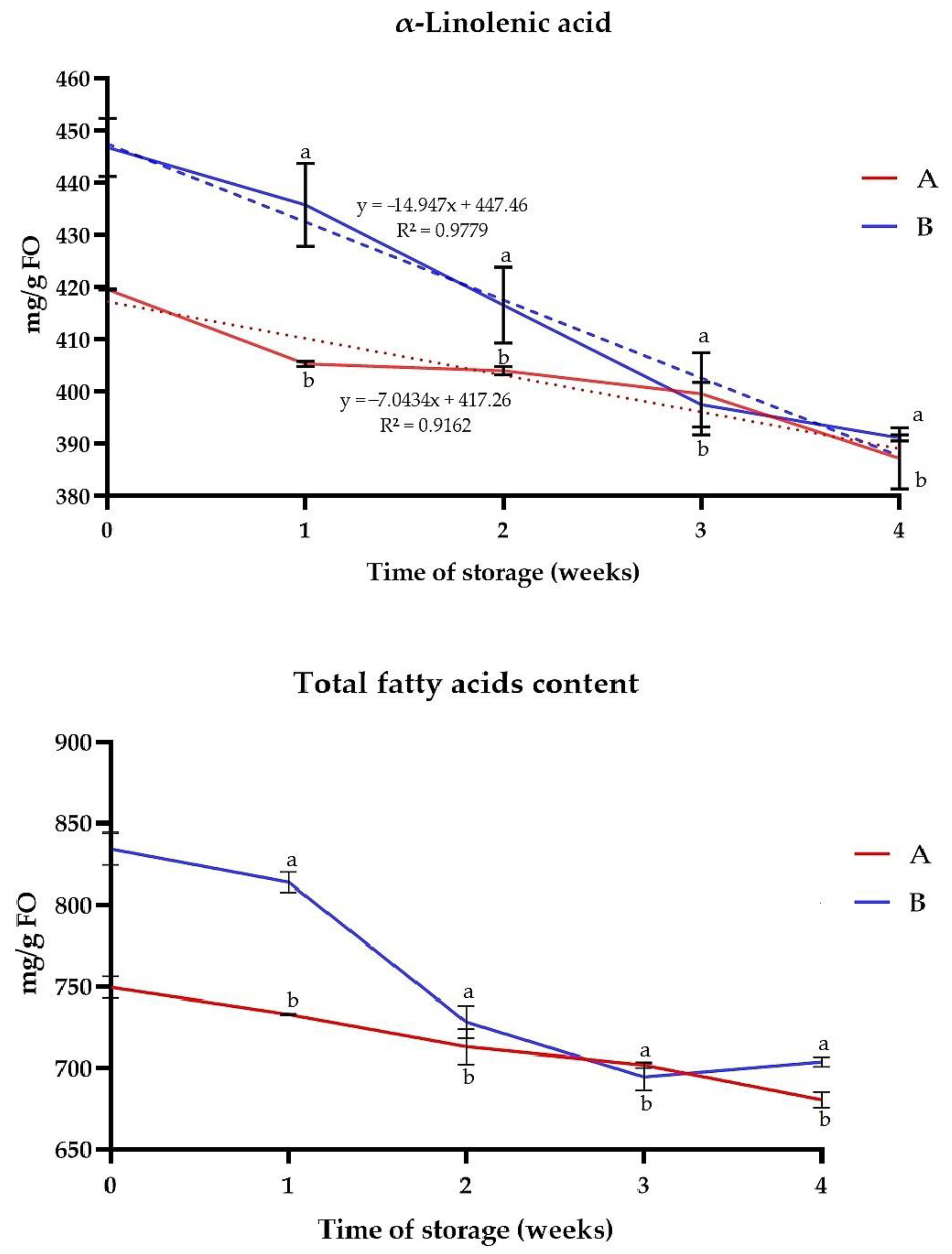
2.8.2. Detection of Fatty Acids and α-Linolenic Acid
2.9. Statistical Analyses
3. Results and Discussion
3.1. Water Activity (aw), Hygroscopicity, Particles Size of Powders and Encapsulation Efficiency of Flaxseed Oil
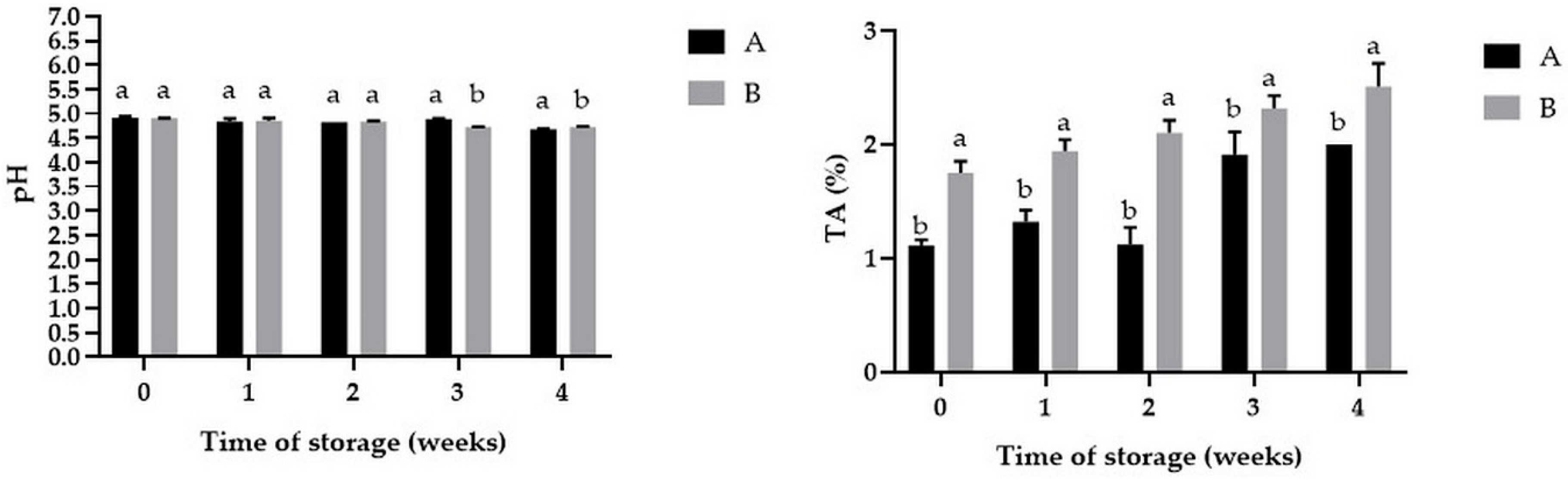
3.2. The Changes of pH and Titrable Acidity of Powders during Storage
3.3. Color Changes
3.4. The Changes in Powder Chemical Composition
3.5. The Changes of Antioxidant Activity, Total Free Amino Acids, and Total Polyphenolic Content
3.6. Oxidative Stability of FO and Changes of Fatty Acid Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michotte, D.; Rogez, H.; Chirinos, R.; Mignolet, E.; Campos, D.; Larondelle, Y. Linseed oil stabilisation with pure natural phenolic compounds. Food Chem. 2011, 129, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-S.; Wang, L.-J.; Li, D.; Li, S.-J.; Özkan, N. Characteristics of Flaxseed Oil from Two Different Flax Plants. Int. J. Food Prop. 2011, 14, 1286–1296. [Google Scholar] [CrossRef] [Green Version]
- Domian, E.; Brynda-Kopytowska, A.; Marzec, A. Functional Properties and Oxidative Stability of Flaxseed Oil Microencapsulated by Spray Drying Using Legume Proteins in Combination with Soluble Fiber or Trehalose. Food Bioprocess Technol. 2017, 10, 1374–1386. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Goyal, A.; Sharma, V.; Sihag, M.K.; Arora, S.; Singh, A.K.; Sabikhi, L. Effect of microencapsulation and spray drying on oxidative stability of flaxseed oil and its release behavior under simulated gastrointestinal conditions. Dry. Technol. 2016, 34, 810–821. [Google Scholar] [CrossRef]
- Gumus, C.E.; Decker, E.A.; Mcclements, D.J. Formation and Stability of ω -3 Oil Emulsion-Based Delivery Systems Using Plant Proteins as Emulsifiers: Lentil, Pea, and Faba Bean Proteins. Food Biophys. 2017, 186–197. [Google Scholar] [CrossRef]
- Tzang, B.S.; Yang, S.F.; Fu, S.G.; Yang, H.C.; Sun, H.L.; Chen, Y.C. Effects of dietary flaxseed oil on cholesterol metabolism of hamsters. Food Chem. 2009, 114, 1450–1455. [Google Scholar] [CrossRef]
- Hano, C.; Corbin, C.; Drouet, S.; Quéro, A.; Rombaut, N.; Savoire, R.; Molinié, R.; Thomasset, B.; Mesnard, F.; Lainé, E. The lignan (+)-secoisolariciresinol extracted from flax hulls is an effective protectant of linseed oil and its emulsion against oxidative damage. Eur. J. Lipid Sci. Technol. 2017, 119, 1–9. [Google Scholar] [CrossRef]
