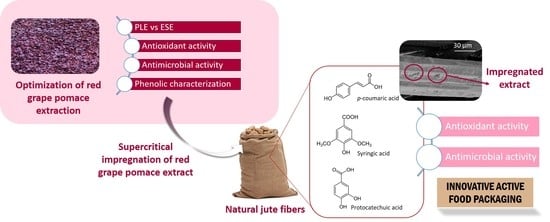
Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents, Raw Materials and Bacterial Samples
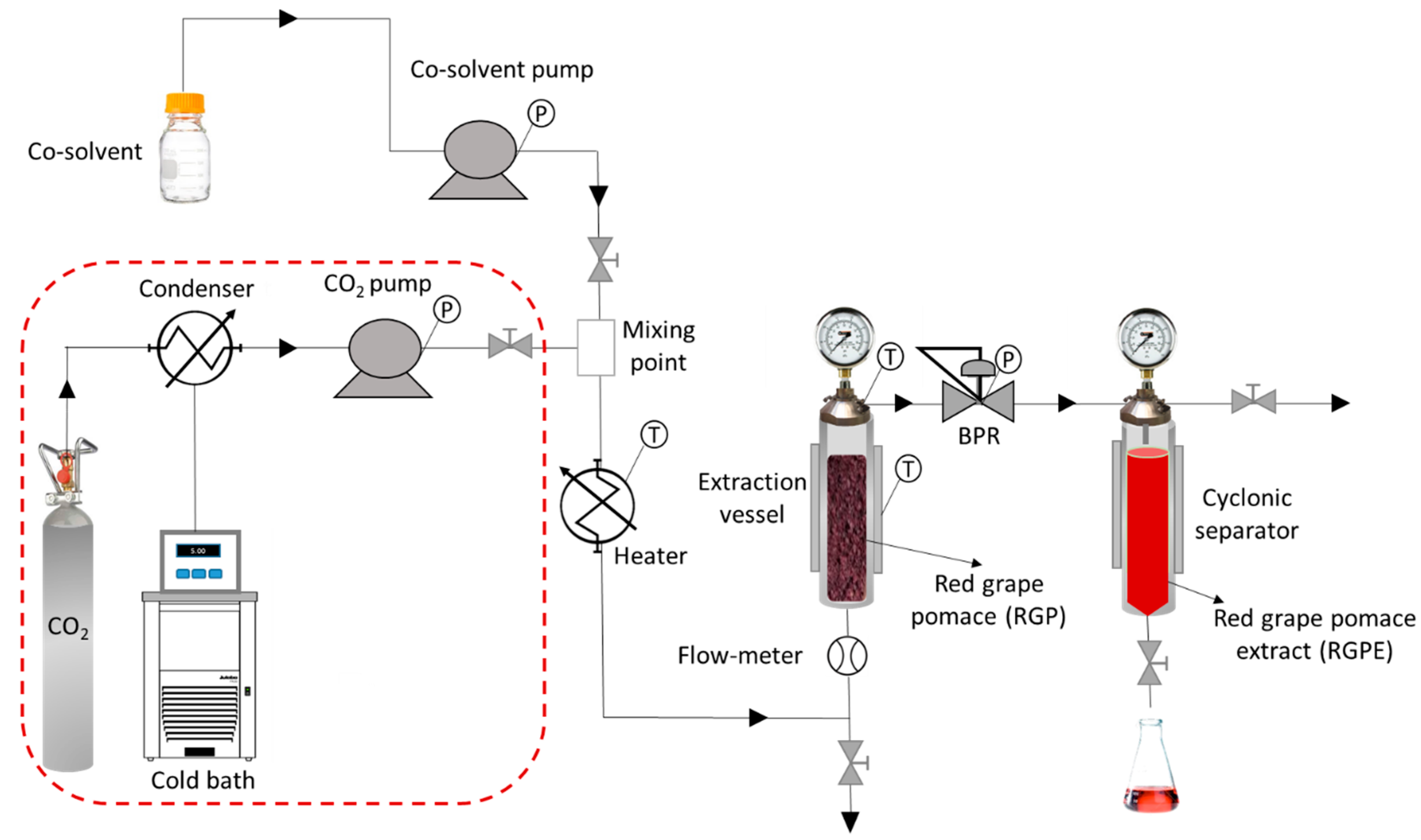
2.2. High-Pressure Extraction of RGPE
2.3. Supercritical Impregnation of Natural Jute Fibers with RGPE
2.4. Analysis of the Bioactivity of the Extracts and the IJF Samples
2.4.1. Antioxidant Capacity
2.4.2. Antibacterial Capacity of the RGPE and the IJF Samples
2.5. Phenolic Characterization of RGPE and Impregnated Jute Fibers by UPLC-ESI-TOF-MS
2.6. Scanning Electron Microscopy (SEM)
3. Results and Discussions
3.1. RGPE Bioactivity
3.2. Bioactivity of RGPE-Impregnated Natural Fibers
3.3. Phenolic Composition of RGPE and Impregnated Jute Fiber
3.4. SEM Images
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of phenolic antioxidants from syrah grape pomace through the optimization of an enzymatic extraction process. Food Chem. 2019, 283, 257–264. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- Oladele, A.K.; Duodu, K.G.; Emmambux, N.M. Pasting, flow, thermal and molecular properties of maize starch modified with crude phenolic extracts from grape pomace and sorghum bran under alkaline conditions. Food Chem. 2019, 297, 124879. [Google Scholar] [CrossRef]
- Trikas, E.D.; Melidou, M.; Papi, R.M.; Zachariadis, G.A.; Kyriakidis, D.A. Extraction, separation and identification of anthocyanins from red wine by-product and their biological activities. J. Funct. Foods 2016, 25, 548–558. [Google Scholar] [CrossRef]
- Mohd Maidin, N.; Michael, N.; Oruna-Concha, M.J.; Jauregi, P. Polyphenols extracted from red grape pomace by a surfactant based method show enhanced collagenase and elastase inhibitory activity. J. Chem. Technol. Biotechnol. 2018, 93, 1916–1924. [Google Scholar] [CrossRef]
- Andrés, A.I.; Petrón, M.J.; Adámez, J.D.; López, M.; Timón, M.L. Food by-products as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Sci. 2017, 129, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baaka, N.; Ben Ticha, M.; Haddar, W.; Amorim, M.T.P.; Mhenni, M.F. Upgrading of uv protection properties of several textile fabrics by their dyeing with grape pomace colorants. Fibers Polym. 2018, 19, 307–312. [Google Scholar] [CrossRef]
- Karaboyacı, M.; Uğur, Ş.S. Ecological wool dyeing with pulps of lavender, broom, and red wine. J. Text. Inst. 2014, 105, 821–827. [Google Scholar] [CrossRef]
- Mansour, R.; Ezzili, B.; Farouk, M. The use of response surface method to optimize the extraction of natural dye from winery waste in textile dyeing. J. Text. Inst. 2017, 108, 528–537. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent ph indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Liang, S.; Wang, L. A natural antibacterial-antioxidant film from soy protein isolate incorporated with cortex phellodendron extract. Polymers 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Qin, Y.; Bai, R.; Zhang, X.; Yuan, L.; Liu, J. Preparation of ph-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract. Int. J. Biol. Macromol. 2019, 134, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Kurek, M.; Hlupić, L.; Elez Garofulić, I.; Descours, E.; Ščetar, M.; Galić, K. Comparison of protective supports and antioxidative capacity of two bio-based films with revalorised fruit pomaces extracted from blueberry and red grape skin. Food Packag. Shelf Life 2019, 20, 100315. