Antioxidant, Pancreatic Lipase Inhibitory, and Tyrosinase Inhibitory Activities of Extracts of the Invasive Plant Spartina anglica (Cord-Grass)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Extraction and Fractionation
2.4. DPPH Free Radical-Scavenging Activity
2.5. ABTS Assay
2.6. Tyrosinase Inhibition Assay
2.7. Pancreatic Lipase Inhibition Assay
2.8. Measurement of Total Phenol Contents
2.9. Chemical Profiles of the Biologically Active Fractions (EA-a and MC-bg)
2.10. Isolation of Major Compounds from the Bioactive Fractions (EA-a and MC-bg)
2.11. Quantitative Analysis of the Major Compounds in EA-a and MC-bg
2.12. Statistical Analysis
3. Results
3.1. Biological Activities of the Extracts and Fractions of S. anglica
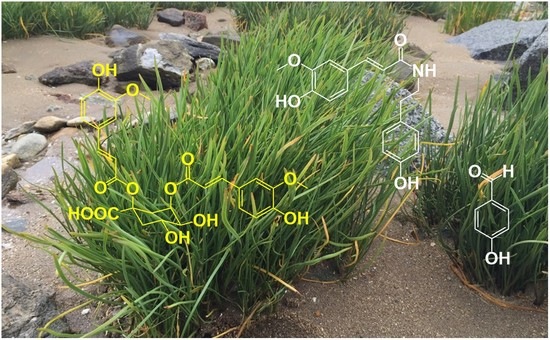
3.2. Structural Determination of the Major Compounds of Bioactive Fractions
3.3. Quantitative Analysis of Compounds 1 to 3 in EA-a and MC-bg
3.4. Biological Activities of Compounds 1 to 3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hubbard, J.C.E. Spartina marshes in southern England. IV. Pattern of invasive in Poole Harbour. J. Ecol. 1965, 53, 799–813. [Google Scholar] [CrossRef]
- Baumel, A.; Ainouche, M.L.; Misset, M.T.; Gourret, J.P.; Bayer, R. Genetic evidence for hybridization between the native Spartina maritima and the introduced Spartina alterniflora (Poaceae) in South-West France: Spartina x neyrautii re-examined. Plant Syst. Evol. 2003, 237, 87–97. [Google Scholar] [CrossRef]
- Millard, A.V.; Evans, P.R. Spartina Anglica in Great Britain. Focus on Nature Conservation, Liverpool University, United Kingdom, 10th November 1982; Doody, P., Ed.; Nature Conservancy Council: Cambridgeshire, UK, 1984; Volume 5, pp. 41–48. [Google Scholar]
- Shimeta, J.; Saint, L.; Verspaandonk, E.R.; Nugegoda, D.; Howe, S. Long-term ecological consequences of herbicide treatment to control the invasive grass, Spartina anglica, in an Australian saltmarsh. Estuar. Coast. Shelf Sci. 2016, 176, 58–66. [Google Scholar] [CrossRef]
- Otte, M.L.; Haarsma, M.S.; Broekman, R.A.; Rozema, J. Relation between heavy metal concentrations in salt marsh plants and soil. Environ. Pollut. 1993, 82, 13–22. [Google Scholar] [CrossRef]
- Thompson, J.D. The Biology of an Invasive Plant: What makes Spartina anglica so successful? BioScience 1991, 41, 393–401. [Google Scholar] [CrossRef]
- Strong, D.R.; Ayres, D.R. Ecological and evolutionary misadventures of Spartina. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 389–410. [Google Scholar] [CrossRef]
- Kim, E.K.; Kil, J.H.; Joo, Y.K.; Jung, Y.S. Distribution and botanical characteristics of unrecorded alien weed Spartina anglica in Korea. Weed Turfgrass Sci. 2015, 4, 65–70. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef]
- Baranowska, M.; Bartoszek, A. Antioxidant and antimicrobial properties of bioactive phytochemicals from cranberry. Postepy Hig. Med. Dosw. 2016, 70, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Djaoudene, O.; López, V.; Cásedas, G.; Les, F.; Schisano, C.; Bachir Bey, M.; Tenore, G.C. Phoenix dactylifera L. seeds: A by-product as a source of bioactive compounds with antioxidant and enzyme inhibitory properties. Food Funct. 2019, 10, 4953–4965. [Google Scholar] [CrossRef]
- Larher, F.; Hamelin, F.; Stewart, G.R. L’acide diméthylsulfonium-3 propanoïque de Spartina anglica. Phytochemistry 1977, 16, 2019–2020. [Google Scholar] [CrossRef]
- van Diggelen, J.; Rozema, J.; Dickson, D.M.J.; Broekman, R. Beta-3-dimethylsulphoniopropionate, proline and quaternary ammonium-compounds in Spartina anglica in relation to sodium chloride, nitrogen and sulfur. New Phytol. 1986, 103, 573–586. [Google Scholar] [CrossRef]
- Grignon-Dubois, M.; Echmak, C. First evaluation of the marine invasive species Spartina anglica as a potential renewable source of glycine betaine. J. Appl. Pharm. Sci. 2013, 3, 29–34. [Google Scholar]
