Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) Burs, Leaves, and Chestnuts Extracts and Their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction of “Marrone di Roccadaspide” Leaves, Burs, and Fruits
2.4. Determination of Total Phenolic, Total Tannin, and Total Flavonoid Content
2.5. Determination of DPPH Radical Scavenging Activity, Antioxidant Activity by TEAC Assay, and Ferric Reducing Antioxidant Power (FRAP)
2.6. Biological Assays
2.6.1. Determination of Cytotoxicity by WST-1 Assay
2.6.2. Determination of Intracellular ROS Scavenging Activity
2.6.3. Determination of Intracellular NF-κB Activity
2.6.4. Determination of Cytotoxicity by MTT Assay
2.6.5. Analysis of Nitrite Production
2.7. LC-ESI/LTQOrbitrap/MS/MS Analysis
2.8. LC-ESI/QTrap/MS/MS Analysis
2.9. Calibration and Quantification
2.10. Statistical Analysis
3. Results
3.1. Evaluation of Total Phenolic, Tannin, and Flavonoid Content of C. sativa “Marrone di Roccadaspide” Burs, Leaves, and Chestnuts by Spectrophotometric Methods
3.2. Evaluation of the Antioxidant Activity of C. sativa “Marrone di Roccadaspide” Burs, Leaves and Chestnuts by Spectrophotometric Methods
3.3. In Vitro Cytotoxicity Analysis
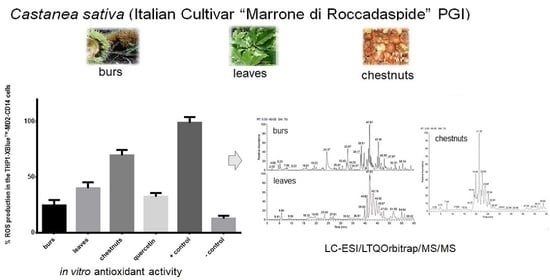
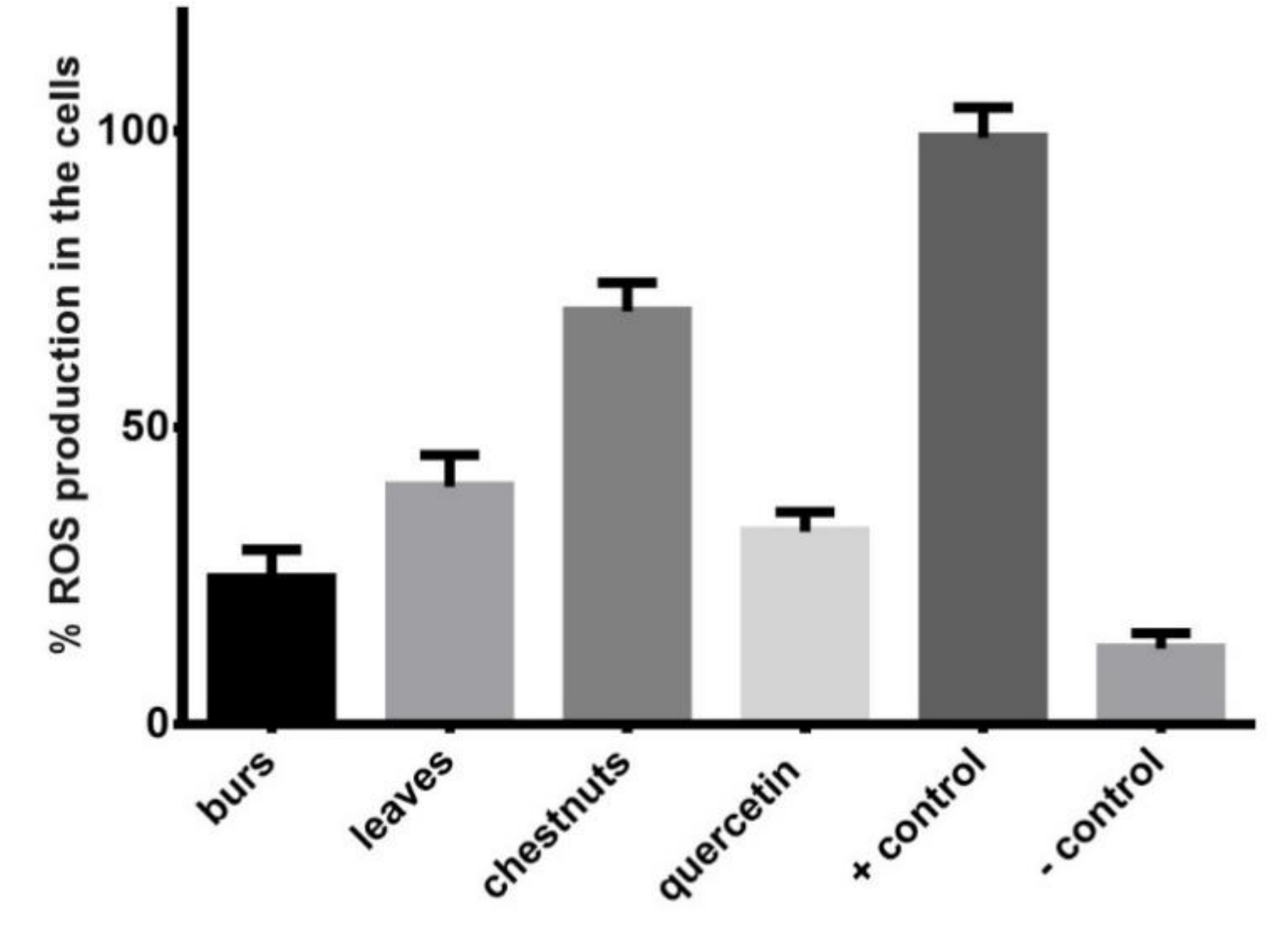
3.4. Evaluation of In Vitro Antioxidant Activity
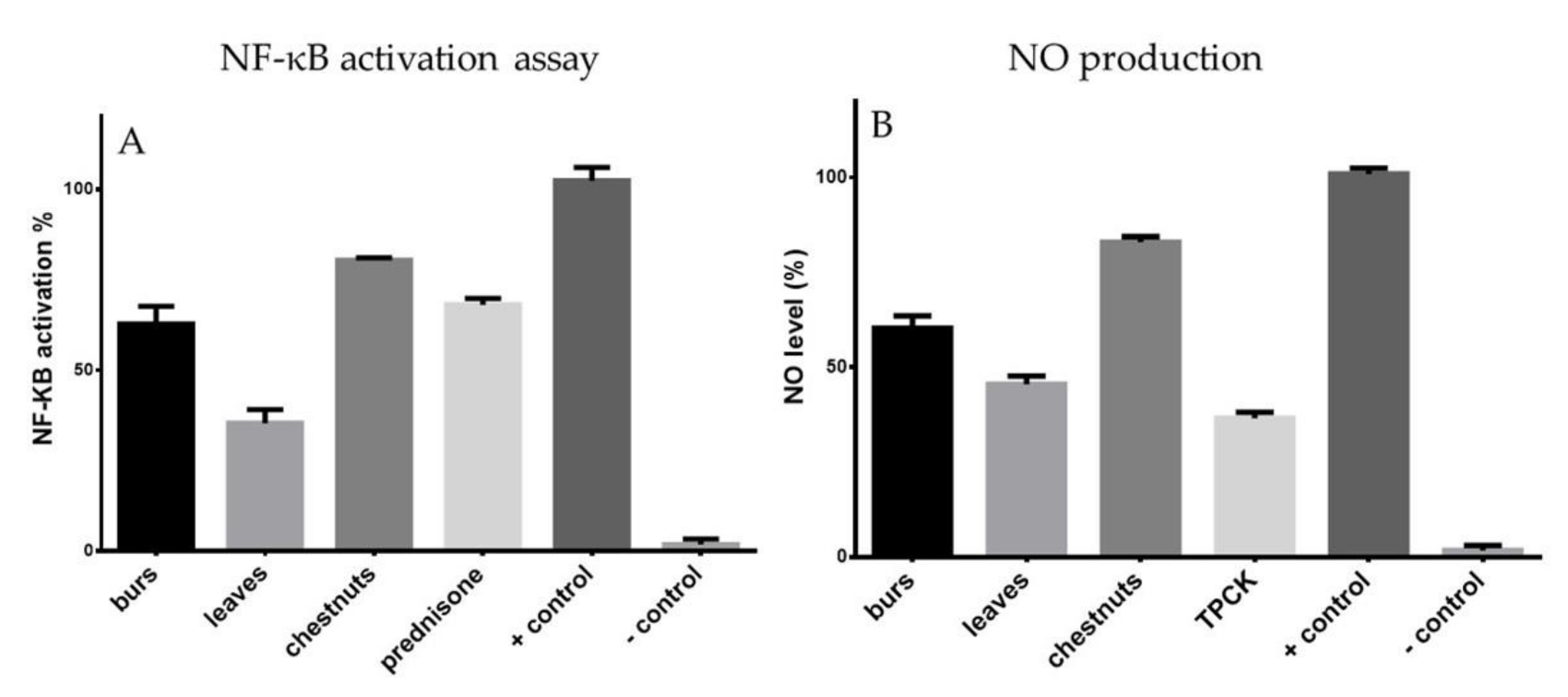
3.5. Effects of Extracts on NF-κB Activation
3.6. Effects of Extracts on NO Production
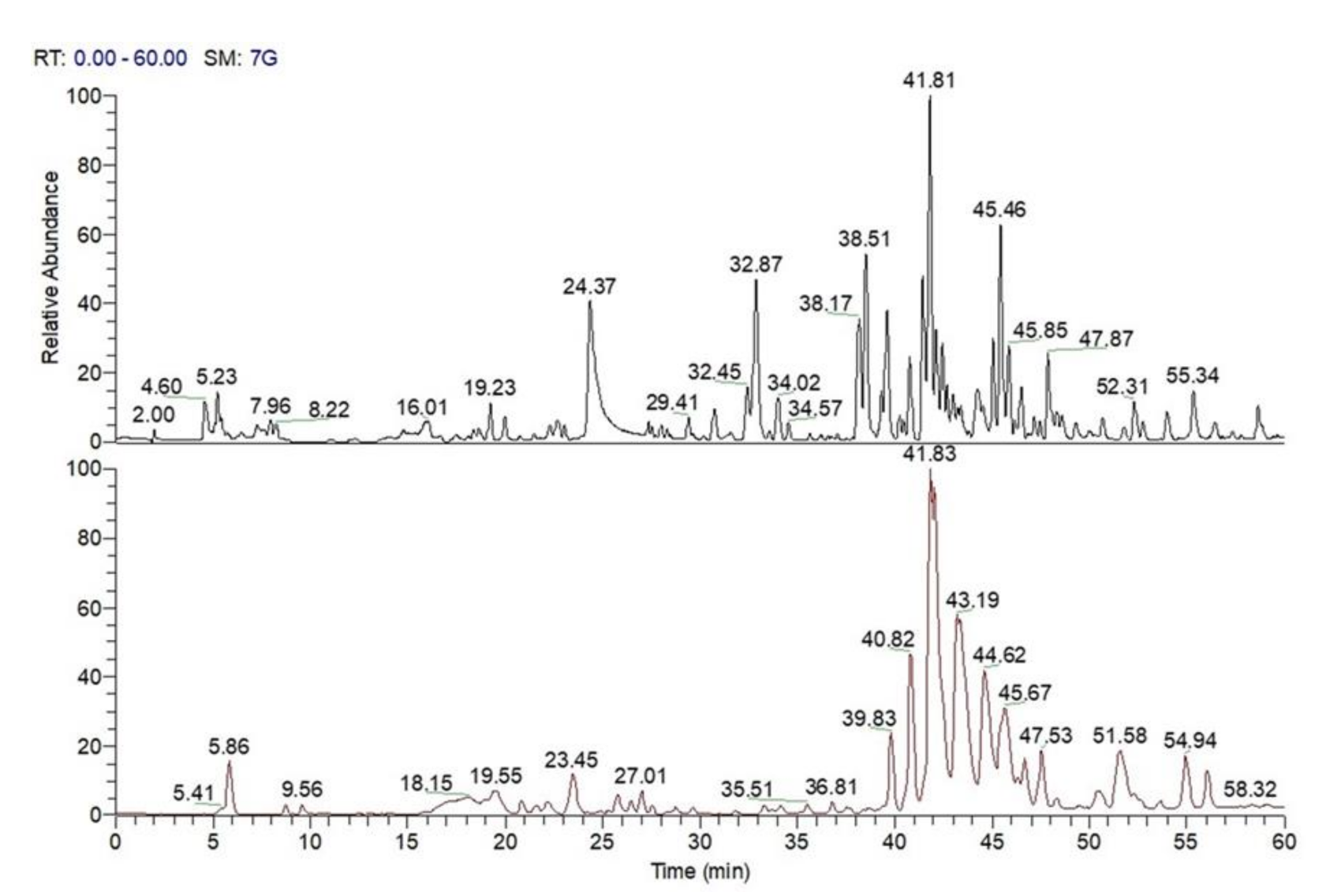
3.7. LC-MS Analysis of C. sativa Leaves, Burs, and Chestnuts
3.7.1. LC-MS Analysis of Specialized Metabolites Occurring in MeOH Extract of C. sativa Leaves, Burs, and Chestnuts
3.7.2. LC-MS Qualitative Analysis of Polar Lipids in MeOH Extract of C. sativa Burs, Leaves, and Chestnuts
3.8. Quantitative Analysis by LC-ESI/QTrap/MS/MS
4. Discussion
4.1. Evaluation of Antioxidant and Anti-Inflammatory Activity
4.2. LC-MS Analysis of Specialized Metabolites and Polar Lipids in C. sativa Burs, Leaves, and Chestnuts
4.3. Quantitative Analysis of Phenolic Compounds in Burs and Leaves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braga, N.; Rodrigues, F.; Oliveira, P.P.M.B. Castanea sativa by-products: A review on added value and sustainable application. Nat. Prod. Res. 2015, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Boscaino, F.; Cammarota, G.; Ottombrino, A.; Nazzaro, M.; Siano, F.; Volpe, M.G.; Sorrentino, A. Chemical, volatile profile and shelf life of muffin enriched with supplementation chestnut cream. J. Food Process. Preserv. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Krist, S.; Unterweger, H.; Bandion, F.; Buchbauer, G. Volatile compound analysis of SPME headspace and extract samples from roasted Italian chestnuts (Castanea sativa Mill.) using GC-MS. Eur. Food Res. Technol. 2004, 219, 470–473. [Google Scholar] [CrossRef]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential anticancer effects of polyphenols from chestnut shell extracts: Modulation of cell growth, and cytokinomic and metabolomic profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef]
- De Vasconcelos, M.d.C.B.M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Evaluating the potential of chestnut (Castanea sativa Mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crops Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the phenolic profile of Castanea sativa mill. By-products and their antioxidant and antimicrobial activity against multiresistant bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) burs extracts and functional compounds: UHPLC-UV-HRMS profiling, antioxidant activity, and inhibitory effects on Phytopathogenic fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [Green Version]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant potential of chestnut (Castanea sativa L.) and almond (Prunus dulcis L.) by-products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Health-promoting effects of Thymus phenolic-rich extracts: Antioxidant, anti-inflammatory and antitumoral properties. Antioxidants 2020, 9, 814. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Masullo, M.; Hosek, J.; Pizza, C.; Piacente, S. Chestnut shells (Italian cultivar “Marrone di Roccadaspide” PGI): Antioxidant activity and chemical investigation with in depth LC-HRMS/MSn rationalization of tannins. Food Res. Int. 2020, 129, 108787. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, A.; Masullo, M.; Mari, A.; Balato, A.; Filosa, R.; Lembo, S.; Napolitano, A.; Piacente, S. Phenolics from Castanea sativa leaves and their effects on UVB-induced damage. Nat. Prod. Res. 2018, 32, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Masullo, M.; Cerulli, A.; Mari, A.; Santos, C.C.D.; Pizza, C.; Piacente, S. LC-MS profiling highlights hazelnut (Nocciola di Giffoni PGI) shells as a byproduct rich in antioxidant phenolics. Food Res. Int. 2017, 101, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Kluwer Academic Publishers: Dodrecht, The Netherlands, 2003; p. 102. [Google Scholar]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Zamuz, S.; Lopez-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Dominguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, A.; Masullo, M.; Montoro, P.; Hosek, J.; Pizza, C.; Piacente, S. Metabolite profiling of “green” extracts of Corylus avellana leaves by 1H NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. Anal. 2018, 160, 168–178. [Google Scholar] [CrossRef]
- Iranshahi, M.; Masullo, M.; Asili, A.; Hamedzadeh, A.; Jahanbin, B.; Festa, M.; Capasso, A.; Piacente, S. Sesquiterpene Coumarins from Ferula gumosa. J. Nat. Prod. 2010, 73, 1958–1962. [Google Scholar] [CrossRef]
- Masullo, M.; Cantone, V.; Cerulli, A.; Lauro, G.; Messano, F.; Russo, G.L.; Pizza, C.; Bifulco, G.; Piacente, S. Giffonins J–P, highly hydroxylated cyclized diarylheptanoids from the leaves of Corylus avellana cultivar “Tonda di Giffoni”. J. Nat. Prod. 2015, 78, 2975–2982. [Google Scholar] [CrossRef]
- Masullo, M.; Montoro, P.; Autore, G.; Marzocco, S.; Pizza, C.; Piacente, S. Quali-quantitative determination of triterpenic acids of Ziziphus jujuba fruits and evaluation of their capability to interfere in macrophages activation inhibiting NO release and iNOS expression. Food Res. Int. 2015, 77, 109–117. [Google Scholar] [CrossRef]
- Napolitano, A.; Cerulli, A.; Pizza, C.; Piacente, S. Multi-class polar lipid profiling in fresh and roasted hazelnut (Corylus avellana cultivar “Tonda di Giffoni”) by LC-ESI/LTQOrbitrap/MS/MSn. Food Chem. 2018, 269, 125–135. [Google Scholar] [CrossRef]
- Mari, A.; Napolitano, A.; Masullo, M.; Pizza, C.; Piacente, S. Identification and quantitative determination of the polar constituents in Helichrysum italicum flowers and derived food supplements. J. Pharm. Biomed. Anal. 2014, 96, 249–255. [Google Scholar] [CrossRef]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Masullo, M.; Cerulli, A.; Montoro, P.; Pizza, C.; Piacente, S. In depth LC-ESIMSn-guided phytochemical analysis of Ziziphus jujuba Mill. leaves. Phytochemistry 2019, 159, 148–158. [Google Scholar] [CrossRef]
- Benavides, A.; Montoro, P.; Bassarello, C.; Piacente, S.; Pizza, C. Catechin derivatives in Jatropha macrantha stems: Characterisation and LC/ESI/MS/MS quali-quantitative analysis. J. Pharm. Biomed. Anal. 2006, 40, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Pacente, S.; Burke, A.; Khan, A.; Pizaa, C. Constituents of Cuscuta reflexa are anti-HIV agents. Antivir. Chem. Chemother. 1997, 8, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Perez, A.J.; Pecio, L.; Kowalczyk, M.; Kontek, R.; Gajek, G.; Stopinsek, L.; Mirt, I.; Oleszek, W.; Stochmal, A. Triterpenoid Components from Oak Heartwood (Quercus robur) and Their Potential Health Benefits. J. Agric. Food Chem. 2017, 65, 4611–4623. [Google Scholar] [CrossRef]