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.; Liu, J.; Liu, J. Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and characterization of antioxidant, antimicrobial and ph-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from lycium ruthenicum murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- Xu, Y.; Willis, S.; Jordan, K.; Sismour, E. Chitosan nanocomposite films incorporating cellulose nanocrystals and grape pomace extracts. Packag. Technol. Sci. 2018, 31, 631–638. [Google Scholar] [CrossRef]
- Vidal, G.; Hormazabal, S. Las Fibras Vegetales y sus Aplicaciones. Innovación en su Generación a Partir de la Depuración de Agua; Universidad de Concepción: Concepción, Chile, 2016. [Google Scholar]
- Yashas Gowda, T.G.; Sanjay, M.R.; Subrahmanya Bhat, K.; Madhu, P.; Senthamaraikannan, P.; Yogesha, B. Polymer matrix-natural fiber composites: An overview. Cogent Eng. 2018, 5, 1446667. [Google Scholar] [CrossRef]
- Ivanovic, J.; Milovanovic, S.; Stamenic, M.; Fanovich, M.A.; Jaeger, P.; Zizovic, I. Application of an integrated supercritical extraction and impregnation process for incorporation of thyme extracts into different carriers. In Handbook on Supercritical Fluids: Fundamentals, Properties and Applications; Osborne, J., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 258–280. [Google Scholar]
- Binti Mohd Nor Hamin, N.S.; Sahadan, M.Y.; Rozman, N.A.S.; Wen Nee, T.; Woei Yenn, T.; Zahan, K.A.; Suzana, W.; Chean Ring, L. Development of medical cotton fabrics with punica granatum l extract finishing for nosocomial infections control. J. Nat. Fibers 2019, 16, 404–411. [Google Scholar] [CrossRef]
- Sharaf, S.; Higazy, A.; Hebeish, A. Propolis induced antibacterial activity and other technical properties of cotton textiles. Int. J. Biol. Macromol. 2013, 59, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Studer, J.; Dransfeld, C.; Jauregui Cano, J.; Keller, A.; Wink, M.; Masania, K.; Fiedler, B. Effect of fabric architecture, compaction and permeability on through thickness thermoplastic melt impregnation. Compos. Part A Appl. Sci. Manuf. 2019, 122, 45–53. [Google Scholar] [CrossRef]
- Lau, K.-t.; Hung, P.-y.; Zhu, M.-H.; Hui, D. Properties of natural fibre composites for structural engineering applications. Compos. Part B Eng. 2018, 136, 222–233. [Google Scholar] [CrossRef]
- Majeed, K.; Jawaid, M.; Hassan, A.; Abu Bakar, A.; Abdul Khalil, H.P.S.; Salema, A.A.; Inuwa, I. Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater. Des. 2013, 46, 391–410. [Google Scholar] [CrossRef]
- Chan, K.-Y.; Jia, B.; Lin, H.; Hameed, N.; Lee, J.-H.; Lau, K.-T. A critical review on multifunctional composites as structural capacitors for energy storage. Compos. Struct. 2018, 188, 126–142. [Google Scholar] [CrossRef]
- Jawaid, M.; Khalil, H.P.S.A.; Bakar, A.A.; Khanam, P.N. Chemical resistance, void content and tensile properties of oil palm/jute fibre reinforced polymer hybrid composites. Mater. Des. 2011, 32, 1014–1019. [Google Scholar] [CrossRef]
- Gangopadhyay, U.K.; Mathur, M.R.; Singh, R.P. Moisture managing technical textiles for packaging of horticultural products. Man-Made Text. India 2018, 46, 259–262. [Google Scholar]
