Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
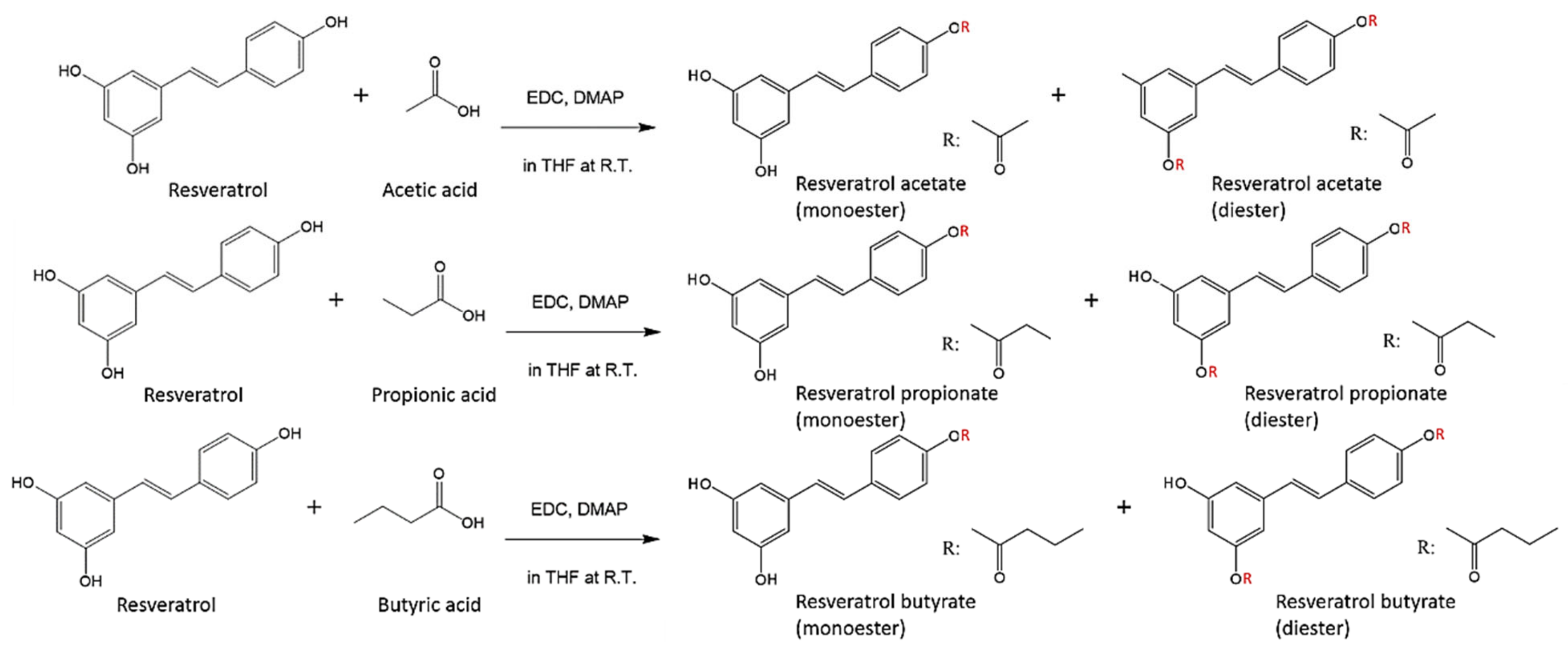
2.2. Synthesis of RE-SCFA Esters
2.3. Physical Properties and Chemical Compositions of RE and RE-SCFA Esters
2.4. Antioxidant Activity of RE-SCFA Esters in Bulk Oil
2.5. Antioxidant Activity of RE-SCFA Esters in Oil-in-Water Emulsion (β-Carotene Bleaching Assay)
2.6. Ability of RE-SCFA to Inhibit Cu2+-Induced LDL Oxidation
2.7. Ability of RE-SCFA to Inhibit Hydroxyl Radical-Induced DNA Scission
2.8. Statistical Analyses
3. Results
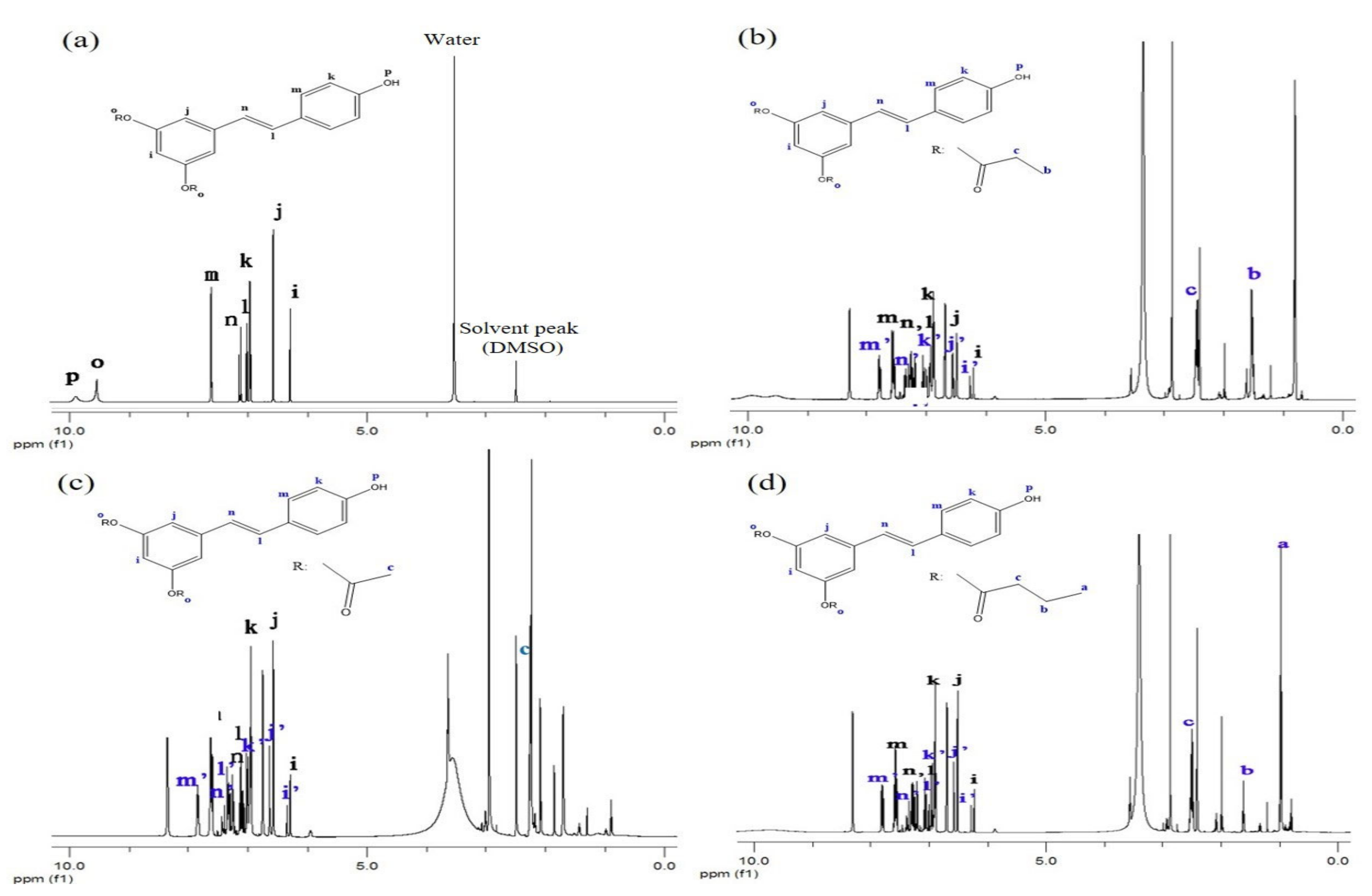
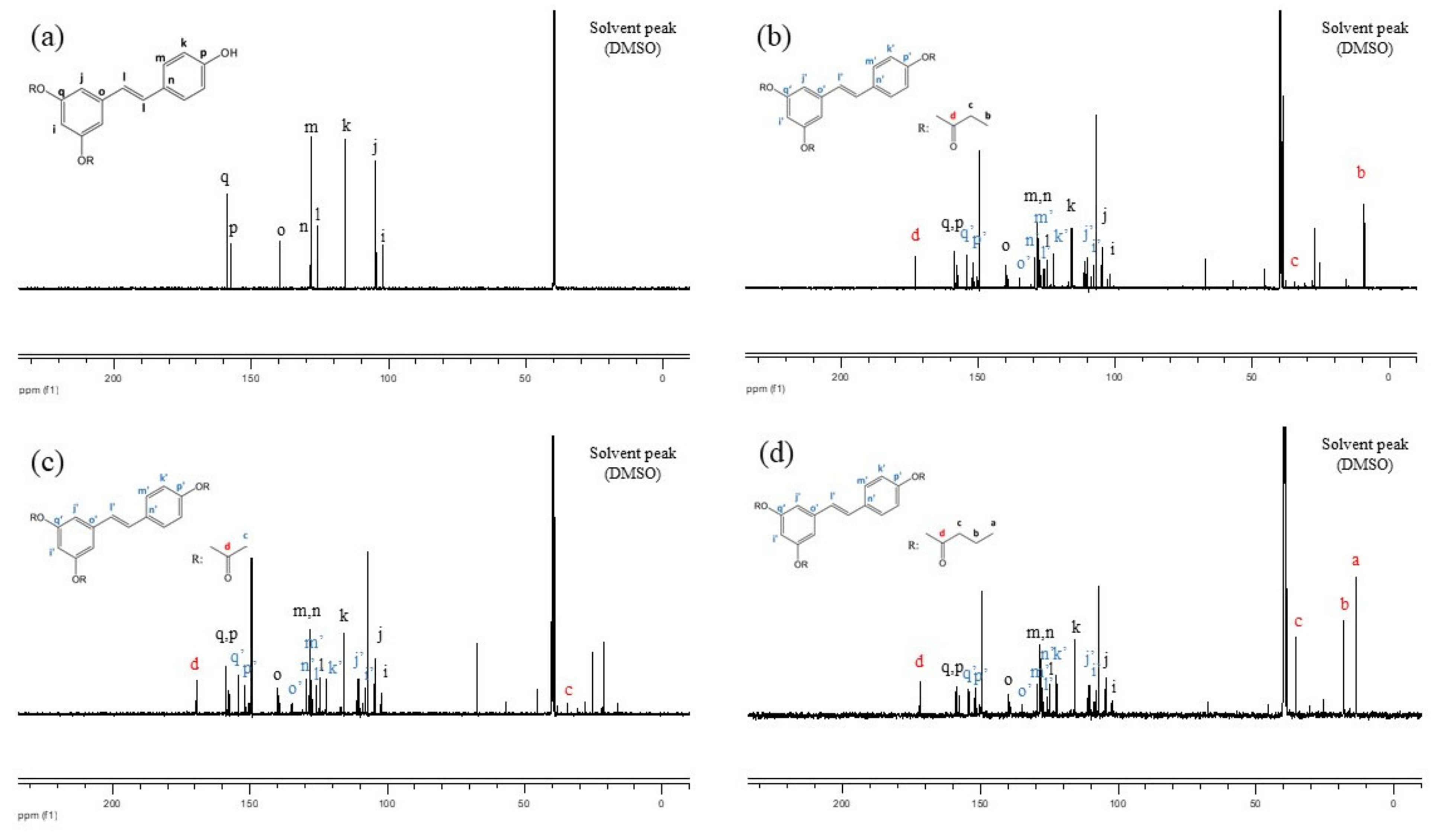
3.1. Synthesis of RE-SCFA Esters
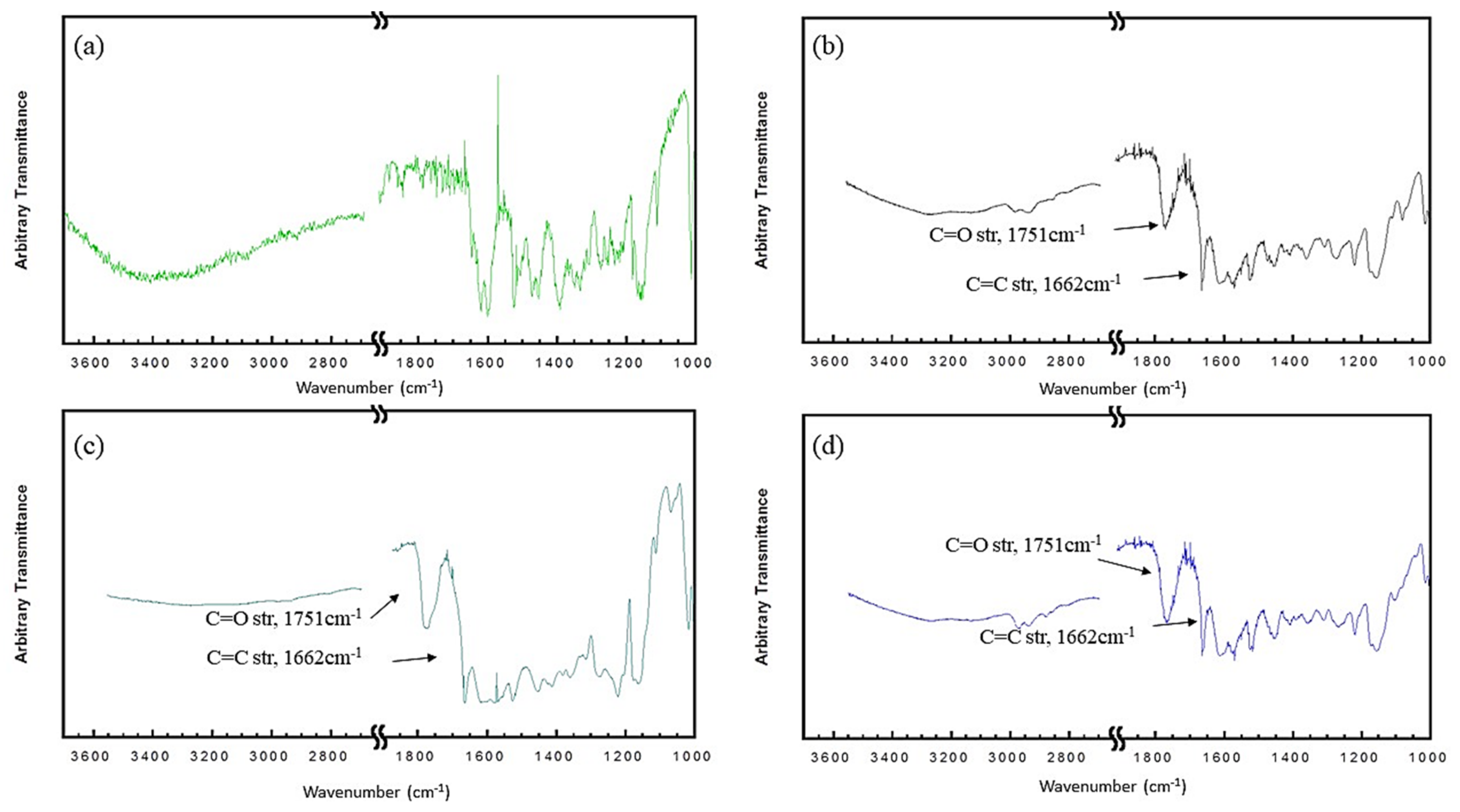
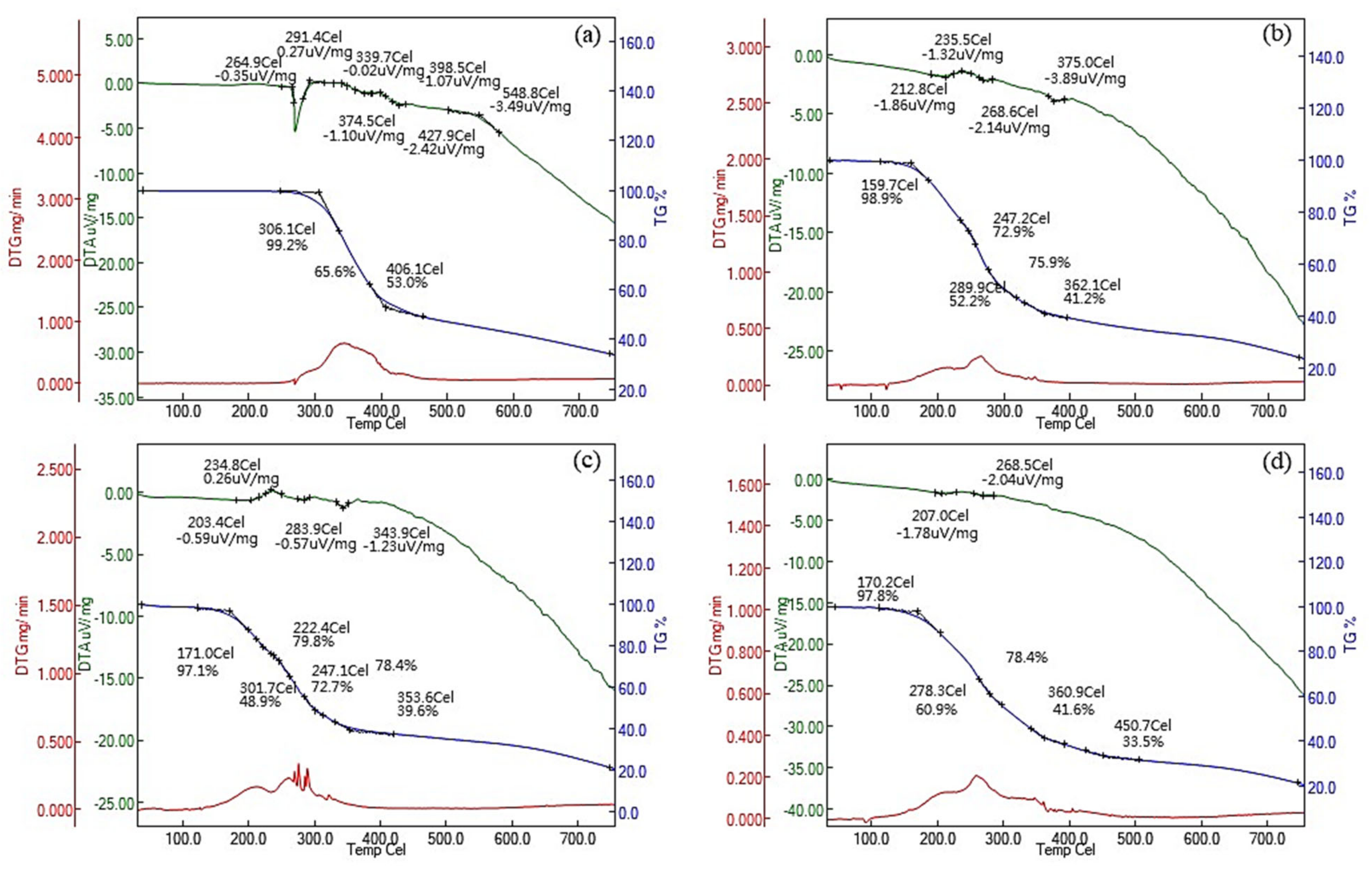
3.2. Physical Properties and Chemical Composition of RE and RE-SCFA Esters
3.3. Antioxidant Activity of RE Esters in Bulk Oil
3.4. Antioxidant Activity of RE Esters in Oil-in-Water Emulsion (β-Carotene Bleaching Assay)
3.5. Inhibition of Cu2+-Induced Low-Density Lipoprotein Oxidation
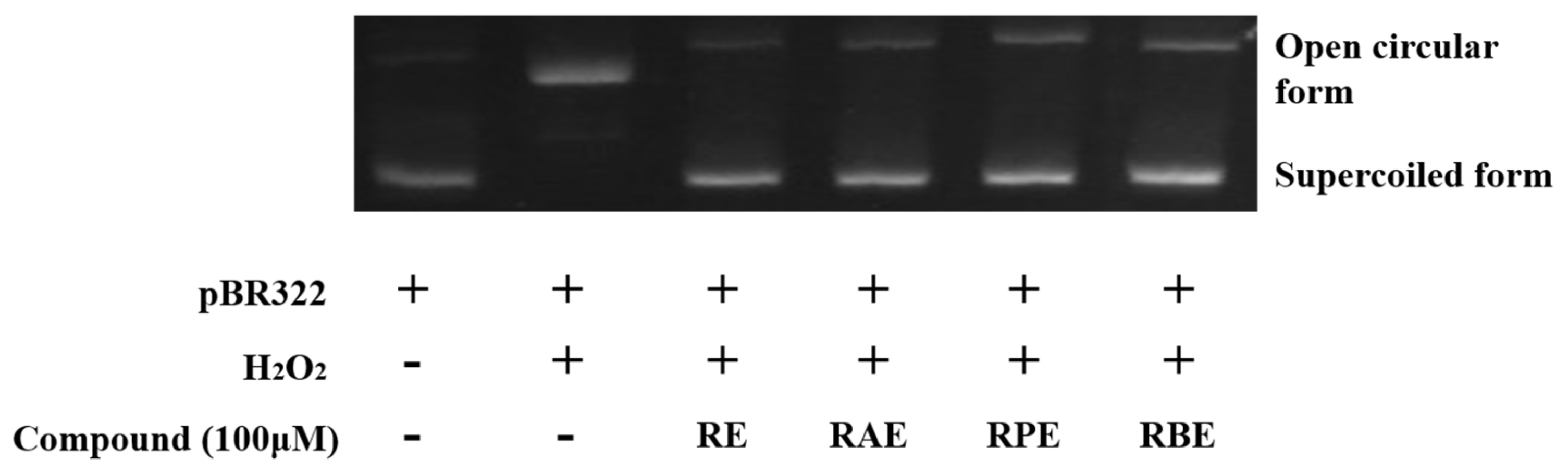
3.6. Inhibition of Hydroxyl Radical-Induced DNA Scission
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Jeandet, P.; Vannozzi, A.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Martínez-Márquez, A.; Clément, C.; Cordelier, S.; Manayi, A.; Nabavi, S.F.; et al. Phytostilbenes as agrochemicals: Biosynthesis, bioactivity, metabolic engineering and biotechnology. Nat. Prod. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Gurusamy, N.; Das, D.K. Resveratrol in cardiovascular health and disease. Ann. N. Y. Acad. Sci. 2011, 1215, 22–33. [Google Scholar] [CrossRef]