- Wensbo Posaric, D.; Andersson, A.; Bergquist, K.-E.; Isaksson, A. Differentiation and quantification of synthetic phosphatidylethanol (PEth) homologues by 1H- and 13C-NMR in polar organic solvents. Anal. Bioanal. Chem. 2014, 406, 4735–4744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnann, H.; Engelmann, C.; Skopp, G.; Winkler, M.; Auwaerter, V.; Dresen, S.; Ferreiros, N.; Wurst, F.M.; Weinmann, W. Identification of 48 homologues of phosphatidylethanol in blood by LC-ESI-MS/MS. Anal. Bioanal. Chem. 2010, 396, 2415–2423. [Google Scholar] [CrossRef]
- D’Urso, G.; Napolitano, A.; Cannavacciuolo, C.; Masullo, M.; Piacente, S. Okra fruit: LC-ESI/LTQOrbitrap/MS/MSn based deep insight on polar lipids and specialized metabolites with evaluation of anti-oxidant and anti-hyperglycemic activity. Food Funct. 2020, 11, 7856–7865. [Google Scholar] [CrossRef]
- Napolitano, A.; Carbone, V.; Saggese, P.; Takagaki, K.; Pizza, C. Novel galactolipids from the leaves of Ipomoea batatas L.: Characterization by liquid chromatography coupled with electrospray ionization-quadrupole time-of-flight tandem mass spectrometry. J. Agric. Food Chem. 2007, 55, 10289–10297. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Benavides, A.; Pizza, C.; Piacente, S. Qualitative on-line profiling of ceramides and cerebrosides by high performance liquid chromatography coupled with electrospray ionization ion trap tandem mass spectrometry: The case of Dracontium loretense. J. Pharm. Biomed. Anal. 2011, 55, 23–30. [Google Scholar] [CrossRef]
- Ito, H. Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Med. 2011, 77, 1110–1115. [Google Scholar] [CrossRef] [Green Version]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Inoue, M.; Adachi, M.; Shimizu, Y.; Tsutsumi, T.; Tokumura, A. Comparison of Lysophospholipid Levels in Rat Feces with Those in a Standard Chow. J. Agric. Food Chem. 2011, 59, 7062–7067. [Google Scholar] [CrossRef] [PubMed]
- Damnjanovic, J.; Kuroiwa, C.; Tanaka, H.; Ishida, K.; Nakano, H.; Iwasaki, Y. Directing positional specificity in enzymatic synthesis of bioactive 1-phosphatidylinositol by protein engineering of a phospholipase D. Biotechnol. Bioeng. 2016, 113, 62–71. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.V.; Welti, R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016, 39, 787–803. [Google Scholar] [CrossRef] [Green Version]
- Masullo, M.; Montoro, P.; Mari, A.; Pizza, C.; Piacente, S. Medicinal plants in the treatment of women’s disorders: Analytical strategies to assure quality, safety and efficacy. J. Pharm. Biomed. Anal. 2015, 113, 189–211. [Google Scholar] [CrossRef]
- Da Costa, E.; Amaro, H.M.; Melo, T.; Guedes, A.C.; Domingues, M.R. Screening for polar lipids, antioxidant, and anti-inflammatory activities of Gloeothece sp. lipid extracts pursuing new phytochemicals from cyanobacteria. J. Appl. Phycol. 2020, 32, 3015–3030. [Google Scholar] [CrossRef]

| C. sativa MeOH Extracts | Total Phenol Content (mg GAE/g ± SD a) | Total Tannin Content (mg GAE/g ± SD a) | Total Flavonoid Content (mg rutin/g ± SD a) |
|---|---|---|---|
| burs | 580.44 ± 29.63 | 276.44 ± 13.52 | 87.19 ± 1.98 |
| leaves | 298.96 ± 14.81 | 105.25 ± 9.95 | 45.54 ± 0.99 |
| chestnuts | N.D. | N.D. | N.D. |
| C. sativa MeOH Extracts | Free Radical Scavenging Activity (DPPH) EC50 (µg/mL ± SD a) b | (TEAC) (mg/mL ± SD a) c | FRAP (mmol ferric sulfate/g ± SD a) d |
|---|---|---|---|
| burs | 4.21 ± 0.01 | 3.03 ± 0.03 | 2.96 ± 0.02 |
| leaves | 3.06 ± 0.01 | 3.01 ± 0.02 | 1.48 ± 0.02 |
| chestnuts | 34.64 ± 0.01 | 0.57 ± 0.01 | 0.18 ± 0.02 |
| N. | Compound | Rt (min) a | Molecular Formula | [M-H]− a | [M-2H]2− a | [(M+FA)-H]− a | Delta (ppm) a | Product ions (m/z) a | CSB | CSL |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sucrose | 5.10 | C12H22O11 | 341.1083 | 387.1137 | 1.38 | 179, 161 | X | X | |
| 2 | Sorbitol/glucitol | 5.22 | C6H14O6 | 227.0766 | 2.05 | 181 | X | - | ||
| 3 | Monogalloyl glucose | 6.44 | C13H16O10 | 331.0665 | 1.59 | 313, 271, 211, 193, 169, 125 | X | X | ||
