Identifying a Role of Red and White Wine Extracts in Counteracting Skin Aging: Effects of Antioxidants on Fibroblast Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Wine Extracts: Spray Dryer
2.2. Preparation of Wine Extracts: Rotavapor
2.3. HPLC Analysis
2.4. MTT Viability Assay
2.5. Antioxidant Activity
2.6. β-Galactosidase Senescence Assay
2.7. Statistical Analysis
3. Results
3.1. Wine Extraction by Rotavapor Improves Quality of Extraction Maintaining the Phenolic Profiles of Wines
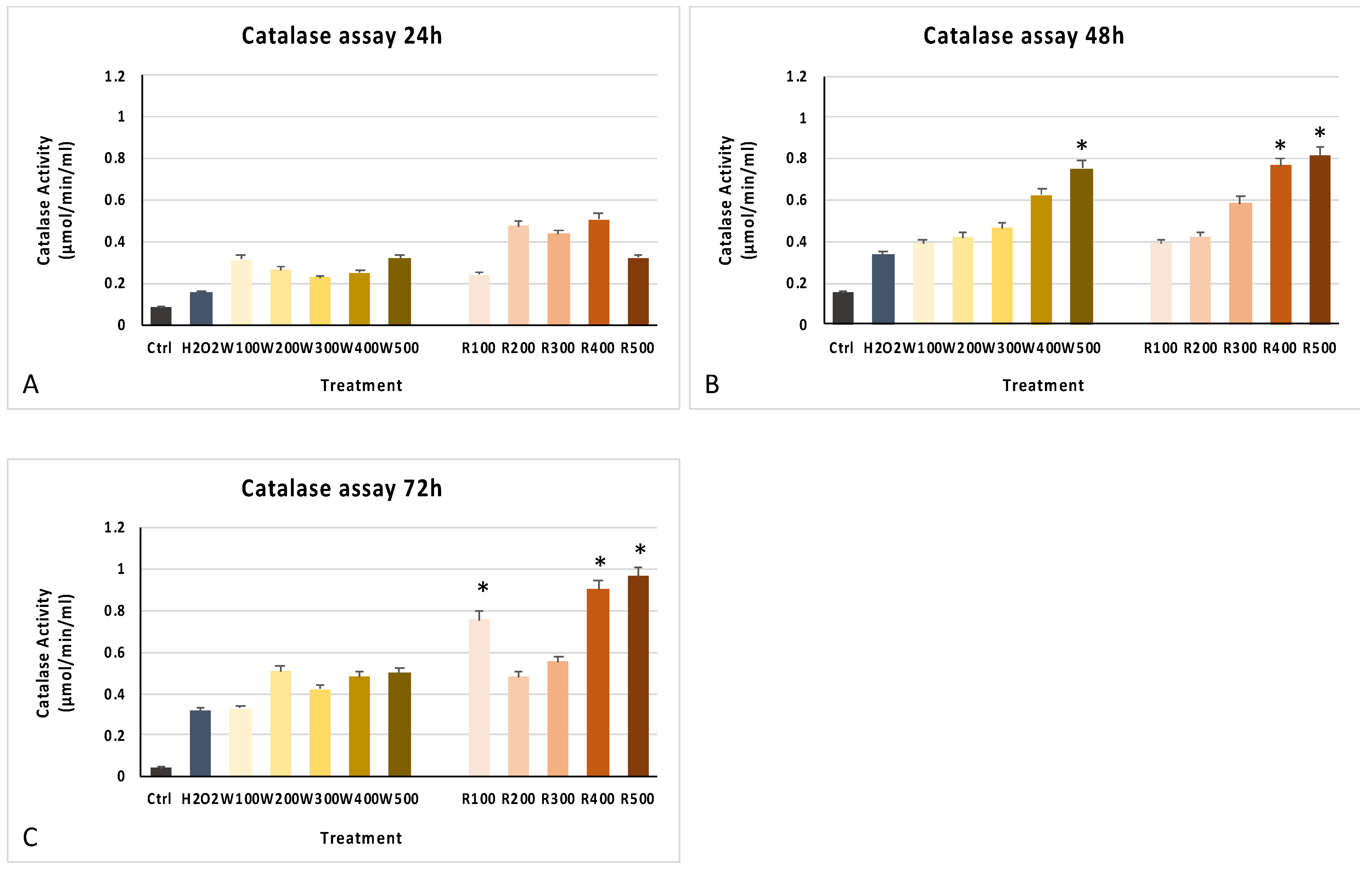
3.2. Wine Extracts Improve Cell Viability and Antioxidant Response
3.3. The Wine Extracts Counteract Cellular Aging, Despite Exposure to a Strong Stressful Event
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol. Alergol. 2016, 33, 1. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Jemec, G.B.E. Mechanical properties and barrier function of healthy human skin. Acta Derm. Venereol. 2006, 86, 308–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, J.; Lee, M.G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006, 52, 24–35. [Google Scholar]
- Wang, Z.; Man, M.Q.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-associated alterations in epidermal function and their clinical significance. Aging 2020, 12, 5551. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. J. Signal Transduct. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Murat, U.; Md, S.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. WAO J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Shim, S.Y.; Kim, H.S. Oxidative stress and the antioxidant enzyme system in the developing brain. Korean J. Pediatrics 2013, 56, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxidative Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, M.A.; Aldosari, S.R.; Abid, M.R. Genetic Alterations in Oxidant and Anti-Oxidant Enzymes in the Vascular System. Front. Cardiovasc. Med. 2018, 5, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- O’Byrne, P. Red Wine and Health; Chapter: 6-Biogenic Amines in Wine: A Review; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009. [Google Scholar]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Boix, N.; Piqué, E.; Gómez-Catalán, J.; Remon, A.M.; Sasot, G.; Mercader-Martí, M.; Mallafre, J.M.L.; Lamuela-Raventós, R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food Chem. 2015, 181, 146–151. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Pandel, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Dermatol. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.E.A.; Pinto, C.A.S.D.O.; De Oliveira, A.C.; Velasco, M.V.R.; Guitiérrez, A.R.G.; Rafael, M.F.C.; Tarazona, J.P.H.; Retuerto-Figueroa, M.G. Contribution of topical antioxidants to maintain healthy skin—A review. Sci. Pharm. 2020, 88, 27. [Google Scholar] [CrossRef]