- Li, H.; Xia, N.; Förstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide Biol. Chem. 2012, 26, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Hsieh, T.-C.; Wang, Z. Cardioprotection by resveratrol: A review of effects/targets in cultured cells and animal tissues. Am. J. Cardiovasc. Dis. 2011, 1, 38–47. [Google Scholar]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Sobarzo-Sánchez, E.; Silva, A.S.; Clément, C.; Nabavi, S.F.; Battino, M.; Rasekhian, M.; Belwal, T.; Habtemariam, S.; Koffas, M.; et al. Whole-cell biocatalytic, enzymatic and green chemistry methods for the production of resveratrol and its derivatives. Biotechnol. Adv. 2020, 39. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Jheng, L.-C.; Chang, S.K.C.; Chen, Y.-W.; Huang, L.-T.; Liao, J.-X.; Hou, C.-Y. Synthesis and Characterization of Novel Resveratrol Butyrate Esters That Have the Ability to Prevent Fat Accumulation in a Liver Cell Culture Model. Molecules 2020, 25, 4199. [Google Scholar] [CrossRef]
- Szekeres, T.; Fritzer-Szekeres, M.; Saiko, P.; Jäger, W. Resveratrol and resveratrol analogues—Structure-activity relationship. Pharm. Res. 2010, 27, 1042–1048. [Google Scholar] [CrossRef]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure-activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Savio, M.; Coppa, T.; Bianchi, L.; Vannini, V.; Maga, G.; Forti, L.; Cazzalini, O.; Lazzè, M.C.; Perucca, P.; Prosperi, E.; et