- Kchaou, H.; Jridi, M.; Nasri, M.; Debeaufort, F. Design of Gelatin Pouches for the Preservation of Flaxseed Oil during Storage. Coatings 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Herchi, W.; Bouali, I.; Bahashwan, S.; Rochut, S.; Boukhchina, S.; Kallel, H.; Pepe, C. Changes in phospholipid composition, protein content and chemical properties of flaxseed oil during development. Plant Physiol. Biochem. 2012, 54, 1–5. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Development of a Method Suitable for High-Throughput Screening to Measure Antioxidant Activity in a Linoleic Acid Emulsion. Antioxidants 2019, 8, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waszkowiak, K.; Mikołajczak, B. The Effect of Roasting on the Protein Profile and Antiradical Capacity of Flaxseed Meal. Foods 2020, 9, 1383. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Consoli, L.; Hubinger, M.D. Spray drying of mono- and double-layer emulsions of PUFA-rich vegetable oil homogenized by ultrasound. Dry. Technol. 2020, 1–14. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes 2020, 8, 889. [Google Scholar] [CrossRef]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef]
- Fruehwirth, S.; Steinschaden, R.; Woschitz, L.; Richter, P.; Schreiner, M.; Hoffmann, B.; Hoffmann, W.; Pignitter, M. Oil-assisted extraction of polyphenols from press cake to enhance oxidative stability of flaxseed oil. LWT 2020, 133. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The effect of native and denaturated flaxseed meal extract on physiochemical properties of low fat mayonnaises. J. Food Meas. Charact. 2020, 14, 1135–1145. [Google Scholar] [CrossRef]
- Drozłowska, E.; Bartkowiak, A.; Łopusiewicz, Ł. Characterization of Flaxseed Oil Bimodal Emulsions Prepared with Flaxseed Oil Cake Extract Applied as a Natural Emulsifying Agent. Polymers 2020, 12, 2207. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Jarzębski, M.; Smułek, W.; Siejak, P.; Rezler, R.; Pawlicz, J.; Trzeciak, T.; Jarzębska, M.; Majchrzak, O.; Kaczorek, E.; Kazemian, P. Aesculus hippocastanum L. as a Stabilizer in Hemp Seed Oil Nanoemulsions for Potential Biomedical and Food Applications. Int. J. Mol. Sci. 2021, 22, 887. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Calvo, P.; Castaño, Á.L.; Hernández, M.T.; González-Gómez, D. Effects of microcapsule constitution on the quality of microencapsulated walnut oil. Eur. J. Lipid Sci. Technol. 2011, 113, 1273–1280. [Google Scholar] [CrossRef]