| 4 | NHTP-glucose (vescalin) | 7.20 | C27H20O18 | 631.0561 | −0.69 | 613, 587, 569, 551, 467, 441, 425 | X | X | ||
| 5 | HHDP-glucose isomer | 7.27 | C20H18O14 | 481.0610 | −0.61 | 421, 301, 275 | X | X | ||
| 6 | HHDP-glucose isomer | 7.60 | C20H18O14 | 481.0601 | −0.48 | 421, 301, 275 | X | - | ||
| 7 | Phenol glucoside (crenatin) # | 7.88 | C13H18O9 | 317.0872 | 1.61 | 299, 155 | X | X | ||
| 8 | Digalloyl glucose isomer | 8.31 | C20H20O14 | 483.0771 | −0.87 | 465, 331, 313, 169 | X | X | ||
| 9 | Hexahydroxydiphenic acid | 8.55 | C14H10O10 | 337.0192 | 0.44 | 293, 249, 169, 167 | X | - | ||
| 10 | Digalloyl glucose isomer | 11.07 | C20H20O14 | 483.0772 | 0.58 | 465, 331, 313, 169 | X | - | ||
| 11 | Digalloyl glucose isomer | 12.29 | C20H20O14 | 483.0763 | −1.33 | 465, 331, 313, 169 | X | - | ||
| 12 | NHTP-valoneoyl-glucose (castavaloninic acid) | 14.01 | C48H30O31 | 1101.0671 | 550.0282 | −1.44 | 1057, 933, 931, 631, 587, 449, 441, 425 | X | - | |
| 13 | Castacrenin C | 14.05 | C27H18O17 | 613.0456 | −0.71 | 595, 493, 301, 299 | X | - | ||
| 14 | Castacrenin B | 14.87 | C27H18O17 | 613.0456 | −0.71 | 595, 493, 301, 299 | X | - | ||
| 15 | Chebulic acid isomer | 14.94 | C14H12O11 | 355.0297 | 0.45 | 337, 311, 267, 191 | X | - | ||
| 16 | Chebulic acid isomer | 15.45 | C14H12O11 | 355.0296 | 0.04 | 337, 311, 267, 191 | X | - | ||
| 17 | NHTP-HHDP-glucose (vescalagin) | 15.67 | C41H26O26 | 933.0611 | 466.0271 | −1.88 | 915, 631, 613, 587, 569, 551, 467, 441, 425, 301 | X | - | |
| 18 | Castamollissin/ maplexin D | 15.73 b | C20H20O13 | 467.0821 b | 0.28 b | 449, 423, 315, 169 | - | X | ||
| 19 | Castacrenin A | 15.97 | C27H18O17 | 613.0453 | −1.10 | 595, 523, 493, 301, 299 | X | - | ||
| 20 | Bis-HHDP-glucose isomer (casuariin/pedunculagin isomer) | 16.45 | C34H24O22 | 783.0669 | 391.0299 | −0.88 | 765, 481, 301, 275 | X | X | |
| 21 | Chesnatin | 16.68 | C27H26O18 | 637.1030 | −0.82 | 593, 467, 305 | X | - | ||
| 22 | HHDP-Valoneoyl-glucose isomer (praecoxin A/platycariin isomer) | 17.13 | C41H28O27 | 951.0714 | 475.0315 | −2.11 | 907, 783, 465, 453 | X | - | |
| 23 | Galloyl-HHDP-glucose isomer (punicacortein A-B/pterocaryanin B/gemin D) | 17.46 | C27H22O18 | 633.0718 | 316.0328 | −0.76 | 615, 589, 481, 463, 301, 275, 257 | X | - | |
| 24 | Bis-HHDP-glucose isomer (pedunculagin/casuariin isomer) | 17.56 | C34H24O22 | 783.0672 | 391.0298 | −0.42 | 765, 721, 481, 301, 275 | X | - | |
| 25 | Trigalloyl glucose isomer | 17.71 | C27H24O18 | 635.0878 | −0.08 | 483, 465, 331, 313, 271, 211 | X | - | ||
| 26 | Galloyl-chebuloyl-HHDP-glucose (chebulagic acid) | 18.03 | C41H30O27 | 953.0881 | 476.0402 | −1.03 | 909, 785 | X | - | |
| 27 | Isochesnatin | 18.05 | C27H26O18 | 637.1030 | −0.80 | 593, 469 | X | - | ||
| 28 | Digalloyl-HHDP-glucose isomer (1-desgalloyleugeniin isomer) | 18.64 | C34H26O22 | 785.0834 | 392.0372 | 0.22 | 633, 615, 483, 313, 301, 275 | X | X | |
| 29 | Galloyl-bis-HHDP-glucose (stachyurin) | 18.71 | C41H28O26 | 935.0787 | 467.0347 | 0.25 | 917, 783, 633 | X | - | |
| 30 | Methylvaloneoyl–NHTP–glucose (vescavaloninic/castavaloninic acid methyl ester) | 18.86 | C49H32O31 | 1115.0844 | −0.01 | 1053, 933, 569 | X | - | ||
| 31 | Galloyl phenol glucoside dimer (chestanin) # | 19.21 | C40H42O26 | 937.1825 | 468.0897 | −0.59 | 893, 637, 635, 467, 301 | X | X | |
| 32 | Galloyl phenol glucoside (cretanin) # | 19.22 | C20H22O13 | 469.0974 | −0.55 | 169 | X | X | ||
| 33 | Galloyl-chebuloyl-HHDP-glucose (chebulagic acid) | 19.25 | C41H30O27 | 953.0881 | 0.98 | 909, 785 | X | - | ||
| 34 | Trigalloyl glucose isomer | 19.41 | C27H24O18 | 635.0870 | −1.34 | 483, 465, 331, 313, 271, 211 | X | - | ||
| 35 | Digalloyl-HHDP-glucose isomer (tellimagrandin I isomer) | 19.97 | C34H26O22 | 785.0825 | 392.0377 | −0.69 | 633, 615, 483, 313, 301, 275 | X | X | |
| 36 | Trigalloyl glucose isomer | 20.20 | C20H22O13 | 635.0873 | 0.75 | 483, 465, 313, 271, 211 | X | X | ||