- Li, Y.L.; Chen, J.H.; Song, X.K.; Sun, J.Y.; Zhan, Y.C. Optimization of microwave extraction of polysaccharides from Spartina anglica using RSM. Lishizhen Med. Mater. Med. Res. 2013, 24, 2126–2128. [Google Scholar]
- Xu, N.J.; Tang, J.; Zhang, Z.W.; Yan, X.J. Inhibitory effects of Spartina anglica on Heterosigma akashiwo and Prorocenrum micans and the isolation and identification of the algicidal compounds. Chin. J. Appl. Ecol. 2009, 20, 2563–2568. [Google Scholar]
- Sun, X.; Tang, J.; Hu, W.; Xu, N. Antioxidant flavonol compounds from the marine cordgrass Spartina anglica. Food Sci. Technol. Res. 2013, 19, 1093–1097. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Tanaka, T.; Kim, H.Y. Protective effects of Corni Fructus against advanced glycation endoproducts and radical scavenging. Evid. Based Complementary Altern. Med. 2012, 2012, 418953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.J.; Park, H.W.; Youn, S.H.; Choi, D.Y.; Shin, C.S. Development of inhibitors against lipase and α-glucosidase from derivatives of monascus pigment. FEMS Microbiol. Lett. 2007, 276, 93–98. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. On phosphotungtic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912, 12, 239–243. [Google Scholar] [CrossRef]
- Scholz-Böttcher, B.M.; Ernst, L.; Maier, H.G. New stereoisomers of quinic acid and their lactones. Liebigs Ann. Chem. 1991, 1991, 1029–1036. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kim, Y.; Jang, H.J.; Oh, H.M.; Lim, C.H.; Lee, S.W.; Rho, M.C. Study of the UV light conversion of feruloyl amides from Portulaca oleracea and their inhibitory effect on IL-6-induced STAT3 activation. Molecules 2016, 21, 865. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I. 1,5-Di-O-isoferuloylquinic acid and other phenolic compounds from pollen of Calendula officinalis. Chem. Nat. Compd. 2014, 50, 589–593. [Google Scholar] [CrossRef]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Kinst-Hori, I. Tyrosinase inhibitors from cumin. J. Agric. Food Chem. 1998, 46, 5338–5341. [Google Scholar] [CrossRef]
- Okombi, S.; Rival, D.; Bonnet, S.; Mariotte, A.M.; Perrier, E.; Boumendjel, A. Analogues of N-hydroxycinnamoylphenalkylamides as inhibitors of human melanocyte-tyrosinase. Bioorg. Med. Chem. Lett. 2006, 16, 2252–2255. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Ryu, J.H.; Yoon, K.D.; Shin, S.S. Effect of Aurea helianthus stem extract on anti-melanogenesis. Biosci. Biotech. Biochem. 2018, 82, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Adegbaju, O.D.; Otunola, G.A.; Afolayan, A.J. Effects of growth stage and seasons on the phytochemical content and antioxidant activities of crude extracts of Celosia argentea L. Heliyon 2020, 6, e04086. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Li, P. Effect of cultivation years on saponins in Paris Polyphylla var. yunnanensis using ultra-high liquid chromatography–tandem mass spectrometry and Fourier transform infrared spectroscopy. Plant. Growth Regul. 2018, 84, 373–381. [Google Scholar] [CrossRef]
- Wang, D.; Calabrese, E.J.; Lian, B.; Lin, Z.; Calabrese, V. Hormesis as a mechanistic approach to understanding herbal treatments in traditional Chinese medicine. Pharm. Ther. 2018, 184, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Hormetic and mitochondria-related mechanisms of antioxidant action of phytochemicals. Antioxidants 2019, 8, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]

 ); 1 (
); 1 (  ); 2 (
); 2 (  ); 3 (
); 3 (  )]: (a) DPPH radical-scavenging activity (control: l-ascorbic acid); (b) ABTS radical-scavenging activity (control: l-ascorbic acid); (c) Pancreatic lipase activity (control: orlistat).