- Chegini, G.; HamidiSepehr, A.; Dizaji, M.F.; Mirnezami, S.V. Study of physical and chemical properties of spray drying whey powder. Int. J. Recycl. Org. Waste Agric. 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, S.; Yang, H. Evaluation of Thermal Effects on the Bioactivity of Curcumin Microencapsulated with Porous Starch-Based Wall Material Using Spray Drying. Processes 2020, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Wu, Z.; Xie, Q.T.; Li, X.M.; Meng, R.; Zhang, B.; Jin, Z.Y. Insight into the stabilization mechanism of emulsions stabilized by Maillard conjugates: Protein hydrolysates-dextrin with different degree of polymerization. Food Hydrocoll. 2020, 99. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and Functional Properties of Seed Proteins from Six Pea (Pisum sativum) Genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef] [Green Version]
- Tong, T.; Liu, Y.-J.; Kang, J.; Zhang, C.-M.; Kang, S.-G. Antioxidant Activity and Main Chemical Components of a Novel Fermented Tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Łopusiewicz, Ł.; Drozłowska, E.; Tarnowiecka-Kuca, A.; Bartkowiak, A.; Mazurkiewicz-Zapałowicz, K.; Salachna, P. Biotransformation of Flaxseed Oil Cake into Bioactive Camembert-Analogue Using Lactic Acid Bacteria, Penicillium camemberti and Geotrichum candidum. Microorganisms 2020, 8, 1266. [Google Scholar] [CrossRef]
- Fioramonti, S.A.; Stepanic, E.M.; Tibaldo, A.M.; Pavón, Y.L.; Santiago, L.G. Spray dried flaxseed oil powdered microcapsules obtained using milk whey proteins-alginate double layer emulsions. Food Res. Int. 2019, 119, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Aberkane, L.; Roudaut, G.; Saurel, R. Encapsulation and Oxidative Stability of PUFA-Rich Oil Microencapsulated by Spray Drying Using Pea Protein and Pectin. Food Bioprocess Technol. 2014, 7, 1505–1517. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; McClements, D.J. Stability and rheology of corn oil-in-water emulsions containing maltodextrin. Food Res. Int. 2004, 37, 851–859. [Google Scholar] [CrossRef]
- Fernandes, R.V.D.B.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.M.; Taip, F.S.; Abdulla, H.Z. Effectiveness of additives in spray drying performance: A review. Food Res. 2018, 2, 486–499. [Google Scholar] [CrossRef]
- Samsu, Z.A.; Mohamad Zahir, A.Z. Production of oil palm milk powder by spray drying technique. Mater. Today Proc. 2020, 31, 306–312. [Google Scholar] [CrossRef]
- Chang, Y.I.; Scire, J.; Jacobs, B. Effect of Particle Size and Microstructure Properties on Encapsulated Orange Oil. In Flavor Encapsulation; ACS: Washington, DC, USA, 1988; pp. 87–102. [Google Scholar]