al. The resveratrol analogue 4,4′-dihydroxy-trans-stilbene inhibits cell proliferation with higher efficiency but different mechanism from resveratrol. Int. J. Biochem. Cell Biol. 2009, 41, 2493–2502. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of Foods and Beverages as a Tool for Increasing Availability of Bioactive Compounds. Focus on Short-Chain Fatty Acids. Foods 2020, 9. [Google Scholar] [CrossRef]
- Tonetti, M.; Eftimiadi, C.; Damiani, G.; Buffa, P.; Buffa, D.; Botta, G.A. Short chain fatty acids present in periodontal pockets may play a role in human periodontal diseases. J. Periodontal Res. 1987, 22, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid–Mediated Activation of G Protein–Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kuwabara, R.; deHaan, B.J.; Smink, A.M.; de Vos, P. Acetate and Butyrate Improve β-cell Metabolism and Mitochondrial Respiration under Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem. Int. Ed. Engl. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Faas, N.; Röcker, B.; Smrke, S.; Yeretzian, C.; Yildirim, S. Prevention of lipid oxidation in linseed oil using a palladium-based oxygen scavenging film. Food Packag. Shelf Life 2020, 24. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilised epigallocatechin gallate (EGCG) derivatives and their antioxidant potential in food and biological systems. Food Chem. 2012, 131, 22–30. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Shahidi, F. Antioxidant Potential of Date (Phoenix dactylifera L.) Seed Protein Hydrolysates and Carnosine in Food and Biological Systems. J. Agric. Food Chem. 2015, 63, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Porto, I.; Nascimento, T.; Oliveira, J.M.; Freitas, P.; Haimeur, A.; França, R. Use of polyphenols as a strategy to prevent bond degradation in the dentin-resin interface. Eur. J. Oral Sci. 2018, 126. [Google Scholar] [CrossRef] [PubMed]
- Gilles, V.; Vieira, M.A.; Lacerda, V., Jr.; Castro, E.V.R.; Santos, R.B.; Orestes, E.; Carneiro, J.W.M.; Greco, S.J. A New, Simple and Efficient Method of Steglich Esterification of Juglone with Long-Chain Fatty Acids: Synthesis of a New Class of Non-Polymeric Wax Deposition Inhibitors for Crude Oil. J. Braz. Chem. Soc. 2015, 26, 74–83. [Google Scholar] [CrossRef]
- Lu, D.-L.; Ding, D.; Yan, W.; Li, R.-W.; Dai, F.; Wang, Q.; Yu, S.-S.; Li, Y.; Jin, X.-L.; Zhou, B. Influence of Glucuronidation and Reduction Modifications of Resveratrol on its Biological Activities. Chembiochem 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-G.; Lu, M.; Chen, Z.-H.; Zhu, H.-H.; Li, Y.; Yang, L.; Wu, L.-M.; Liu, Z.-L. Antioxidant effects of resveratrol and its analogues against the free-radical-induced peroxidation of linoleic acid in micelles. Chemistry 2002, 8, 4191–4198. [Google Scholar] [CrossRef]