- D’Urso, G.; Montoro, P.; Piacente, S. Detection and comparison of phenolic compounds in different extracts of black currant leaves by liquid chromatography coupled with high-resolution ESI-LTQ-Orbitrap MS and high-sensitivity ESI-Qtrap MS. J. Pharm. Biomed. Anal. 2020, 179, 112926. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Maioli, M.; Basoli, V.; Carta, P.; Fabbri, D.; Dettori, M.A.; Cruciani, S.; Serra, P.A.; Delogu, G. Synthesis of magnolol and honokiol derivatives and their effect against hepatocarcinoma cells. PLoS ONE 2018, 13, e0192178. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bacun-Druzina, V.; Komes, D. Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–44. [Google Scholar]
- Ferhi, S.; Santaniello, S.; Zerizer, S.; Cruciani, S.; Fadda, A.; Sanna, D.; Dore, A.; Maioli, M.; D’Hallewin, G. Total Phenols from Grape Leaves Counteract Cell Proliferation and Modulate Apoptosis-Related Gene Expression in MCF-7 and HepG2 Human Cancer Cell Lines. Molecules 2019, 24, 612. [Google Scholar] [CrossRef] [Green Version]
- Cruciani, S.; Santaniello, S.; Garroni, G.; Fadda, A.; Balzano, F.; Bellu, E.; Maioli, M. Myrtus Polyphenols, from Antioxidants to Anti-Inflammatory Molecules: Exploring a Network Involving Cytochromes P450 and Vitamin D. Molecules 2019, 24, 1515. [Google Scholar] [CrossRef] [Green Version]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Da Luz, P.L.; Coimbra, S.R. Wine, alcohol and atherosclerosis: Clinical evidences and mechanisms. Braz. J. Med Biol. Res. 2004, 37, 1275–1295. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 683. [Google Scholar] [PubMed]
- Khan, M.I.; Ahhmed, A.; Shin, J.H.; Baek, J.S.; Kim, M.Y.; Kim, J.D. Green Tea Seed Isolated Saponins Exerts Antibacterial Effects against Various Strains of Gram Positive and Gram Negative Bacteria, a Comprehensive Study in Vitro and in Vivo. Evid. Based Complement. Altern. Med. 2018, 2018, 3486106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid. Based Complement. Altern. Med. 2020, 2020, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndiaye, M.; Philippe, C.; Mukhtar, H.; Ahmad, N. The grape antioxidant resveratrol for skin disorders: Promise, prospects, and challenges. Arch. Biochem. Biophys. 2011, 508, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannelli, L.; Pitozzi, V.; Jacomelli, M.; Mulinacci, N.; Laurenzana, A.; Dolara, P.; Mocali, A. Protective effects of resveratrol against senescence-associated changes in cultured human fibroblasts. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 66, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Lila, M.A. Anthocyanins and human health: An in vitro investigative approach. J. Biomed. Biotechnol. 2004, 2004, 306–313. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Winter, A.N.; Bickford, P.C. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef] [Green Version]