- Kumar, L.R.G.; Chatterjee, N.S.; Tejpal, C.S.; Vishnu, K.V.; Anas, K.K.; Asha, K.K.; Anandan, R.; Mathew, S. Evaluation of chitosan as a wall material for microencapsulation of squalene by spray drying: Characterization and oxidative stability studies. Int. J. Biol. Macromol. 2017, 104, 1986–1995. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Encapsulation of Flaxseed Oil Using a Benchtop Spray Dryer for Legume Protein−Maltodextrin Microcapsule Preparation. J. Agric. Food Chem. 2013. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Springer: New York, NY, USA, 2005; pp. 157–173. ISBN 978-0-306-47806-2. [Google Scholar]
- Tonon, R.V.; Pedro, R.B.; Grosso, C.R.F.; Hubinger, M.D. Microencapsulation of Flaxseed Oil by Spray Drying: Effect of Oil Load and Type of Wall Material. Dry. Technol. 2012, 30, 1491–1501. [Google Scholar] [CrossRef]
- Badke, L.B.; da Silva, B.C.; de Carvalho-Jorge, A.R.; Taher, D.M.; Riegel-Vidotti, I.C.; Marino, C.E.B. Synthesis and characterization of microalgae fatty acids or Aloe vera oil microcapsules. Polimeros 2019, 29. [Google Scholar] [CrossRef]
- Muzaffar, K.; Kumar, P. Moisture sorption isotherms and storage study of spray dried tamarind pulp powder. Powder Technol. 2016, 291, 322–327. [Google Scholar] [CrossRef]
- Stoll, L.; Silva, A.M.D.; Iahnke, A.O.E.S.; Costa, T.M.H.; Flôres, S.H.; Rios, A.D.O. Active biodegradable film with encapsulated anthocyanins: Effect on the quality attributes of extra-virgin olive oil during storage. J. Food Process. Preserv. 2017, 41, e13218. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yi, B.R.; Lee, C.; Gim, S.Y.; Kim, M.J.; Lee, J.H. Effects of pH on the rates of lipid oxidation in oil–water system. Appl. Biol. Chem. 2016, 59, 157–161. [Google Scholar] [CrossRef]
- Du, J.; Ge, Z.Z.; Xu, Z.; Zou, B.; Zhang, Y.; Li, C.M. Comparison of the Efficiency of Five Different Drying Carriers on the Spray Drying of Persimmon Pulp Powders. Dry. Technol. 2014, 32, 1157–1166. [Google Scholar] [CrossRef]
- Tambade, P.B.; Sharma, M.; Singh, A.K.; Surendranath, B. Flaxseed Oil Microcapsules Prepared Using Soy Protein Isolate and Modified Starch: Process Optimization, Characterization and In Vitro Release Behaviour. Agric. Res. 2020, 9, 652–662. [Google Scholar] [CrossRef]
- Grehk, T.M.; Berger, R.; Bexell, U. Investigation of the drying process of linseed oil using FTIR and ToF-SIMS. J. Phys. Conf. Ser. 2008, 100, 012019. [Google Scholar] [CrossRef]
- Palanisamy, K.L.; Devabharathi, V.; Sundaram, N.M. The utility of magnetic iron oxide nanoparticles stabilized by carrier oils in removal of heavy metals from waste water. IJRANS 2013, 1, 15–22. [Google Scholar]