- Liu, W.B.; Hu, L.; Hu, Q.; Chen, N.N.; Yang, Q.S.; Wang, F.F. New resveratrol oligomer derivatives from the roots of rheum lhasaense. Molecules 2013, 18, 7093–7102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erfando, T.; Khalid, I.; Bahari, R. Experimental of alternative demulsifier formulation from corn oil in overcoming water–oil emulsion. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Monitoring of minor compounds in corn oil oxidation by direct immersion-solid phase microextraction-gas chromatography/mass spectrometry. New oil oxidation markers. Food Chem. 2019, 290, 286–294. [Google Scholar] [CrossRef]
- Ferrari, R.A.; Schulte, E.; Esteves, W.; Brühl, L.; Mukherjee, K.D. Minor constituents of vegetable oils during industrial processing. J. Am. Oil Chem. Soc. 1996, 73, 587–592. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Influence of some experimental variables and matrix components in the determination of antioxidant properties by β-carotene bleaching assay: Experiments with BHT used as standard antioxidant. Eur. Food Res. Technol. 2010, 231, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Porter, W.L. Recent Trends in Food Applications of Antioxidants BT—Autoxidation in Food and Biological Systems; Simic, M.G., Karel, M., Eds.; Springer: Boston, MA, USA, 1980; pp. 295–365. ISBN 978-1-4757-9351-2. [Google Scholar]
