Rolipram Prevents the Formation of Abdominal Aortic Aneurysm (AAA) in Mice: PDE4B as a Target in AAA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Animal Handling
2.3. Non-Invasive Measurement of Systolic Blood Pressure
2.4. Ultrasound Measurement of the Abdominal Aortic Diameter
2.5. Basic Measurements of Cardiac Function by Echocardiography (M-mode and Doppler)
2.6. Real-Time Polymerase Chain Reaction (PCR)
2.7. Western Blotting
2.8. Histological and Immunohistochemical Analysis
2.9. In Situ Zymography
2.10. In Situ Detection of Vascular O2·− Production
2.11. Statistical Analysis
3. Results
3.1. Vascular PDE4B Expression Is Enhanced in Mouse and Human AAA
3.2. Treatment of ApoE−/− Mice with Rolipram Prevents AAA Formation Induced by AngII Infusion
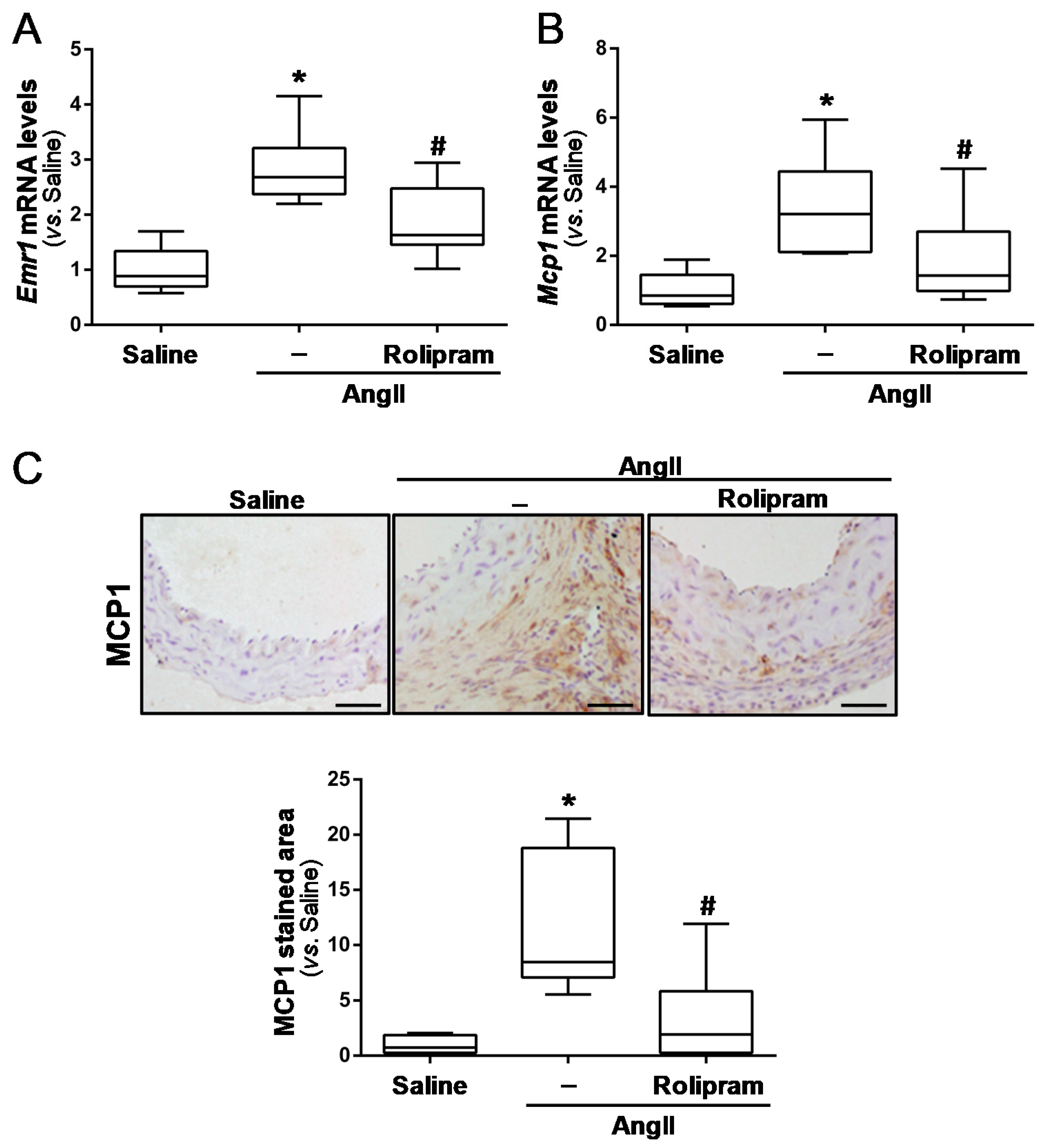
3.3. Rolipram Ameliorates AngII-Induced Inflammation and Oxidative Stress
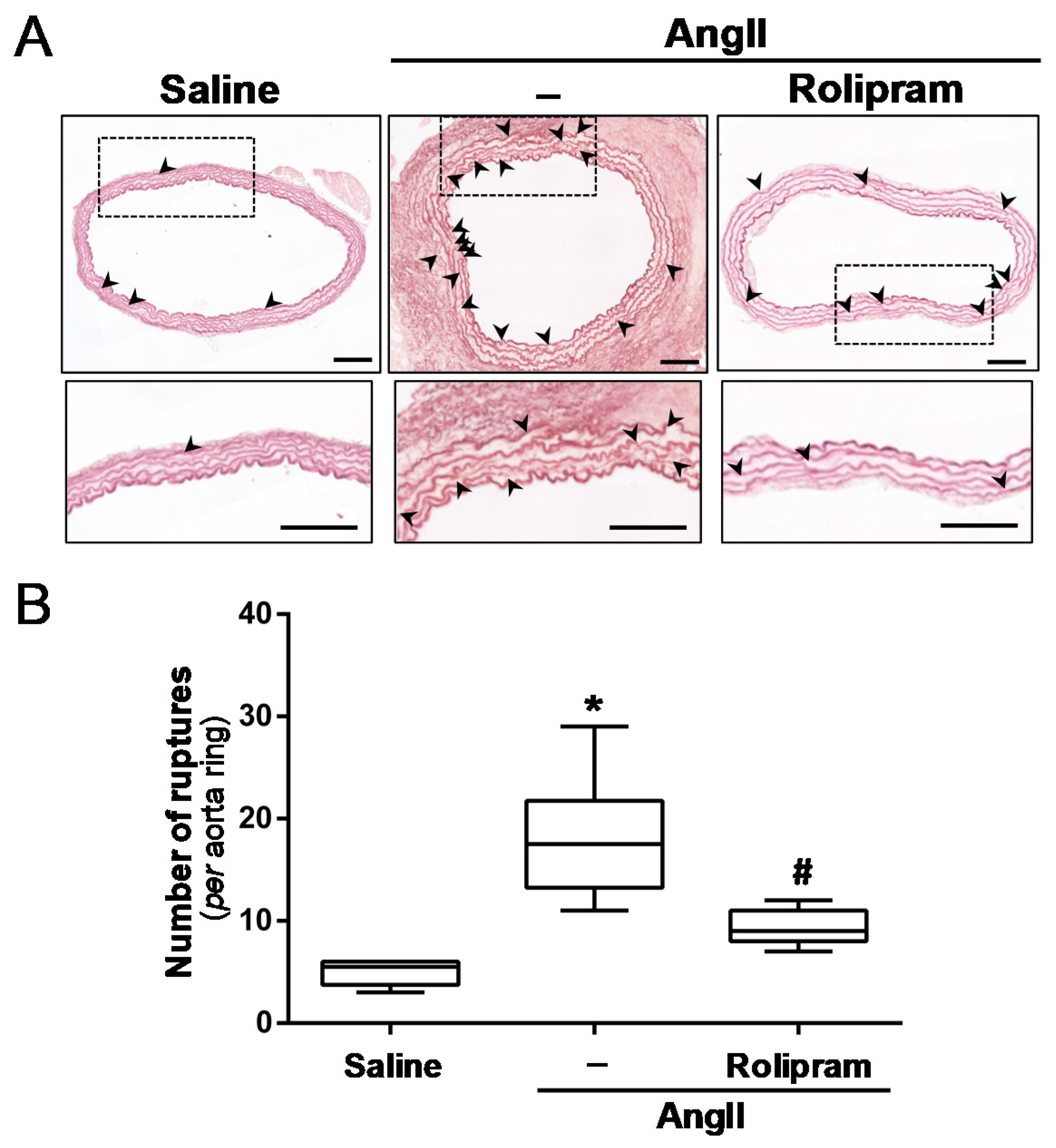
3.4. Rolipram Attenuates the Destructive Vascular Remodelling Triggered by AngII, and Limits MMP Expression and Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 92–102. [Google Scholar] [CrossRef]
- Kent, K.C. Clinical practice. Abdominal aortic aneurysms. N. Engl. J. Med. 2014, 371, 2101–2108. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2019, 16, 225–242. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuivaniemi, H.; Ryer, E.J.; Elmore, J.R.; Tromp, G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev. Cardiovasc. Ther. 2015, 13, 975–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golledge, J.; Muller, J.; Daugherty, A.; Norman, P. Abdominal aortic aneurysm: Pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2605–2613. [Google Scholar] [CrossRef] [Green Version]
- Camacho, M.; Dilmé, J.; Solà-Villà, D.; Rodríguez, C.; Bellmunt, S.; Siguero, L.; Alcolea, S.; Romero, J.M.; Escudero, J.R.; Martínez-González, J.; et al. Microvascular COX-2/mPGES-1/EP-4 axis in human abdominal aortic aneurysm. J. Lipid. Res. 2013, 54, 3506–3515. [Google Scholar] [CrossRef] [Green Version]
- Torres-Fonseca, M.; Galan, M.; Martinez-Lopez, D.; Cañes, L.; Roldan-Montero, R.; Alonso, J.; Reyero-Postigo, T.; Orriols, M.; Mendez-Barbero, N.; Sirvent, M.; et al. Pathophisiology of abdominal aortic aneurysm: Biomarkers and novel therapeutic targets. Clin. Investig. Arterioscler. 2019, 31, 166–177. [Google Scholar]