- Sitarek, P.; Merecz-Sadowska, A.; Kowalczyk, T.; Wieczfinska, J.; Zajdel, R.; Śliwiński, T. Potential synergistic action of bioactive compounds from plant extracts against skin infecting microorganisms. Int. J. Mol. Sci. 2020, 21, 5105. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Idelchik, M.d.S.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Soelaiman, I.-N.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and Anti-Inflammatory Properties of Anthocyanins Extracted from Oryza sativa L. in Primary Dermal Fibroblasts. Oxid. Med. Cell. Longev. 2019, 2019, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysocki, A.B. Skin anatomy, physiology, and pathophysiology. Nurs. Clin. N. Am. 1999, 34, 777–797. [Google Scholar] [PubMed]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflamm. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Obrenovich, M.E.; Nair, N.G.; Beyaz, A.; Aliev, G.; Reddy, V.P. The role of polyphenolic antioxidants in health, disease, and aging. Rejuvenation Res. 2010, 13, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Byun, H.O.; Lee, Y.K.; Kim, J.M.; Yoon, G. From cell senescence to age-related diseases: Differential mechanisms of action of senescence-associated secretory phenotypes. BMB Rep. 2015, 48, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bloom, S.I.; Donato, A.J. The role of senescence, telomere dysfunction and shelterin in vascular aging. Microcirculation 2019, 26, e12487. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, S.; Santaniello, S.; Fadda, A.; Sale, L.; Sarais, G.; Sanna, D.; Mulas, M.; Ginesu, G.C.; Cossu, M.L.; Serra, P.A.; et al. Extracts from Myrtle Liqueur Processing Waste Modulate Stem Cells Pluripotency under Stressing Conditions. Biomed Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruciani, S.; Garroni, G.; Ginesu, G.C.; Fadda, A.; Ventura, C.; Maioli, M. Unravelling cellular mechanisms of stem cell senescence: An aid from natural bioactive molecules. Biology 2020, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, H.N.; Hardman, M.J. Senescence in Wound Repair: Emerging Strategies to Target Chronic Healing Wounds. Front. Cell Dev. Biol. 2020, 8, 773. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- And Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Escribano, A.; Amor, M.; Pastor, S.; Castillo, S.; Sanz, F.; Codoñer-Franch, P.; Dasí, F. Decreased glutathione and low catalase activity contribute to oxidative stress in children with α-1 antitrypsin deficiency. Thorax 2014, 70, 82–83. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.; Lee, J.; Huh, J.Y.; Park, J.; Lee, H.B.; Ho, Y.-S.; Ha, H. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes 2012, 61, 728–738. [Google Scholar] [CrossRef] [Green Version]
- Matés, J.M.; Pérez-Gómez, C.; de Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar]