- Souza, C.R.F.; Georgetti, S.R.; Salvador, M.J.; José, M.; Fonseca, V.; Oliveira, W.P. Antioxidant activity and physical-chemical properties of spray and spouted bed dried extracts of Bauhinia forficata. Braz. J. Pharm. Sci. 2009, 45, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Malcolmson, L.J.; Przybylski, R.; Daun, J.K. Storage stability of milled flaxseed. J. Am. Oil Chem. Soc. 2000, 77, 235–238. [Google Scholar] [CrossRef]
- Pag, A.I.; Radu, D.G.; Draganescu, D.; Popa, M.I.; Sîrghie, C. Flaxseed cake—A sustainable source of antioxidant and antibacterial extracts. Cellul. Chem. Technol. 2014, 48, 265–273. [Google Scholar]
- Gerstenmeyer, E.; Reimer, S.; Berghofer, E.; Schwartz, H.; Sontag, G. Effect of thermal heating on some lignans in flax seeds, sesame seeds and rye. Food Chem. 2013, 138, 1847–1855. [Google Scholar] [CrossRef]
- Imran, M.; Ahmad, N.; Anjum, F.M.; Khan, M.K.; Mushtaq, Z.; Nadeem, M.; Hussain, S. Potential protective properties of flax lignan secoisolariciresinol diglucoside. Nutr. J. 2015, 14, 71. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, A.; Fliniaux, O.; Quéro, A.; Molinié, R.; Demailly, H.; Hano, C.; Paetz, C.; Roscher, A.; Grand, E.; Kovensky, J.; et al. Kinetics of the incorporation of the main phenolic compounds into the lignan macromolecule during flaxseed development. Food Chem. 2017, 217, 1–8. [Google Scholar] [CrossRef]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.P.; Ferroud, C.; Doussot, J. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef]
- Renouard, S.; Hano, C.; Corbin, C.; Fliniaux, O.; Lopez, T.; Montguillon, J.; Barakzoy, E.; Mesnard, F.; Lamblin, F.; Lainé, E. Cellulase-assisted release of secoisolariciresinol from extracts of flax (Linum usitatissimum) hulls and whole seeds. Food Chem. 2010, 122, 679–687. [Google Scholar] [CrossRef]
- Socrier, L.; Quéro, A.; Verdu, M.; Song, Y.; Molinié, R.; Mathiron, D.; Pilard, S.; Mesnard, F.; Morandat, S. Flax phenolic compounds as inhibitors of lipid oxidation: Elucidation of their mechanisms of action. Food Chem. 2019, 274, 651–658. [Google Scholar] [CrossRef]
- Cheng, C.; Yu, X.; McClements, D.J.; Huang, Q.; Tang, H.; Yu, K.; Xiang, X.; Chen, P.; Wang, X.; Deng, Q. Effect of flaxseed polyphenols on physical stability and oxidative stability of flaxseed oil-in-water nanoemulsions. Food Chem. 2019, 301, 125207. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Durante, M.; Milano, F.; De Caroli, M.; Giotta, L.; Piro, G.; Mita, G.; Frigione, M.; Lenucci, M.S. Tomato Oil Encapsulation by α-, β-, and γ-Cyclodextrins: A Comparative Study on the Formation of Supramolecular Structures, Antioxidant Activity, and Carotenoid Stability. Foods 2020, 9, 1553. [Google Scholar] [CrossRef]