- Decker, E.A.; McClements, D.J.; Bourlieu-Lacanal, C.; Durand, E.; Figueroa-Espinoza, M.C.; Lecomte, J.; Villeneuve, P. Hurdles in Predicting Antioxidant Efficacy in Oil-in-water emulsions. Trends Food Sci. Technol. 2017, 67, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Nègre-Salvayre, A.; Augé, N.; Camaré, C.; Bacchetti, T.; Ferretti, G.; Salvayre, R. Dual signaling evoked by oxidized LDLs in vascular cells. Free Radic. Biol. Med. 2017, 106, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, V.F.; Re, N.; Coletti, C.; Defant, A.; Mancini, I.; Tosi, P. A joint experimental and theoretical investigation on the oxidative coupling of resveratrol induced by copper and iron ions. Int. J. Mass Spectrom. 2012, 319–320, 55–63. [Google Scholar] [CrossRef]
- Zheng, L.-F.; Wei, Q.-Y.; Cai, Y.-J.; Fang, J.-G.; Zhou, B.; Yang, L.; Liu, Z.-L. DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu (II) ions: Mechanism and structure-activity relationship. Free Radic. Biol. Med. 2006, 41, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Poetsch, A.R. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Živković, L.; Bajić, V.; Bruić, M.; Borozan, S.; Popić, K.; Topalović, D.; Santibanez, J.; Spremo-Potparević, B. Antigenotoxic and antioxidant potential of medicinal mushrooms (Immune Assist) against DNA damage induced by free radicals-an in vitro study. Mutat. Res. Toxicol. Environ. Mutagen. 2019, 845, 403078. [Google Scholar] [CrossRef]
- Sun, R.; Vuillier, L.; Deakin, J.; Kogan, A. Oxytocin increases emotional theory of mind, but only for low socioeconomic status individuals. Heliyon 2020, 6. [Google Scholar] [CrossRef]