)]: (a) DPPH radical-scavenging activity (control: l-ascorbic acid); (b) ABTS radical-scavenging activity (control: l-ascorbic acid); (c) Pancreatic lipase activity (control: orlistat).
 ); 1 (
); 1 (  ); 2 (
); 2 (  ); 3 (
); 3 (  )]: (a) DPPH radical-scavenging activity (control: l-ascorbic acid); (b) ABTS radical-scavenging activity (control: l-ascorbic acid); (c) Pancreatic lipase activity (control: orlistat).
)]: (a) DPPH radical-scavenging activity (control: l-ascorbic acid); (b) ABTS radical-scavenging activity (control: l-ascorbic acid); (c) Pancreatic lipase activity (control: orlistat).
| Sample | Radical-Scavenging Activity Against DPPH (%) | Radical-Scavenging Activity Against ABTS (%) | Inhibition of l-DOPA Oxidation (%) | Inhibition of Pancreatic Lipase (%) |
|---|---|---|---|---|
| Control | 98.5 ± 0.2 1 | 99.5 ± 0.3 1 | 98.6 ± 0.4 2 | 67.8 ± 0.0 3 |
| EtOH-a | 18.5 ± 4.4 | 39.4 ± 0.4 | 30.4 ± 1.0 | 47.7 ± 0.4 |
| Hex-a | 2.0 ± 0.6 | 5.9 ± 0.5 | 36.8 ± 0.6 | 36.6 ±0.3 |
| MC-a | 38.4 ± 2.7 | 58.8 ± 0.8 | 38.2 ± 1.5 | 38.4 ± 0.5 |
| EA-a | 55.9 ± 0.6 | 68.8 ± 0.3 | 43.9 ± 0.5 | 51.2 ± 0.5 |
| BuOH-a | 41.7 ± 1.4 | 47.3 ± 0.3 | 42.5 ± 1.2 | 32.7 ± 0.7 |
| H2O-a | 8.3 ± 1.8 | 11.8 ± 0.4 | 44.0 ± 1.6 | 22.7 ± 0.8 |
| EtOH-bg | 11.1 ± 1.4 | 19.6 ± 0.5 | 29.5 ± 0.5 | 49.5 ± 0.5 |
| Hex-bg | 6.7 ± 1.2 | 10.2 ± 0.7 | 67.9 ± 0.3 | 46.0 ± 1.2 |
| MC-bg | 38.1 ± 1.5 | 50.1 ± 0.4 | 85.0 ± 1.8 | 33.3 ± 0.8 |
| EA-bg | 28.6 ± 1.1 | 44.2 ± 1.2 | 69.4 ± 0.8 | 38.6 ± 0.9 |
| BuOH-bg | 5.0 ± 6.2 | 12.2 ± 0.2 | 43.4 ± 0.6 | 0.0 ± 0.7 |
| H2O-bg | − 0.4 ± 0.4 | 3.0 ± 0.7 | 41.6 ± 0.3 | 26.4 ± 0.6 |
| Sample | Total Phenols (mg/g) 1 |
|---|---|
| EtOH-a | 24.91 ± 0.11 |
| Hex-a | 15.60 ± 0.14 |
| MC-a | 55.18 ± 0.13 |
| EA-a | 155.28 ± 0.30 |
| BuOH-a | 33.03 ± 0.13 |
| H2O-a | 24.94 ± 0.10 |
| EtOH-bg | 19.56 ± 0.06 |
| Hex-bg | 15.12 ± 0.05 |
| MC-bg | 68.26 ± 0.10 |
| EA-bg | 105.62 ± 0.36 |
| BuOH-bg | 13.87 ± 0.07 |
| H2O-bg | 11.69 ± 0.08 |
| Unit and Position | δC (62.5 MHz) | δH (600 MHz) |
|---|---|---|
| (-)-Quinic acid | ||
| 1 | 81.12 1, C | |
| 2 | 32.74, CH2 | 2.31 (1H, dd, J = 16.2, 3.2, H-2ax) 2.97 (1H, dt, J = 16.2, 3.2, H-2eq) |
| 3 | 73.21, CH | 5.36 (1H, dt, J = 3.6, 3.2) |
| 4 | 75.31, CH | 3.64 (1H, dd, J = 9.6, 3.6) |
| 5 | 67.80, CH | 4.28 (1H, ddd, J = 11.3, 9.6, 4.5) |
| 6 | 41.46, CH2 | 1.84 (1H, dd, J = 13.6, 11.3, H-6ax) 2.54 (1H, ddd, J = 13.6, 4.5, 3.2, H-6eq) |
| 7 | 174.58 1, C | |