- Mrkìc, V.; Cocci, E.; Rosa, M.D.; Sacchetti, G. Effect of drying conditions on bioactive compounds and antioxidant activity of broccoli (Brassica oleracea L.). J. Sci. Food Agric. 2006, 86, 1559–1566. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Sopelana, P.; Nakashima, F.; Shibata, T.; Uchida, K.; Guillén, M.D. A dual perspective of the action of lysine on soybean oil oxidation process obtained by combining 1H NMR and LC-MS: Antioxidant effect and generation of Lysine-Aldehyde adducts. Antioxidants 2019, 8, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.; Winkler-Moser, J.K.; Doll, K.M.; Gadgil, M.; Liu, S.X. Factors Affecting Antioxidant Activity of Amino Acids in Soybean Oil at Frying Temperatures. Eur. J. Lipid Sci. Technol. 2019, 121, 1900091. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Wu, J.; A’yun, Q.; Van der Meeren, P. Maillard conjugation as an approach to improve whey proteins functionality: A review of conventional and novel preparation techniques. Trends Food Sci. Technol. 2019, 91, 1–11. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Mahdi Jafari, S.; Sarabandi, K.; Mohammadi, M.; Khakbaz Heshmati, M.; Pezeshki, A. Influence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhao, M.; Cui, C.; Zhao, H.; Yang, B. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innov. Food Sci. Emerg. Technol. 2009, 10, 235–240. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, Y.; Zhu, X.; Li, S.; Zhou, C. L-lysine and L-arginine inhibit the oxidation of lipids and proteins of emulsion sausage by chelating iron ion and scavenging radical. Asian-Australas. J. Anim. Sci. 2018, 31, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Filippenko, T.A.; Gribova, N.Y. Antioxidant activity of amino acids during oxidation of sunflower oil in an emulsion. Pharm. Chem. J. 2011, 45, 296–298. [Google Scholar] [CrossRef]
- Gong, K.J.; Shi, A.M.; Liu, H.Z.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2015, 170, 33–40. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.J.; Xu, W. Comparisons on the Functional Properties and Antioxidant Activity of Spray-Dried and Freeze-Dried Egg White Protein Hydrolysate. Food Bioprocess Technol. 2012, 5, 2342–2352. [Google Scholar] [CrossRef]