- Chatterjee, A.; Kumar, S.; Singh, H. Tensile strength and thermal behavior of jute fibre reinforced polypropylene laminate composite. Compos. Commun. 2020, 22, 100483. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical impregnation of food packaging films to provide antioxidant properties. J. Supercrit. Fluids 2017, 128, 200–207. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Ivanovic, J.; Zizovic, I.; Misic, D.; Jaeger, P. Functionalization of polycaprolactone/hydroxyapatite scaffolds with usnea lethariiformis extract by using supercritical co2. Mater. Sci. Eng. C 2016, 58, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, S.; Tadic, V.; Ivanovic, J.; Radmanovic, T.; Milovanovic, S.; Stankovic, M.; Zizovic, I. Utilization of the integrated process of supercritical extraction and impregnation for incorporation of helichrysum italicum extract into corn starch xerogel. Chem. Ind. Chem. Eng. Q. 2018, 24, 191–200. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical co2 impregnation of pla/pcl films with natural substances for bacterial growth control in food packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.R.; Ferreira, S.R.S.; Carciofi, B.A.M. High pressure carbon dioxide for impregnation of clove essential oil in lldpe films. Innov. Food Sci. Emerg. Technol. 2017, 41, 206–215. [Google Scholar] [CrossRef]
- Medeiros, G.R.; Guimarães, C.; Ferreira, S.R.S.; Carciofi, B.A.M. Thermomechanical and transport properties of lldpe films impregnated with clove essential oil by high-pressure CO2. J. Supercrit. Fluids 2018, 139, 8–18. [Google Scholar] [CrossRef]
- Almeida, A.P.; Rodríguez-Rojo, S.; Serra, A.T.; Vila-Real, H.; Simplicio, A.L.; Delgadilho, I.; Beirão Da Costa, S.; Beirão Da Costa, L.; Nogueira, I.D.; Duarte, C.M.M. Microencapsulation of oregano essential oil in starch-based materials using supercritical fluid technology. Innov. Food Sci. Emerg. Technol. 2013, 20, 140–145. [Google Scholar] [CrossRef]
- Pires, F.C.S.; e Silva, A.P.D.S.; Salazar, M.D.L.A.R.; da Costa, W.A.; da Costa, H.S.C.; Lopes, A.S.; Rogez, H.; de Carvalho Junior, R.N. Determination of process parameters and bioactive properties of the murici pulp (byrsonima crassifolia) extracts obtained by supercritical extraction. J. Supercrit. Fluids 2019, 146, 128–135. [Google Scholar] [CrossRef]
- Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; De La Ossa, E.J.M. Green extraction of antioxidants from different varieties of red grape pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; de la Ossa, E.J.M. Impregnation of mango leaf extract into a polyester textile using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (aai) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.T.; Mantell Serrano, C.M.; de la Ossa-Fernández, E.J. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into pet/pp food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- de Peredo AV, G.; Vázquez-Espinosa, M.; Piñeiro, Z.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Development of a rapid and accurate uhplc-pda-fl method for the quantification of phenolic compounds in grapes. Food Chem. 2021, 334, 127569. [Google Scholar] [CrossRef] [PubMed]