- Furusho, A.; Aoki, H.; Ohno-Urabe, S.; Nishihara, M.; Hirakata, S.; Nishida, N.; Ito, S.; Hayashi, M.; Imaizumi, T.; Hiromatsu, S.; et al. Involvement of B cells, immunoglobulins, and syk in the pathogenesis of abdominal aortic aneurysm. J. Am. Heart Assoc. 2018, 7, e007750. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, W.; Lindholt, J.S.; Sukhova, G.K.; Libby, P.; Yu, X.; Shi, G.P. Regulatory T cells in human and angiotensin II-induced mouse abdominal aortic aneurysms. Cardiovasc. Res. 2015, 107, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuruda, T.; Kato, J.; Hatakeyama, K.; Kojima, K.; Yano, M.; Yano, Y.; Nakamura, K.; Nakamura-Uchiyama, F.; Matsushima, Y.; Imamura, T.; et al. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ. Res. 2008, 102, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kotera, J. Overview of PDEs and their regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, A.T.; Beavo, J.A. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol. Rev. 2006, 58, 488–520. [Google Scholar] [CrossRef] [PubMed]
- Bobin, P.; Belacel-Ouari, M.; Bedioune, I.; Zhang, L.; Leroy, J.; Leblais, V.; Fischmeister, R.; Vandecasteele, G. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Arch. Cardiovasc. Dis. 2016, 109, 431–443. [Google Scholar] [CrossRef]
- Fertig, B.A.; Baillie, G.S. PDE4-mediated cAMP signalling. J. Cardiovasc. Dev. Dis. 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houslay, M.D.; Baillie, G.S.; Maurice, D.H. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: A molecular toolbox for generating compartmentalized cAMP signaling. Circ. Res. 2007, 100, 950–966. [Google Scholar] [CrossRef]
- Azam, M.A.; Tripuraneni, N.S. Selective phosphodiesterase 4B inhibitors: A review. Sci. Pharm. 2014, 82, 453–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houslay, M.D.; Adams, D.R. PDE4 cAMP phosphodiesterases: Modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 2003, 370, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Qin, Y.; Li, D.; Cai, N.; Wu, J.; Jiang, L.; Jie, L.; Zhou, Z.; Xu, J.; Wang, H. Inhibition of PDE4 protects neurons against oxygen-glucose deprivation-induced endoplasmic reticulum stress through activation of the Nrf-2/HO-1 pathway. Redox Biol. 2020, 28, 101342. [Google Scholar] [CrossRef]
- Zhong, J.; Yu, H.; Huang, C.; Zhong, Q.; Chen, Y.; Xie, J.; Zhou, Z.; Xu, J.; Wang, H. Inhibition of phosphodiesterase 4 by FCPR16 protects SH-SY5Y cells against MPP+-induced decline of mitochondrial membrane potential and oxidative stress. Redox Biol. 2018, 16, 47–58. [Google Scholar] [CrossRef]