- Koç, M.; Güngör, Ö.; Zungur, A.; Yalçın, B.; Selek, İ.; Ertekin, F.K.; Ötles, S. Microencapsulation of Extra Virgin Olive Oil by Spray Drying: Effect of Wall Materials Composition, Process Conditions, and Emulsification Method. Food Bioprocess Technol. 2015, 8, 301–318. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission; FAO; WHO. Standard for Fish Oils; CXS 329-2017; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Nawar, W.W. Lipids in Food Chemistry; Fennema, O.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 225–314. [Google Scholar]
- Gallardo, G.; Guida, L.; Martinez, V.; López, M.C.; Bernhardt, D.; Blasco, R.; Pedroza-Islas, R.; Hermida, L.G. Microencapsulation of linseed oil by spray drying for functional food application. Food Res. Int. 2013, 52, 473–482. [Google Scholar] [CrossRef]
- Mao, X.; Chen, W.; Huyan, Z.; Sherazi, S.T.H.; Yu, X. Impact of linolenic acid on oxidative stability of rapeseed oils. J. Food Sci. Technol. 2020, 57, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Revisiting the polar paradox theory: A critical overview. J. Agric. Food Chem. 2011, 59, 3499–3504. [Google Scholar] [CrossRef]
- Porter, W.L.; Black, E.D.; Drolet, A.M. Use of Polyamide Oxidative Fluorescence Test on Lipid Emulsions: Contrast in Relative Effectiveness of Antioxidants in Bulk Versus Dispersed Systems. J. Agric. Food Chem. 1989, 37, 615–624. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.W.; Kanner, J.; German, J.B. Interfacial Phenomena in the Evaluation of Antioxidants: Bulk Oils vs Emulsions. J. Agric. Food Chem. 1994, 42, 1054–1059. [Google Scholar] [CrossRef]
| Sample | aw (-) | Hygroscopicity (%) | D4,3 (µm) | EE (%) |
|---|---|---|---|---|
| A | 0.41 ± 0.01 a | 15.48 ± 0.88 a | 28.39 ± 0.23 b | 22.00 ± 3.24 a |
| B | 0.36 ± 0.00 b | 14.75 ± 0.35 b | 31.55 ± 0.67 a | 39.00 ± 0.02 b |
| L * | |||||
| 0 | 1 | 2 | 3 | 4 | |
| A | 95.04 ± 0.00 Aa | 95.06 ± 0.00 Ba | 94.53 ± 0.00 Ca | 95.14 ± 0.01 Da | 95.22 ± 0.02 Ea |
| B | 94.63 ± 0.00 Ab | 94.21 ± 0.00 Bb | 94.27 ± 0.00 Cb | 94.05 ± 0.00 Db | 93.92 ± 0.01 Eb |
| a * | |||||
| A | −1.33 ± 0.00 Ab | −1.42 ± 0.00 Bb | −1.26 ± 0.03 Cb | −1.25 ± 0.02 Db | −1.23 ± 0.00 Eb |
| B | −1.13 ± 0.01 Aa | −1.09 ± 0.01 Ba | −1.08 ± 0.02 Ca | −1.02 ± 0.00 Da | −1.15 ± 0.00 Ea |
| b * | |||||
| A | 9.35 ± 0.01 Ab | 10.00 ± 0.01 Bb | 8.84 ± 0.03 Bb | 8.81 ± 0.01 Bb | 8.31 ± 0.01 Cb |
| B | 10.37 ± 0.01 Aa | 10.50 ± 0.01 Ba | 10.37 ± 0.01 Ca | 10.20 ± 0.05 Da | 9.67 ± 0.01 Ea |
| YI | |||||
| A | 13.06 ± 0.01 Ab | 13.02 ± 0.01 Bb | 13.36 ± 0.01 Cb | 13.23 ± 0.02 Db | 13.46 ± 0.08 Eb |
| B | 15.66 ± 0.01 Aa | 15.92 ± 0.01 Ba | 15.71 ± 0.01 Ca | 16.26 ± 0.01 Da | 16.72 ± 0.01 Ea |
| WI | |||||
| A | 89.33 ± 0.01 Aa | 88.76 ± 0.00 Ba | 89.53 ± 0.03 Ca | 89.86 ± 0.01 Da | 89.34 ± 0.05 Ea |