- Tamires Vitor Pereira, D.; Vollet Marson, G.; Fernández Barbero, G.; Gadioli Tarone, A.; Baú Betim Cazarin, C.; Dupas Hubinger, M.; Martínez, J. Concentration of bioactive compounds from grape marc using pressurized liquid extraction followed by integrated membrane processes. Sep. Purif. Technol. 2020, 250, 117206. [Google Scholar] [CrossRef]
- Pöhler, H.; Kiran, E. Volumetric properties of carbon dioxide + ethanol at high pressures. J. Chem. Eng. Data 1997, 42, 384–388. [Google Scholar] [CrossRef]
- Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E. A screening analysis of the high-pressure extraction of anthocyanins from red grape pomace with carbon dioxide and cosolvent. Eng. Life Sci. 2003, 3, 38–42. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Sannita, E.; Perego, P. High-pressure high-temperature extraction of phenolic compounds from grape skins. Int. J. Food Sci. Technol. 2012, 47, 399–405. [Google Scholar] [CrossRef]
- Luque-Rodríguez, J.M.; Luque de Castro, M.D.; Pérez-Juan, P. Dynamic superheated liquid extraction of anthocyanins and other phenolics from red grape skins of winemaking residues. Bioresour. Technol. 2007, 98, 2705–2713. [Google Scholar]
- Simonetti, G.; Brasili, E. Antifungal activity of phenolic and polyphenolic compounds from different matrices of Vitis vinifera L. Against human pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.E.-D.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- Kitrytė, V.; Kavaliauskaitė, A.; Tamkutė, L.; Pukalskienė, M.; Syrpas, M.; Rimantas Venskutonis, P. Zero waste biorefining of lingonberry (Vaccinium vitis-idaea L.) pomace into functional ingredients by consecutive high pressure and enzyme assisted extractions with green solvents. Food Chem. 2020, 322, 126767. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.H.; Baggio Ribeiro, D.H.; Micke, G.A.; Vitali, L.; Hense, H. Extraction of bioactive compounds from feijoa (acca sellowiana (o. Berg) burret) peel by low and high-pressure techniques. J. Supercrit. Fluids 2019, 145, 219–227. [Google Scholar] [CrossRef]
- del Valle, J.M.; Martín, Á.; Cocero, M.J.; de la Fuente, J.C.; de la Cruz-Quiroz, R. Supercritical co2 extraction of solids using aqueous ethanol as static modifier is a two-step mass transfer process. J. Supercrit. Fluids 2019, 143, 179–190. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Alarcón, M.; Marchante, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Extraction of natural flavorings with antioxidant capacity from cooperage by-products by green extraction procedure with subcritical fluids. Ind. Crops Prod. 2017, 103, 222–232. [Google Scholar] [CrossRef]
- Ghai, I.; Ghai, S. Understanding antibiotic resistance via outer membrane permeability. Infect. Drug Resist. 2018, 11, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.G.P.; Porto, E.; Corrêa, C.B.; Alencar, S.M.; Gloria, E.M.; Cabral, I.S.R.; Aquino, L.M. Antimicrobial potential and chemical composition of agro-industrial wastes. J. Natural Prod. 2012, 5, 27–36. [Google Scholar]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef]