| 37 | 3-O-SSupp-coumaroylquinic acid | 20.21 | C16H18O8 | 337.0920 | 0.55 | 191, 179 | X | - | ||
| 38 | Galloyl phenol glucoside | 20.58 b | C20H22O13 | 469.0980 b | 0.75 b | 169 | - | X | ||
| 39 | coumaroylquinic acid | 20.72 | C16H18O8 | 337.0919 | 0.37 | 191, 179 | X | X | ||
| 40 | Galloyl-methylchebuloyl-HHDP-glucose (chebulagic acid methyl ester) | 20.74 | C42H32O27 | 967.1033 | 483.0481 | −1.50 | 785, 765 | X | X | |
| 41 | Galloyl phenol glucoside dimer (isochestanin) | 20.75 b | C40H42O26 | 937.1877 b | −0.40 b | 637, 467 | - | X | ||
| 42 | Ellagic acid pentoside | 20.84 | C19H14O12 | 433.0402 | 0.09 | 301, 300 | X | X | ||
| 43 | Euphorbin A/B | 21.15 | C82H58O53 | 944.0816 | 1419, 922, 860, 783, 467, 301 | X | - | |||
| 44 | Nobotanin A/Malabathrin B/1-Desgalloylrugosin F/Zeylaniin A isomer | 21.35 | C75H52O48 | 859.0722 | 0.41 | 1417, 1115, 1085, 937, 917, 783, 767, 301 | X | - | ||
| 45 | Galloyl-methylchebuloyl-HHDP-glucose (chebulagic acid methyl ester) | 21.44 | C42H32O27 | 967.1038 | 483.0478 | −0.99 | 785, 765 | X | X | |
| 46 | Rugosin E/Camptothin B | 21.56 | C75H54O48 | 860.0796 | −1.40 | 1419, 1089, 953, 937, 935, 917, 909, 785, 767, 699, 615, 597, 465, 301, 275, 249 | X | - | ||
| 47 | Digalloyl-HHDP-glucose isomer (tellimagrandin I isomer) | 21.77 | C34H26O22 | 785.0833 | 392.0378 | 0.03 | 633, 615, 483, 313, 301 | X | X | |
| 48 | Quercetin galloyl hexoside | 21.99 | C28H24O16 | 615.0969 | −1.95 | 463, 301 | X | X | ||
| 49 | Valoneic acid dilactone | 22.21 | C21H10O13 | 469.0035 | −0.55 | 425, 301, 299, 169 | X | - | ||
| 50 | Rugosin E/Camptothin B | 22.42 | C75H54O48 | 860.0798 | 1.26 | 1419, 1089, 953, 937, 935, 917, 909, 785, 767, 699, 615, 597, 465, 301, 275, 249 | X | - | ||
| 51 | Isorhamnetin deoxyhexose hexoside | 22.52 | C28H32O16 | 623.1603 | −0.61 | 315, 300, 271 | X | X | ||
| 52 | Kaempferol deoxyhexose hexoside | 22.57 | C27H30O15 | 593.1500 | −0.13 | 285 | X | X | ||
| 53 | Methylellagic acid hexoside | 22.62 b | C21H18O13 | 477.0662 b | −0.24 b | 315, 301 | - | X | ||
| 54 | Quercetin 3-O-β-D-glucopyranoside # | 22.74 | C21H20O12 | 463.0869 | −0.19 | 301 | X | X | ||
| 55 | Quercetin hexuronoside | 22.80 | C21H18O13 | 477.0660 | −0.75 | 301 | X | - | ||
| 56 | Galloyl phenol glucoside gallate (galloyl-cretanin) | 22.97 | C27H26O17 | 621.1082 | −0.38 | 577, 469, 451, 317, 313 | X | X | ||
| 57 | Trigalloyl-HHDP-glucose (Tellimagrandin II) | 23.05 | C41H30O26 | 937.0933 | 468.0430 | 0.03 | MS2 (468): 767, 635, 633, 617, 392 [M−2H−152]2−, 313, 301, 169 | X | X | |
| 58 | Tetragalloyl glucose isomer | 23.14 | C34H28O22 | 787.0987 | 393.0453 | −0.14 | 635, 617, 483, 465, 447, 295 | X | X | |
| 59 | Kaempferol hexoside (astragalin) | 23.97 | C21H20O11 | 447.0922 | 0.07 | 327, 285 | X | X | ||
| 60 | Ellagic acid # | 24.37 | C14H6O8 | 300.9989 | 0.47 | 284, 257, 229, 201, 185, 145 | X | X | ||
| 61 | Isorhamnetin 3-O-β-D-glucopyranoside # | 24.60 | C22H22O12 | 477.1029 | 0.33 | 357, 315, 314 | X | X | ||
| 62 | Quercetin pentoside | 24.68 b | C20H18 O11 | 433.0769 b | 0.77 b | 301, 300 | - | X | ||
| 63 | Quercetin 3-O-α-L-rhamnopyranoside # | 25.06 b | C21H20O11 | 447.0924 b | 0.50 b | 301 | - | X | ||
| 64 | Methyl coumaroyl quinate | 25.11 | C17H20O8 | 351.1082 | 2.29 | 163 | X | X | ||
| 65 | Methylellagic acid pentoside | 25.51 | C20H16O12 | 447.0553 | −1.12 | 315, 301, 300 | X | X | ||
| 66 | Isorhamnetin hexuronoside | 26.05 | C22H20O13 | 491.0818 | −0.40 | 315, 301 | X | X | ||
| 67 | Lignan hexoside | 27.01 | C26H32O11 | 519.1862 | 0.14 | 357 | X | X | ||
| 68 | Valoneic acid dilactone methyl ester | 27.14 | C22H12O13 | 483.01902 | −0.53 | 451, 301 | X | X | ||
| 69 | Bartogenic acid hexoside | 27.33 | C36H56O12 | 679.3693 | 725.3736 | 0.67 | 559, 517, 455 | X | - | |
| 70 | Lignan hexoside | 27.34 | C26H34O11 | 521.2018 | 567.2074 | 0.19 | 359, 341, 177 | X | X | |