- Bhat, A.; Tan, V.; Heng, B.; Lovejoy, D.B.; Sakharkar, M.K.; Essa, M.M.; Chidambaram, S.B.; Guillemin, G.J. Roflumilast, a cAMP-specific phosphodiesterase-4 inhibitor, reduces oxidative stress and improves synapse functions in human cortical neurons exposed to the excitotoxin quinolinic acid. ACS Chem. Neurosci. 2020, 11, 4405–4415. [Google Scholar] [CrossRef]
- Orriols, M.; Varona, S.; Martí-Pàmies, I.; Galán, M.; Guadall, A.; Escudero, J.R.; Martín-Ventura, J.L.; Camacho, M.; Vila, L.; Martínez-González, J.; et al. Down-regulation of Fibulin-5 is associated with aortic dilation: Role of inflammation and epigenetics. Cardiovasc. Res. 2016, 110, 431–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, J.; Galán, M.; Martí-Pàmies, I.; Romero, J.M.; Camacho, M.; Rodríguez, C.; Martínez-González, J. NOR-1/NR4A3 regulates the cellular inhibitor of apoptosis 2 (cIAP2) in vascular cells: Role in the survival response to hypoxic stress. Sci. Rep. 2016, 6, 34056. [Google Scholar] [CrossRef] [Green Version]
- Daugherty, A.; Manning, M.W.; Cassis, L.A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Investig. 2000, 105, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañes, L.; Martí-Pàmies, I.; Ballester-Servera, C.; Alonso, J.; Serrano, E.; Briones, A.M.; Rodríguez, C.; Martínez-González, J. High neuron derived orphan receptor-1 (NOR-1) expression strengthens the vascular wall response to angiotensin II leading to aneurysm formation in mice. Hypertension 2021, 77, 557–570. [Google Scholar] [CrossRef]
- Cañes, L.; Martí-Pàmies, I.; Ballester-Servera, C.; Herraiz-Martínez, A.; Alonso, J.; Galán, M.; Nistal, J.F.; Muniesa, P.; Osada, J.; Hove-Madsen, L.; et al. Neuron-derived orphan receptor-1 modulates cardiac gene expression and exacerbates angiotensin II-induced cardiac hypertrophy. Clin. Sci. 2020, 134, 359–377. [Google Scholar] [CrossRef]
- Galán, M.; Varona, S.; Guadall, A.; Orriols, M.; Navas, M.; Aguiló, S.; de Diego, A.; Navarro, M.A.; García-Dorado, D.; Rodríguez-Sinovas, A.; et al. Lysyl oxidase overexpression accelerates cardiac remodeling and aggravates angiotensin II-induced hypertrophy. FASEB J. 2017, 31, 3787–3799. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; O’Callaghan, J.P.; O’Donnell, J.M. Effects of repeated treatment with phosphodiesterase-4 inhibitors on cAMP signaling, hippocampal cell proliferation, and behavior in the forced-swim test. J. Pharmacol. Exp. Ther. 2011, 338, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Manning, M.W.; Cassis, L.A.; Daugherty, A. Differential effects of doxycycline, a broadspectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jover, E.; Silvente, A.; Marín, F.; Martínez-González, J.; Orriols, M.; Martinez, C.M.; Puche, C.M.; Valdés, M.; Rodriguez, C.; Hernández-Romero, D. Inhibition of enzymes involved in collagen cross-linking reduces vascular smooth muscle cell calcification. FASEB J. 2018, 32, 4459–4469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Revelles, S.; García-Redondo, A.B.; Avendaño, M.S.; Varona, S.; Palao, T.; Orriols, M.; Roque, F.R.; Fortuño, A.; Touyz, R.M.; Martínez-González, J.; et al. Lysyl oxidase induces vascular oxidative stress and contributes to arterial stiffness and abnormal elastin structure in hypertension: Role of p38MAPK. Antioxid. Redox Signal. 2017, 27, 379–397. [Google Scholar] [CrossRef]
- Wang, P.; Ohleth, K.M.; Wu, P.; Billah, M.M.; Egan, R.W. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol. Pharmacol. 1999, 56, 170–174. [Google Scholar] [CrossRef]
- Castro, A.; Jerez, M.J.; Gil, C.; Martinez, A. Cyclic nucleotide phosphodiesterases and their role in immunomodulatory responses: Advances in the development of specific phosphodiesterase inhibitors. Med. Res. Rev. 2005, 25, 229–244. [Google Scholar] [CrossRef]
- Richter, W.; Menniti, F.S.; Zhang, H.T.; Conti, M. PDE4 as a target for cognition enhancement. Expert. Opin. Ther. Targets 2013, 17, 1011–1027. [Google Scholar] [CrossRef] [Green Version]
- Hackam, D.G.; Thiruchelvam, D.; Redelmeier, D.A. Angiotensin-converting enzyme inhibitors and aortic rupture: A population-based case-control study. Lancet 2006, 368, 659–665. [Google Scholar] [CrossRef]
- Kiss, T.; Giles, C.B.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Gautam, T.; Csipo, T.; Nyúl-Tóth, Á.; Lipecz, A.; Szabo, C.; et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience 2019, 41, 419–439. [Google Scholar] [CrossRef]
- Yagi, K.; Tada, Y.; Kitazato, K.T.; Tamura, T.; Satomi, J.; Nagahiro, S. Ibudilast inhibits cerebral aneurysms by down-regulating inflammation-related molecules in the vascular wall of rats. Neurosurgery 2010, 66, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Komas, N.; Lugnier, C.; Stoclet, J.C. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br. J. Pharmacol. 