| B | 88.26 ± 0.00 Ab | 87.96 ± 0.00 Bb | 88.11 ± 0.01 Cb | 87.71 ± 0.00 Db | 87.96 ± 0.00 Eb |
| ΔE | |||||
| A | Used as a standard | 0.65 ± 0.01 Aa | 0.73 ± 0.01 Ba | 0.67 ± 0.00 Ca | 1.06 ± 0.05 Da |
| B | Used as a standard | 0.44 ± 0.01 Ab | 0.36 ± 0.01 Bb | 0.55 ± 0.01 Cb | 0.70 ± 0.01 Db |
| ABTS (%) | |||||
| Sample | 0 | 1 | 2 | 3 | 4 |
| A | 74.15 ± 0.08 Aa | 61.47 ± 0.14 Bb | 58.22 ± 0.16 Cb | 54.50 ± 0.01 Da | 53.35 ± 0.08 Ea |
| B | 74.01 ± 0.08 Ab | 66.67 ± 0.08 Ba | 61.05 ± 0.00 Ca | 58.50 ± 0.08 Da | 54.21 ± 0.16 Eb |
| DPPH (%) | |||||
| A | 65.27 ± 0.05 Aa | 58.57 ± 0.00 Ba | 54.66 ± 0.03 Ca | 52.24 ± 0.06 Db | 51.21 ± 0.05 Eb |
| B | 63.03 ± 0.02 Ab | 55.24 ± 0.05 Bb | 53.39 ± 0.07 Cb | 52.67 ± 0.07 Da | 51.71 ± 0.02 Ea |
| TPC (mg GAE/mL) | |||||
| A | 19.08 ± 0.01 Ab | 18.84 ± 0.02 Bb | 17.80 ± 0.02 Cb | 16.01 ± 0.02 Db | 15.06 ± 0.00 Ea |
| B | 20.05 ± 0.01 Aa | 19.69 ± 0.05 Ba | 18.15 ± 0.02 Ca | 17.59 ± 0.00 Da | 16.98 ± 0.02 Eb |
| TFAA (mg Gly/mL) | |||||
| Sample | 0 | 1 | 2 | 3 | 4 |
| A | 5.74 ± 0.06 Ab | 10.68 ± 0.04 Bb | 11.19 ± 0.02 Cb | 12.23 ± 0.07 Db | 12.32 ± 0.04 Eb |
| B | 9.03 ± 0.13 Aa | 12.36 ± 0.04 Ba | 12.93 ± 0.09 Ca | 14.97 ± 0.06 Da | 15.50 ± 0.12 Ea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozłowska, E.; Bartkowiak, A.; Trocer, P.; Kostek, M.; Tarnowiecka-Kuca, A.; Bienkiewicz, G.; Łopusiewicz, Ł. The Influence of Flaxseed Oil Cake Extract on Oxidative Stability of Microencapsulated Flaxseed Oil in Spray-Dried Powders. Antioxidants 2021, 10, 211. https://doi.org/10.3390/antiox10020211
Drozłowska E, Bartkowiak A, Trocer P, Kostek M, Tarnowiecka-Kuca A, Bienkiewicz G, Łopusiewicz Ł. The Influence of Flaxseed Oil Cake Extract on Oxidative Stability of Microencapsulated Flaxseed Oil in Spray-Dried Powders. Antioxidants. 2021; 10(2):211. https://doi.org/10.3390/antiox10020211
Chicago/Turabian StyleDrozłowska, Emilia, Artur Bartkowiak, Paulina Trocer, Mateusz Kostek, Alicja Tarnowiecka-Kuca, Grzegorz Bienkiewicz, and Łukasz Łopusiewicz. 2021. "The Influence of Flaxseed Oil Cake Extract on Oxidative Stability of Microencapsulated Flaxseed Oil in Spray-Dried Powders" Antioxidants 10, no. 2: 211. https://doi.org/10.3390/antiox10020211
APA StyleDrozłowska, E., Bartkowiak, A., Trocer, P., Kostek, M., Tarnowiecka-Kuca, A., Bienkiewicz, G., & Łopusiewicz, Ł. (2021). The Influence of Flaxseed Oil Cake Extract on Oxidative Stability of Microencapsulated Flaxseed Oil in Spray-Dried Powders. Antioxidants, 10(2), 211. https://doi.org/10.3390/antiox10020211