- Bagheri Hashkavayi, A.; Hashemnia, S.; Osfouri, S. Investigations of antioxidant potential and protective effect of Acanthophora algae on DNA damage: An electrochemical approach. Microchem. J. 2020, 159. [Google Scholar] [CrossRef]
- Rossi, M.; Caruso, F.; Antonioletti, R.; Viglianti, A.; Traversi, G.; Leone, S.; Basso, E.; Cozzi, R. Scavenging of hydroxyl radical by resveratrol and related natural stilbenes after hydrogen peroxide attack on DNA. Chem. Biol. Interact. 2013, 206, 175–185. [Google Scholar] [CrossRef] [PubMed]
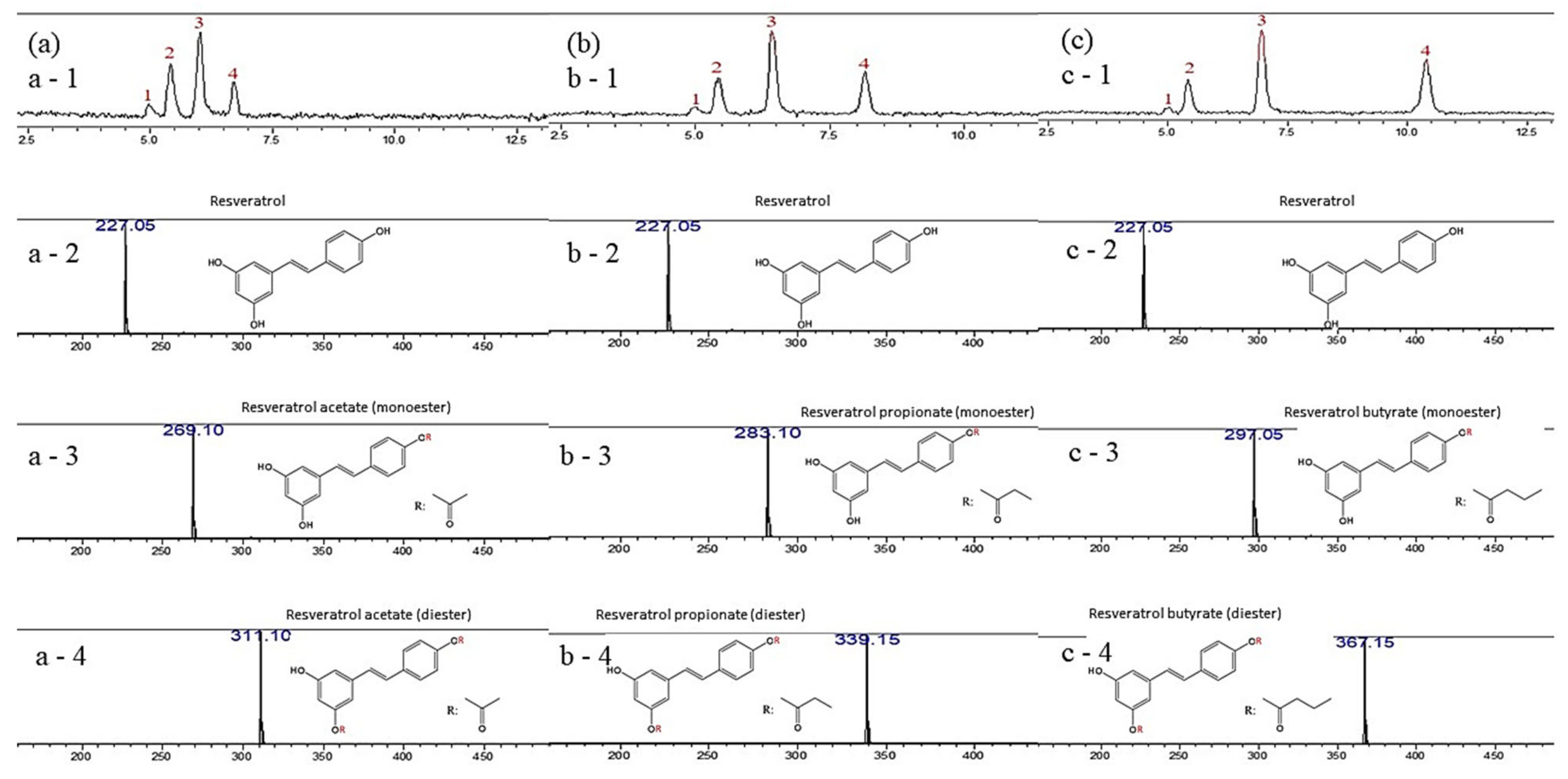
| Sample | Peak 1 a (%) | Peak 2 b (%) | Peak 3 b (%) | Peak 4 b (%) |
|---|---|---|---|---|
| Resveratrol acetate | 1.620 | 25.34 | 49.64 | 23.40 |
| Resveratrol propionate | 1.475 | 19.91 | 45.81 | 32.80 |
| Resveratrol butyrate | 0.768 | 17.11 | 47.12 | 35.00 |
| Sample | Conjugated Diene Content (%) | p-Anisidine Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Storage Period | Storage Period | |||||||
| Day 0 | Day 1 | Day 3 | Day 6 | Day 0 | Day 1 | Day 3 | Day 6 | |
| Resveratrol | 0.09 ± 0.00 a | 0.16 ± 0.00 a | 0.16 ± 0.02 a | 0.28 ± 0.01 a | 8.71 ± 0.12 a | 4.29 ± 0.06 b | 4.69 ± 0.06 b | 16.40 ± 0.55 c |
| Resveratrol acetate | 0.07 ± 0.01 a | 0.14 ± 0.00 a,b | 0.14 ± 0.00 a,b | 0.27 ± 0.01 a,b | 3.93 ± 0.09 a | 4.79 ± 0.09 a | 4.41 ± 0.09 a | 10.71 ± 0.97 b |
| Resveratrol propionate | 0.05 ± 0.01 a | 0.16 ± 0.01 b | 0.17 ± 0.01 b | 0.29 ± 0.01 b | 0.27 ± 0.31 a | 4.13 ± 0.41 b | 9.19 ± 0.16 c | 27.89 ± 0.51 d |