| 71 | Dimethylellagic acid pentoside | 27.53 | C21H18O12 | 461.0713 | −0.35 | 446, 328, 313, 299, 285, 284 | X | X | ||
| 72 | Quercetin coumaroyl hexoside | 29.43 | C30H26O14 | 609.1234 | −0.77 | 463, 301 | X | X | ||
| 73 | Dimethylellagic acid deoxyhexoside | 29.70 | C22H20O12 | 475.0865 | −1.23 | 460, 328, 313, 299, 275, 217, 193 | X | X | ||
| 74 | Trimethylellagic acid hexoside | 30.74 | C23H22O13 | 505.0981 | 551.1031 | 343, 328, 313, 299, 284 | X | X | ||
| 75 | Kaempferol coumaroyl hexoside | 32.45 | C30H26O13 | 593.1285 | −0.72 | 447, 285, 257, 229 | X | - | ||
| 76 | Kaempferol coumaroyl hexoside | 32.87 | C30H26O13 | 593.1285 | −0.72 | 447, 285, 257, 229 | X | - | ||
| 77 | Kaempferol deoxyhexosyl coumaroyl hexoside | 33.27 b | C36H36O17 | 739.1870 b | 0.18 b | 593, 575, 453, 285 | - | X | ||
| 78 | Isorhamnetin coumaroyl hexoside | 33.29 | C31H28O14 | 623.1394 | −0.21 | 477, 315, 300 | X | X | ||
| 79 | Kaempferol coumaroyl hexoside | 34.02 | C30H26O13 | 593.1284 | −0.92 | 447, 285, 257, 229 | X | X | ||
| 80 | Castaartancrenoic acid D/E hexoside | 34.17 b | C36H58O10 | 649.3951 b | 695.3998 b | 0.67 b | 649, 487 | - | X | |
| 81 | Kaempferol coumaroyl hexoside | 34.57 | C30H26O13 | 593.1285 | −0.82 | 447, 285, 257, 229 | X | X | ||
| 82 | Roburgenic acid isomer | 35.58 | C30H46O8 | 533.3109 | 0.03 | 485, 471, 457, 453 | X | - | ||
| 83 | Isorhamnetin coumaroyl hexoside | 37.04 b | C31H28O14 | 623.1395 b | −0.10 b | 477, 315, 300 | - | X | ||
| 84 | Dimethylellagic acid | 38.17 | C16H10O8 | 329.0296 | 1.33 | 314, 299, 285 | X | - | ||
| 85 | Trimethylellagic acid deoxyhexoside | 38.51 | C23H22O12 | 535.1085 | 0.43 | 343, 328, 313, 299 | X | - | ||
| 86 | l-PI (18:3) | 39.28 | C27H47O12P | 593.2720 | −0.18 | 413, 315, 277, 241 | X | X | ||
| 87 | Kaempferol acetyl coumaroyl hexoside | 39.33 | C32H28O14 | 635.1392 | −0.58 | 575, 489, 285 | X | X | ||
| 88 | Castaartancrenoic acid B | 39.33 | C27H44O5 | 447.31039 | −0.25 | 429, 401, 365 | X | - | ||
| 89 | Dimethylellagic acid | 39.60 | C16H10O8 | 329.0297 | 1.42 | 314, 299, 285 | X | - | ||
| 90 | l-PI (18:2) | 40.20 | C27H49O12P | 595.2876 | −0.84 | 415, 315, 279, 241, 179 | X | X | ||
| 91 | Quercetin dicoumaroyl hexoside | 40.21 | C39H32O16 | 755.1605 | −0.19 | 609, 463, 301 | X | X | ||
| 92 | SQMG (18:3) | 40.32 | C27H46O11S | 577.2676 | −0.22 | 299, 277, 225 | X | X | ||
| 93 | l-PI (16:0) | 40.43 | C25H49O12P | 571.2873 | −0.77 | 409, 391, 333, 315, 255, 241, 223, 171 | X | X | ||
| 94 | Roburgenic acid isomer | 40.43 | C30H46O8 | 533.3104 | −0.89 | 485, 471 | X | - | ||
| 95 | NA-GPE (18:2) | 40.45 | C23H44O7NP | 476.2767 | −0.98 | 415, 279, 214, 196, 153 | X | X | ||
| 96 | Bartogenic acid # | 40.78 | C30H46O7 | 517.3156 | −0.72 | 499, 455, 437 | X | - | ||
| 97 | SQMG (18:2) | 41.37 | C27H48O11S | 579.2831 | −0.48 | 299, 279, 225 | X | X | ||
| 98 | SQMG (16:0) | 41.55 | C25H48O11S | 555.2833 | −0.05 | 299, 255, 225 | X | X | ||
| 99 | Kaempferol dicoumaroyl hexoside | 41.81 | C39H32O15 | 739.1650 | −1.04 | 593, 575, 453, 285, | X | X | ||
| 100 | DGMG (18:3) | 42.16 | C33H56O14 | 675.3594 | 1.19 | 415, 397 | X | X | ||
| 101 | l-PG (16:0) | 42.29 | C22H45O9P | 483.2722 | 0.92 | 391, 255, 245, 227, 153 | X | X | ||
| 102 | l-PG (18:1) | 43.32 b | C24H47O9P | 509.2876 b | 0.34 b | 417, 281, 245, 227, 153 | - | X | ||
| 103 | l-PA (18:3) | 43.78 | C21H37O7P | 431.2194 | 0.24 | 413, 277, 153 | X | X | ||
| 104 | l-PC (16:0) | 44.46 | C25H52O9NP | 540.3298 | 0.18 | 480, 255, 225 | X | X | ||
| 105 | l-PA (18:2) | 44.94 | C21H39O7P | 433.2350 | −1.15 | 171, 153 | X | X | ||
| 106 | Kaempferol acetyl dicoumaroyl hexoside | 45.04 | C41H34O16 | 781.1753 | −0.98 | 635, 617, 495, 435, 285 | X | X | ||
| 107 | l-PA (16:0) | 45.26 | C19H39O7P | 409.2354 | 1.01 | 391, 255, 153 | X | X | ||
| 108 | Kaempferol acetyl dicoumaroyl hexoside | 45.46 | C41H34O16 | 781.1757 | −0.76 | 635, 617, 495, 435, 285 | X | X | ||