1991, 104, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, Y.J.; Xi, L. Pivotal effects of phosphodiesterase inhibitors on myocyte contractility and viability in normal and ischemic hearts. Acta Pharmacol. Sin. 2009, 30, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eschenhagen, T. PDE4 in the human heart—Major player or little helper? Br. J. Pharmacol. 2013, 169, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Totani, L.; Piccoli, A.; Dell’Elba, G.; Concetta, A.; Di Santo, A.; Martelli, N.; Federico, L.; Pamuklar, Z.; Smyth, S.S.; Evangelista, V. Phosphodiesterase type 4 blockade prevents platelet-mediated neutrophil recruitment at the site of vascular injury. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1689–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, M.J.; Cortijo, J.; Taha, M.A.; Cerdá-Nicolás, M.; Schatton, E.; Burgbacher, B.; Klar, J.; Tenor, H.; Schudt, C.; Issekutz, A.C.; et al. Roflumilast inhibits leukocyte-endothelial cell interactions, expression of adhesion molecules and microvascular permeability. Br. J. Pharmacol. 2007, 152, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.G.; Cassali, G.D.; Poole, S.; Teixeira, M.M. Effects of inhibition of PDE4 and TNF-alpha on local and remote injuries following ischaemia and reperfusion injury. Br. J. Pharmacol. 2001, 134, 985–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumer, Y.; McCurdy, S.; Weatherby, T.M.; Mehta, N.N.; Halbherr, S.; Halbherr, P.; Yamazaki, N.; Boisvert, W.A. Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat. Commun. 2017, 8, 1129. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.J.; Reynolds, L.J.; Toward, T.J. Elastolytic activity and alveolar epithelial type-1 cell damage after chronic LPS inhalation: Effects of dexamethasone and rolipram. Toxicol. Appl. Pharmacol. 2005, 207, 257–265. [Google Scholar] [CrossRef]
- Growcott, E.J.; Spink, K.G.; Ren, X.; Afzal, S.; Banner, K.H.; Wharton, J. Phosphodiesterase type 4 expression and anti-proliferative effects in human pulmonary artery smooth muscle cells. Respir. Res. 2006, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Chouly, C.A.; Astier, A.; Jacob, C.; Pruniaux, M.P.; Bertrand, C.; Lagente, V. Modulation of matrix metalloproteinase production from human lung fibroblasts by type 4 phosphodiesterase inhibitors. Life Sci. 2004, 75, 823–840. [Google Scholar] [CrossRef]