| tR (min) | Compound | Formula | Precursor Ion [M−H]−m/z | Dppm | Fragments [M−H]−m/z | |
|---|---|---|---|---|---|---|
| 1 | 4.49 | Gallic acid | C7H6O5 | 169.0141 | 0.93 | 125 |
| 2 | 4.96 | Procyanidin trimer | C45H38O18 | 865.1975 | 0.08 | 847/739/695/577 |
| 3 | 5.81 | Protocatechuic acid | C7H6O4 | 153.0187 | 4.5 | 109 |
| 4 | 6.42 | Quercetin-3-O-galactoside | C21H20O12 | 463.0863 | −1.797 | 301 |
| 5 | 7.24 | Procyanidin B1 | C30H26O12 | 577.1339 | −0.352 | 451/425/289 |
| 6 | 7.47 | Caftaric acid | C13H12O9 | 311.0405 | 2.481 | 179 |
| 7 | 7.50 | p-Coumaric acid | C9H8O3 | 163.0399 | 5.016 | 119 |
| 8 | 8.29 | Procyanidin trimer | C45H38O18 | 865.1975 | −0.694 | 847/739/695/577 |
| 9 | 8.41 | Catechin | C15H14O6 | 289.0711 | 1.541 | 245/205 |
| 10 | 8.65 | Fertaric acid | C14H14O9 | 325.0562 | 2.374 | 193 |
| 11 | 8.65 | Vanillic acid | C8H8O4 | 167.0347 | 4.758 | 152/123 |
| 12 | 8.76 | Procyanidin B2 | C30H26O12 | 577.1345 | 1.018 | 451/425/289 |
| 13 | 9.01 | Coutaric acid | C13H12O8 | 295.0454 | 2.021 | 163 |
| 14 | 9.35 | Epicatechin | C15H14O6 | 289.0711 | 1.541 | 245/205 |
| 15 | 9.37 | Ferulic acid | C10H10O4 | 193.0503 | 4.065 | 178/149 |
| 16 | 9.47 | Rutin | C27H30O16 | 609.1453 | 0.409 | 301 |
| 17 | 9.59 | Caffeic acid | C9H8O4 | 179.0347 | 4.349 | 135 |
| 18 | 10.05 | Myricetin-3-O-glucoside | C21H20O13 | 479.0819 | −0.023 | 317 |
| 19 | 10.27 | Myricetin-3-O-glucuronide | C21H18O14 | 493.0612 | −0.064 | 317 |
| 20 | 11.00 | Kaempferol-3-O-galactoside | C21H20O11 | 447.0921 | −0.263 | 285 |
| 21 | 11.09 | Quercetin-3-O-glucoside | C21H20O12 | 463.0874 | 0.578 | 301 |
| 22 | 11.09 | Quercetin-3-O-Rhamnoside | C21H20O11 | 447.0921 | −0.263 | 301 |
| 23 | 11.31 | Syringic acid | C9H10O5 | 197.0453 | 4.413 | 182/153 |
| 24 | 11.32 | trans-Resveratrol glucoside | C20H22O8 | 389.1233 | 0.427 | 227 |
| 25 | 11.35 | Quercetin glucuronide | C21H18O13 | 477.0665 | 0.279 | 301 |
| 26 | 11.39 | Ethyl gallate | C9H10O5 | 197.0453 | 0.87 | 169 |
| 27 | 11.67 | astilbin | C21H22O11 | 449.1080 | 0.428 | 303 |
| 28 | 12.27 | Kaempferol-3-O-glucoside | C21H20O11 | 447.0921 | −0.263 | 285 |
| 29 | 12.63 | cis-Resveratrol glucoside | C20H22O8 | 389.1233 | 0.427 | 227 |
| 30 | 12.73 | trans Resveratrol | C14H12O3 | 227.0710 | 0.679 | 185 |
| 32 | 16.50 | cis-Resveratrol | C14H12O3 | 227.0706 | 0.319 | 185 |
| 33 | 16.86 | dimetilcaffeic acid | C11H12O4 | 207.0660 | 0.795 | 179 |
| tR (min) | Compound | Formula | Precursor Ion [M−H]−m/z | Dppm | Fragments [M−H]−m/z | |
|---|---|---|---|---|---|---|
| 1 | 4.48 | Gallic acid | C7H6O5 | 169.0135 | 891 | 125 |
| 2 | 4.93 | Procyanidin trimer | C45H38O18 | 865.1984 | 0.855 | 847/739/695/577 |
| 3 | 6.98 | Protocatechuic acid | C7H6O4 | 153.0192 | 5371 | 109 |
| 4 | 7.22 | Procyanidin B1 | C30H26O12 | 577.1340 | −0.039 | 451/425/289 |