| 109 | Kaempferol acetyl dicoumaroyl hexoside | 45.87 | C41H34O16 | 781.1754 | −0.92 | 635, 617, 495, 435, 285 | X | X | ||
| 110 | Kaempferol acetyl dicoumaroyl hexoside | 46.21 | C41H34O16 | 781.1767 | 0.18 | 635, 617, 495, 435, 285 | X | - | ||
| 111 | l-PA (18:1) | 46.36 | C21H41O7P | 435.2510 | 0.83 | 417, 281, 153 | X | X | ||
| 112 | Trimethylellagic acid | 46.43 | C17H12O8 | 343.0452 | 0.31 | 328, 313, 299, 297, 284, 275 | X | - | ||
| 113 | Kaempferol diacetyl dicoumaroyl hexoside | 47.87 | C43H36O17 | 823.1856 | −1.31 | 677, 659, 635, 617, 557, 531, 391, 285 | X | X | ||
| 114 | Kaempferol diacetyl dicoumaroyl hexoside | 48.04 | C43H36O17 | 823.1865 | −0.50 | 677, 659, 617, 391, 285 | X | X | ||
| 115 | Kaempferol diacetyl dicoumaroyl hexoside | 48.34 | C43H36O17 | 823.1862 | 0.83 | 677, 659, 635, 617, 557, 531, 391, 285 | X | X | ||
| 116 | Kaempferol diacetyl dicoumaroyl hexoside | 48.57 | C43H36O17 | 823.1864 | −0.58 | 677, 659, 617, 557, 531, 391, 285 | X | - | ||
| 117 | PI (16:0; 18:3) | 51.26 | C43H77O13P | 831.5017 | −0.11 | 575, 553, 413, 391, 277, 255 | X | X | ||
| 118 | SQDG (16:0; 18:3) | 51.58 b | C43H76O12S | 815.4966 b | −0.90 b | 559, 537, 277, 255 | - | X | ||
| 119 | Hederagenin | 52.31 | C30H40O4 | 471.3466 | −0.50 | 453, 425, 407 | X | X | ||
| 120 | 2-Pentadecanone | 52.72 | C16H32O3 | 271.2270 | 0.92 | 225, 209 | X | - | ||
| 121 | SQDG (16:0; 16:0) | 53.21 b | C41H78O12S | 793.5132 b | 0.20 b | 537, 255, 225 | - | X | ||
| 122 | GlyCer (t18:1;h16:0) | 54.01 | C40H77O10N | 730.5461 | 776.5514 | −0.40 | 568, 550, 326, 271 | X | X | |
| 123 | DGDG (18:3; 18:3) | 54.94 b | C51H84O15 | 935.5726 b | 981.5783 b | −0.08 b | 657, 397, 341, 323, 277 | - | X | |
| 124 | GlyCer (d18:2;h16:0) | 55.34 | C40H75O9N | 712.5351 | 758.54028 | −0.99 | 550, 532, 312, 296, 271, 253, 225 | X | X | |
| 125 | DGDG (18:3, 16:0) | 56.40 | C49H86O15 | 913.5881 | 959.5929 | 2.03 | 657, 635, 379, 277 | X | X | |
| 126 | DGDG (18:2, 16:0) | 57.29 | C49H88O15 | 915.6023 | 961.6087 | −1.81 | 659, 635, 379 | X | X | |
| 127 | Glycerol-ω-hydroxyacid-ferulic acid (22:0) | 57.83 | C35H58O8 | 605.4042 | −0.92 | 531, 513, 193, 175 | X | - | ||
| 128 | GlyCer (t18:1;h22:0) | 58.78 b | C46H89O10N | 814.6398 b | −0.62 b | 652, 634, 410, 355, 337, 309 | - | X |
| N. | Compound | Rt (min) | Molecular Formula | [M-H]− | [(M+FA)-H]− | Delta (ppm) | Product ions (m/z) |
|---|---|---|---|---|---|---|---|
| 60 | Ellagic acid # | 7.28 | C14H6O8 | 300.9989 | 0.47 | 284, 257, 229, 201, 185, 145 | |
| 90 | l-PI (18:2) | 19.06 | C27H49O12P | 595.2880 | 0.28 | 415, 315, 279, 241, 223 | |
| 93 | l-PI (16:0) | 19.11 | C25H49O12P | 571.2883 | 0.95 | 391, 315, 255, 241, 223 | |
| 95 | NA-GPE (18:2) | 19.28 | C23H44O7NP | 476.2774 | 0.43 | 402, 384, 214, 171, 153 | |
| 97 | SQMG (18:2) | 19.62 | C27H48O11S | 579.2836 | 0.36 | 299, 279, 225 | |
| 98 | SQMG (16:0) | 19.68 | C25H48O11S | 555.2839 | 1.05 | 299, 255, 225 | |
| 129 | NA-GPE (18:1) | 19.89 | C23H46O7NP | 478.2931 | 0.57 | 404, 386, 171, 153 | |
| 130 | l-PG (18:2) | 20.06 | C24H45O9P | 507.2719 | 0.26 | 415, 279, 245, 227, 153 | |
| 101 | l-PG (16:0) | 20.17 | C22H45O9P | 483.2722 | 0.86 | 391, 255, 245, 227, 153 | |
| 131 | SQMG (18:1) | 20.17 | C27H50O11S | 581.2994 | 0.64 | 563, 299, 281, 225 | |
| 100 | DGMG (18:3) | 20.52 | C33H56O14 | 675.3587 | 721.3639 | 0.10 | 397 |
| 132 | Bartogenic acid # | 20.69 | C30H46O7 | 517.3163 | 0.58 | 499, 455, | |
| 102 | l-PG (18:1) | 20.80 | C24H47O9P | 509.2878 | 0.81 | 417, 281, | |
| 133 | DGMG (18:2) | 21.20 | C33H58O14 | 677.3751 | 723.3796 | 1.15 | 415, 397, 235 |
| 134 | DGMG (16:0) | 21.31 | C31H58O14 | 653.3751 | 699.3802 | 1.19 | 415, 397, |
| 103 | l-PA (18:3) | 21.65 | C21H37O7P | 431.2193 | 0.03 | 413, 153 | |
| 135 | DGMG (18:1) | 22.11 | C33H60O14 | 679.3905 | 725.3952 | 0.83 | 415, 397, 235 |
| 104 | l-PC (16:0) | 22.45 | C24H50O7NP | 540.3297 | 0.21 | 480, 255 | |
| 105 | l-PA (18:2) | 22.51 | C21H39O7P | 433.2351 | 0.40 | 415, 279, 153 | |