- Zandoná, G.P.; Bagatini, L.; Woloszyn, N.; de Souza Cardoso, J.; Hoffmann, J.F.; Moroni, L.S.; Stefanello, F.M.; Junges, A.; Rombaldi, C.V. Extraction and characterization of phytochemical compounds from araçazeiro (Psidium cattleianum) leaf: Putative antioxidant and antimicrobial properties. Food Res. Int. 2020, 137, 109573. [Google Scholar] [CrossRef] [PubMed]
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J.; Bigger, S.W. Effect of supercritical co2 and olive leaf extract on the structural, thermal and mechanical properties of an impregnated food packaging film. J. Supercrit. Fluids 2019, 145, 181–191. [Google Scholar] [CrossRef]
- García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; Martínez de la Ossa, E.J. Impregnation of mesoporous silica with mangiferin using supercritical co2. J. Supercrit. Fluids 2018, 140, 129–136. [Google Scholar] [CrossRef]
- Kühn, S.; Temelli, F. Recovery of bioactive compounds from cranberry pomace using ternary mixtures of co2+ethanol+water. J. Supercrit. Fluids 2017, 130, 147–155. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical co2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Wenzel, J.E.; Moorman, V.; Wang, L.; Spencer-Williams, I.; Hall, M.; Samaniego, C.S.; Ammerman, M.L. In-situ extraction and impregnation of black walnut husk into polyethylene film using supercritical carbon dioxide with an ethanol modifier. Food Sci. Nutr. 2020, 8, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical impregnation of olive leaf extract to obtain bioactive films effective in cherry tomato preservation. Food Packag. Shelf Life 2019, 21, 100338. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gonçalves, S. Extraction of antioxidants from winemaking byproducts: Effect of the solvent on phenolic composition, antioxidant and anti-cholinesterase activities, and electrochemical behaviour. Antioxidants 2020, 9, 675. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Implementation of subcritical water extraction with natural deep eutectic solvents for sustainable extraction of phenolic compounds from winemaking by-products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef]
- Buratto, R.T.; Hoyos, E.G.; Cocero, M.J.; Martín, Á. Impregnation of açaí residue extracts in silica-aerogel. J. Supercrit. Fluids 2019, 146, 120–127. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Fouad, M.T.; Saleh, H.H.; Kholif, A.E.; Morsy, T.A. Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; León-González, M.E.; Ferreira, I.C.F.R.; Barros, L. Valorisation of black mulberry and grape seeds: Chemical characterization and bioactive potential. Food Chem. 2021, 337, 127998. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ponce, M.T.; Medina-Ruiz, E.; Casas, L.; Mantell, C.; Martínez de la Ossa-Fernández, E.J. Development of cotton fabric impregnated with antioxidant mango polyphenols by means of supercritical fluids. J. Supercrit. Fluids 2018, 140, 310–319. [Google Scholar] [CrossRef]



| Reagent | Supplier |
|---|---|
| Carbon dioxide (99.99%) | Abello-Linde S.A. (Barcelona, Spain). |
| 2,2-diphenyl-1-picrylhydrazyl (DPPH) | Sigma-Aldrich (Steinheim, Germany) |
| Lennox LB agar | Conda Laboratories (Torrejón de Ardoz, Spain) |
| Dimethyl sulfoxide (DMSO) | Panreac (Barcelona, Spain) |
| Phenolic standards (gallic acid, quercetin and cyanidin) | Sigma-Aldrich (Steinheim, Germany) |