- White, W.B.; Cooke, G.E.; Kowey, P.R.; Calverley, P.M.; Bredenbröker, D.; Goehring, U.M.; Zhu, H.; Lakkis, H.; Mosberg, H.; Rowe, P.; et al. Cardiovascular safety in patients receiving roflumilast for the treatment of COPD. Chest 2013, 144, 758–765. [Google Scholar] [CrossRef]
- Lourenço, E.M.G.; Fernandes, J.M.; Carvalho, V.F.; Grougnet, R.; Martins, M.A.; Jordão, A.K.; Zucolotto, S.M.; Barbosa, E.G. Identification of a selective PDE4B inhibitor from bryophyllum pinnatum by target fishing study and in vitro evaluation of quercetin 3-O-α-L-arabinopyranosyl-(1→2)-O-α-L-rhamnopyranoside. Front. Pharmacol. 2020, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Eliason, J.L.; Hannawa, K.K.; Ailawadi, G.; Sinha, I.; Ford, J.W.; Deogracias, M.P.; Roelofs, K.J.; Woodrum, D.T.; Ennis, T.L.; Henke, P.K.; et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation 2005, 112, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Sukhova, G.K.; Yang, M.; Wolters, P.J.; MacFarlane, L.A.; Libby, P.; Sun, C.; Zhang, Y.; Liu, J.; Ennis, T.L.; et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J. Clin. Investig. 2007, 117, 3359–3368. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varona, S.; Puertas, L.; Galán, M.; Orriols, M.; Cañes, L.; Aguiló, S.; Camacho, M.; Sirvent, M.; Andrés, V.; Martínez-González, J.; et al. Rolipram Prevents the Formation of Abdominal Aortic Aneurysm (AAA) in Mice: PDE4B as a Target in AAA. Antioxidants 2021, 10, 460. https://doi.org/10.3390/antiox10030460
Varona S, Puertas L, Galán M, Orriols M, Cañes L, Aguiló S, Camacho M, Sirvent M, Andrés V, Martínez-González J, et al. Rolipram Prevents the Formation of Abdominal Aortic Aneurysm (AAA) in Mice: PDE4B as a Target in AAA. Antioxidants. 2021; 10(3):460. https://doi.org/10.3390/antiox10030460
Chicago/Turabian StyleVarona, Saray, Lídia Puertas, María Galán, Mar Orriols, Laia Cañes, Silvia Aguiló, Mercedes Camacho, Marc Sirvent, Vicente Andrés, José Martínez-González, and et al. 2021. "Rolipram Prevents the Formation of Abdominal Aortic Aneurysm (AAA) in Mice: PDE4B as a Target in AAA" Antioxidants 10, no. 3: 460. https://doi.org/10.3390/antiox10030460
APA StyleVarona, S., Puertas, L., Galán, M., Orriols, M., Cañes, L., Aguiló, S., Camacho, M., Sirvent, M., Andrés, V., Martínez-González, J., & Rodríguez, C. (2021). Rolipram Prevents the Formation of Abdominal Aortic Aneurysm (AAA) in Mice: PDE4B as a Target in AAA. Antioxidants, 10(3), 460. https://doi.org/10.3390/antiox10030460