| 107 | l-PA (16:0) | 22.74 | C19H39O7P | 409.2352 | 0.50 | 391, 255, 153 | |
| 136 | l-PEth (18:2) | 23.42 | C23H43O7P | 461.2664 | 0.33 | 415, 279, 181 | |
| 137 | l-PEth (16:0) | 23.71 | C21H43O7P | 437.2661 | −0.43 | 255, 181 | |
| 111 | l-PA (18:1) | 23.77 | C21H41O7P | 435.2508 | 0.47 | 417, 391, 281, 153 | |
| 138 | MGMG (18:2) | 24.97 | C27H48O9 | 515.3218 | 561.3273 | 0.74 | 279, 253, 235 |
| 139 | MGMG (16:0) | 25.20 | C25H48O9 | 491.3215 | 537.3274 | 0.08 | 255, 253, 235 |
| 140 | l-PEth (18:1) | 24.97 | C23H45O7P | 463.2822 | 0.55 | 281, 181 | |
| 141 | MGMG (18:1) | 26.69 | C27H50O9 | 517.3375 | 563.3439 | 0.68 | 281, 253, 235 |
| 122 | GlyCer (t18:1; h16:0) | 41.88 | C40H77O10N | 730.5461 | 776.5514 | −0.40 | 568, 550, 326, 271 |
| 142 | SQDG (16:0; 18:2) | 43.48 | C43H78O12S | 817.5129 | −0.10 | 723, 561, 537, 279, 225 | |
| 143 | SQDG (16:0; 18:2) | 44.50 | C43H78O12S | 817.5147 | 2.06 | 723, 561, 537, 279, 255, 225 | |
| 144 | PE (18:3, 18:2) | 44.88 | C41H72O8NP | 736.4924 | 1.70 | 458, 456, 279, 277, 255 | |
| 124 | GlyCer (d18:2; h16:0) | 45.44 | C40H75O9N | 712.5363 | 758.5417 | 0.72 | 550, 532, 312, 296, 271, 253, 225 |
| 145 | SQDG (16:0; 18:1) | 46.33 | C43H80O12S | 819.5280 | −0.80 | 563, 537, 281, 255, 225 | |
| 146 | PE (16:0; 18:3) | 46.22 | C39H72O8NP | 712.4899 | −1.76 | 456, 277, 255 | |
| 147 | PE (18:2; 18:2) | 46.81 | C41H74O8NP | 738.5067 | −0.16 | 476, 458, 279 | |
| 148 | DGDG (18:2; 18:2) | 48.10 | C51H88O15 | 939.6033 | 985.6087 | −0.66 | 677, 659, 415, 397, 279 |
| 149 | PG (16:0;18:2) | 48.31 | C40H75O10P | 745.5022 | 1.06 | 507, 489, 483, 465, 415, 391, 279, 255 | |
| 150 | PE (16:0; 18:2) | 48.85 | C39H74O8NP | 714.5063 | −0.76 | 458, 434, 279, 255 | |
| 151 | PE (18:2; 18:1) | 49.28 | C41H76O8NP | 740.5226 | 0.13 | 478, 476, 460, 458, 281, 279 | |
| 152 | MGDG (16:0; 12:2) | 50.44 | C37H66O10 | 669.4580 | 1.17 | 499, 431, 413, 255 |
| Compound | Mrm Transition | R2 | Regression Line | mg/100 g Burs ± SD * | mg/100 g Leaves ± SD * |
|---|---|---|---|---|---|
| Crenatin (7) | 317→155 | 0.9967 | y = 0.000143x + 0.0284 | 0.90 ± 0.09 | 15.77 ± 1.17 |
| chestanin (31) | 937→467 | 0.9962 | y = 0.000228x + 0.000139 | 3.23 ± 0.06 | 85.84 ± 2.18 |
| cretanin (32) | 469→169 | 0.9969 | y = 3.25e−5x-0.00466 | 16.75 ± 2.24 | 95.2 ± 7.21 |
| quercetin 3-O-β-D-glucopyranoside (54) | 463→301 | 0.9930 | y = 2.53x − 0.422 | 0.04 ± 0.002 | 3.37 ± 0.12 |
| ellagic acid (60) | 301→284 | 0.9968 | y = 8.93e−5x − 0.196 | 3.09 ± 0.25 | 7.97 ± 0.59 |
| isorhamnetin 3-O-β-D-glucopyranoside (61) | 477→301 | 0.9977 | y = 5.1e−6x + 0.00474 | 10.61 ± 0.29 | 50.33 ± 1.87 |
| quercetin-3-O-α-L-rhamnopyranoside (63) | 447→301 | 0.9981 | y = 0.00173x − 0.00746 | --- | 3.06 ± 0.24 |
| bartogenic acid (96) | 517→455 | 0.9916 | Y=0.000107x-0.154 | 3.59 ± 0.08 | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) Burs, Leaves, and Chestnuts Extracts and Their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. https://doi.org/10.3390/antiox10020278
Cerulli A, Napolitano A, Hošek J, Masullo M, Pizza C, Piacente S. Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) Burs, Leaves, and Chestnuts Extracts and Their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants. 2021; 10(2):278. https://doi.org/10.3390/antiox10020278
Chicago/Turabian StyleCerulli, Antonietta, Assunta Napolitano, Jan Hošek, Milena Masullo, Cosimo Pizza, and Sonia Piacente. 2021. "Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) Burs, Leaves, and Chestnuts Extracts and Their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS" Antioxidants 10, no. 2: 278. https://doi.org/10.3390/antiox10020278
APA StyleCerulli, A., Napolitano, A., Hošek, J., Masullo, M., Pizza, C., & Piacente, S. (2021). Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) Burs, Leaves, and Chestnuts Extracts and Their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants, 10(2), 278. https://doi.org/10.3390/antiox10020278