| Lysogenic Broth (LB) with 10 g/L tryptone, 5 g/L NaCl and 5 g/L yeast extract | Sigma-Aldrich (Steinheim, Germany) |
| Escherichia coli (CECT101) | Spanish Type Culture Collection (CECT, Valencia, Spain) |
| Pseudomonas aeruginosa (ATCC 9027) | Microbiologics Inc. (Saint Cloud, MN, USA) |
| Staphylococcus aureus (ATCC 6538) | Microbiologics Inc. (Saint Cloud, MN, USA) |
| Experiment | P (MPa) | T (°C) | Dried RGPE (mg) * | % RGPE | % Modifier (v/v) ** (C2H5OH:H2O) | Molar Ratio (n CO2/n Modifier) | Modifier Volume (mL) |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 55 | 28.8 | 9.6 | 2.8 | 6.88 | 3 |
| 50 | 20.09 | ||||||
| 2 | 50 | 55 | 28.8 | 9.6 | 2.8 | 20.09 | 3 |
| 46.1 | 4.8 | 10.73 | 5 | ||||
| 67.2 | 6.7 | 7.89 | 7 | ||||
| 3 | 50 | 55 | 270 | 90 | 2.8 | 20.09 | 3 |
| Bacteria | MIC RGPE (mg/mL) | Concentration of RGPE in Liquid Medium (mg/L) | % Inhibition in RGPE-Jute |
|---|---|---|---|
| Escherichia coli | 12.0 | 0.07 | 8.250 ± 0.496 |
| 0.14 | 14.877 ± 1.385 | ||
| Pseudomonas aeruginosa | 4.0 | 0.07 | 26.045 ± 3.007 |
| 0.14 | 35.471 ± 1.516 | ||
| Staphylococcus aureus | 1.5 | 0.07 | 33.234 ± 1.083 |
| 0.14 | 42.605 ± 1.062 |
| RT | Mass (Da) | RGPE (µg/mL) | IJF (µg/mL) | % Impregnation * | |
|---|---|---|---|---|---|
| Phenolic acids | |||||
| Protocatechuic acid | 0.38 | 153.0188 | 1.45 ± 0.09 | 5.38 ± 0.71 | 6.60 ± 0.47 |
| Caffeic acid | 1.81 | 179.0344 | 0.63 ± 0.90 | nd | nd |
| p-coumaric acid | 1.95 | 163.0395 | 1.27 ± 0.50 | 22.56 ± 3.46 | 33.08 ± 8.19 |
| Syringic acid | 1.98 | 197.0450 | 17.70 ± 1.35 | 22.73 ± 1.31 | 2.29 ± 0.31 |
| Flavanols | |||||
| Catechin | 2.59 | 289.0712 | 0.73 ± 0.04 | 20.08 ± 1.69 | 48.77 ± 1.63 |
| Flavonols | |||||
| Rutin | 1.94 | 609.1456 | 10.03 ± 3.60 | nd | nd |
| Quercetin 3-glucoside | 1.98 | 463.0877 | 43.62 ± 7.90 | nd | nd |
| Quercetin | 2.31 | 301.0348 | 20.12 ± 1.26 | nd | nd |
| Anthocyanins | |||||
| Delphinidin-3-O-glucoside | 1.98 | 463.0877 | 86.28 ± 2.04 | nd | nd |
| Petunidin-3-O-glucoside | 2.07 | 477.1033 | 20.99 ± 4.89 | nd | nd |
| Delphinidin 3-O-(6′′-acetyl)-glucoside | 2.15 | 505.0982 | 8.27 ± 0.30 | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cejudo-Bastante, C.; Arjona-Mudarra, P.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J.; Pereyra, C. Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging. Antioxidants 2021, 10, 216. https://doi.org/10.3390/antiox10020216
Cejudo-Bastante C, Arjona-Mudarra P, Fernández-Ponce MT, Casas L, Mantell C, Martínez de la Ossa EJ, Pereyra C. Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging. Antioxidants. 2021; 10(2):216. https://doi.org/10.3390/antiox10020216
Chicago/Turabian StyleCejudo-Bastante, Cristina, Paloma Arjona-Mudarra, María Teresa Fernández-Ponce, Lourdes Casas, Casimiro Mantell, Enrique J. Martínez de la Ossa, and Clara Pereyra. 2021. "Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging" Antioxidants 10, no. 2: 216. https://doi.org/10.3390/antiox10020216
APA StyleCejudo-Bastante, C., Arjona-Mudarra, P., Fernández-Ponce, M. T., Casas, L., Mantell, C., Martínez de la Ossa, E. J., & Pereyra, C. (2021). Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging. Antioxidants, 10(2), 216. https://doi.org/10.3390/antiox10020216