| 1-O-feruloyl | ||
| 1′ | 127.39, C | |
| 2′ | 111.51, CH | 6.93 (1H, d, J = 1.9) |
| 3′ | 150.63, C | |
| 4′ | 149.19, C | |
| 5′ | 116.39, CH | 6.57 (1H, d, J = 8.2) |
| 6′ | 123.79, CH | 6.75 (1H, dd, J = 8.2, 1.9) |
| 7′ | 147.45, CH | 7.53 (1H, d, J = 15.9) |
| 8′ | 115.58, CH | 6.26 (1H, d, J = 15.9) |
| 9′ | 168.74, C | |
| 3′-OCH3 | 56.18, CH3 | 3.67 (3H, s) |
| 3-O-feruloyl | ||
| 1″ | 127.43, C | |
| 2″ | 111.57, CH | 6.83 (1H, d, J = 1.9) |
| 3″ | 150.35, C | |
| 4″ | 149.02, C | |
| 5″ | 116.28, CH | 6.67 (1H, d, J = 8.2) |
| 6″ | 124.13, CH | 6.88 (1H, dd, J = 8.2, 1.9) |
| 7″ | 146.93, CH | 7.54 (1H, d, J = 15.9) |
| 8″ | 115.70, CH | 6.16 (1H, d, J = 15.9) |
| 9″ | 167.75, C | |
| 3″-OCH3 | 56.08, CH3 | 3.60 (3H, s) |
| Sample | Radical-Scavenging Activity Against DPPH IC50, μg/mL (μM) | Radical Scavenging Activity Against ABTS IC50, μg/mL (μM) | Inhibition of Pancreatic Lipase IC50, μg/mL (μM) |
|---|---|---|---|
| Control | 1.17 (6.64) 1 | 3.50 (19.87) 1 | 0.49 (0.98) 3 |
| 1 | 9.53 (17.50) | 16.37 (30.06) | 131.72 (241.91) |
| 2 | - 2 | - 2 | 86.71 (710.02) |
| 3 | 4.67 (14.90) | 8.19 (26.14) | - 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.J.; Park, S.; Kim, E.; Kwon, H.; Park, H.-J.; Nam, J.-W.; Roh, S.-S.; Choi, H. Antioxidant, Pancreatic Lipase Inhibitory, and Tyrosinase Inhibitory Activities of Extracts of the Invasive Plant Spartina anglica (Cord-Grass). Antioxidants 2021, 10, 242. https://doi.org/10.3390/antiox10020242
Kim GJ, Park S, Kim E, Kwon H, Park H-J, Nam J-W, Roh S-S, Choi H. Antioxidant, Pancreatic Lipase Inhibitory, and Tyrosinase Inhibitory Activities of Extracts of the Invasive Plant Spartina anglica (Cord-Grass). Antioxidants. 2021; 10(2):242. https://doi.org/10.3390/antiox10020242
Chicago/Turabian StyleKim, Geum Jin, Songhee Park, Eonmi Kim, Hyukbean Kwon, Hae-Jin Park, Joo-Won Nam, Seong-Soo Roh, and Hyukjae Choi. 2021. "Antioxidant, Pancreatic Lipase Inhibitory, and Tyrosinase Inhibitory Activities of Extracts of the Invasive Plant Spartina anglica (Cord-Grass)" Antioxidants 10, no. 2: 242. https://doi.org/10.3390/antiox10020242
APA StyleKim, G. J., Park, S., Kim, E., Kwon, H., Park, H.-J., Nam, J.-W., Roh, S.-S., & Choi, H. (2021). Antioxidant, Pancreatic Lipase Inhibitory, and Tyrosinase Inhibitory Activities of Extracts of the Invasive Plant Spartina anglica (Cord-Grass). Antioxidants, 10(2), 242. https://doi.org/10.3390/antiox10020242