| Resveratrol butyrate | 0.09 ± 0.06 a | 0.15 ± 0.01 b | 0.17 ± 0.00 b | 0.29 ± 0.00 b | 3.71 ± 0.03 a | 4.05 ± 0.10 a | 4.91 ± 0.07 a | 18.65 ± 1.53 b |
| Sample | β-Carotene Bleaching (Inhibition %) | LDL (Inhibition %) |
|---|---|---|
| Resveratrol | 60.9 ± 0.51 a | −59.9 ± 4.62 b |
| Resveratrol acetate | 46.7 ± 1.01 b | 74.1 ± 33.2 a |
| Resveratrol propionate | 41.1 ± 0.54 d | 79.0 ± 11.3 a |
| Resveratrol butyrate | 44.9 ± 0.83 c | 64.8 ± 20.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Chang, S.K.C.; Liao, J.-X.; Chen, Y.-W.; Huang, H.-T.; Li, Y.-L.; Hou, C.-Y. Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties. Antioxidants 2021, 10, 420. https://doi.org/10.3390/antiox10030420
Tain Y-L, Chang SKC, Liao J-X, Chen Y-W, Huang H-T, Li Y-L, Hou C-Y. Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties. Antioxidants. 2021; 10(3):420. https://doi.org/10.3390/antiox10030420
Chicago/Turabian StyleTain, You-Lin, Sam K. C. Chang, Jin-Xian Liao, Yu-Wei Chen, Hung-Tse Huang, Yu-Lun Li, and Chih-Yao Hou. 2021. "Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties" Antioxidants 10, no. 3: 420. https://doi.org/10.3390/antiox10030420
APA StyleTain, Y. -L., Chang, S. K. C., Liao, J. -X., Chen, Y. -W., Huang, H. -T., Li, Y. -L., & Hou, C. -Y. (2021). Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties. Antioxidants, 10(3), 420. https://doi.org/10.3390/antiox10030420