| 5 | 7.59 | Caftaric acid | C13H12O9 | 311.0403 | 1.581 | 179 |
| 6 | 8.41 | Procyanidin trimer 4 | C45H38O18 | 865.1984 | 0.855 | 847/739/695/577 |
| 7 | 8.41 | Catechin | C15H14O6 | 289.0710 | 1.299 | 245/205 |
| 8 | 8.77 | Procyanidin B2 | C30H26O12 | 577.1340 | −0.039 | 451/425/289 |
| 9 | 8.77 | Coutaric acid | C13H12O8 | 295.0451 | 0.767 | 163 |
| 10 | 9.49 | Rutin | C27H30O16 | 609.1451 | −0.593 | 301 |
| 11 | 9.37 | Epicatechin | C15H14O6 | 289.0710 | 1299 | 245/205 |
| 12 | 9.37 | Ferulic acid | C10H10O4 | 193.0505 | 5204 | 178/149 |
| 13 | 9.37 | Fertaric acid | C14H14O9 | 325.0558 | 1.174 | 193 |
| 14 | 9.61 | Caffeic acid | C9H8O4 | 179.0346 | 4.048 | 135 |
| 15 | 11.13 | Kaempferol galactoside | C21H20O11 | 447.0920 | −0.465 | 285 |
| 16 | 11.24 | Quercetin-3-O-glucoside | C21H20O12 | 463.0869 | −0.48 | 301 |
| 17 | 11.27 | Syringic acid | C9H10O5 | 197.0452 | 3.655 | 182/153 |
| 18 | 11.34 | trans-Resveratrol glucoside | C20H22O8 | 389.1233 | 0.504 | 227 |
| 19 | 11.72 | Astilbin | C21H22O11 | 449.1078 | −0.04 | 303 |
| 20 | 12.43 | Quercetin-3-O-Rhamnoside | C21H20O11 | 447.0920 | −0.465 | 301 |
| 21 | 12.55 | cis-Resveratrol glucoside | C20H22O8 | 389.1233 | 0.504 | 227 |
| 22 | 12.65 | trans-Resveratrol | C14H12O3 | 227.0706 | 4136 | 185 |
| 23 | 13.03 | Kaempferol-3-O-glucoside | C21H20O11 | 447.0920 | −0.465 | 285 |
| 24 | 16.8 | Kaempferol | C15H10O6 | 285.0395 | 0.335 | 257 |
| 25 | 16.61 | cis-Resveratrol | C14H12O3 | 227.0706 | 3080 | 185 |
| 26 | 16.84 | dimetilcaffeic acid | C11H12O4 | 207.0657 | 2.486 | 179 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruciani, S.; Trenta, M.; Rassu, G.; Garroni, G.; Petretto, G.L.; Ventura, C.; Maioli, M.; Pintore, G. Identifying a Role of Red and White Wine Extracts in Counteracting Skin Aging: Effects of Antioxidants on Fibroblast Behavior. Antioxidants 2021, 10, 227. https://doi.org/10.3390/antiox10020227
Cruciani S, Trenta M, Rassu G, Garroni G, Petretto GL, Ventura C, Maioli M, Pintore G. Identifying a Role of Red and White Wine Extracts in Counteracting Skin Aging: Effects of Antioxidants on Fibroblast Behavior. Antioxidants. 2021; 10(2):227. https://doi.org/10.3390/antiox10020227
Chicago/Turabian StyleCruciani, Sara, Margherita Trenta, Giovanna Rassu, Giuseppe Garroni, Giacomo Luigi Petretto, Carlo Ventura, Margherita Maioli, and Giorgio Pintore. 2021. "Identifying a Role of Red and White Wine Extracts in Counteracting Skin Aging: Effects of Antioxidants on Fibroblast Behavior" Antioxidants 10, no. 2: 227. https://doi.org/10.3390/antiox10020227
APA StyleCruciani, S., Trenta, M., Rassu, G., Garroni, G., Petretto, G. L., Ventura, C., Maioli, M., & Pintore, G. (2021). Identifying a Role of Red and White Wine Extracts in Counteracting Skin Aging: Effects of Antioxidants on Fibroblast Behavior. Antioxidants, 10(2), 227. https://doi.org/10.3390/antiox10020227









