Combined Ubisol-Q10 and Ashwagandha Root Extract Target Multiple Biochemical Mechanisms and Reduces Neurodegeneration in a Paraquat-Induced Rat Model of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethanolic Extraction of Ashwagandha Root and Phytochemical Assessment
2.2. Animal Care
2.3. Injection Regimen
2.4. Drinking Water Treatment
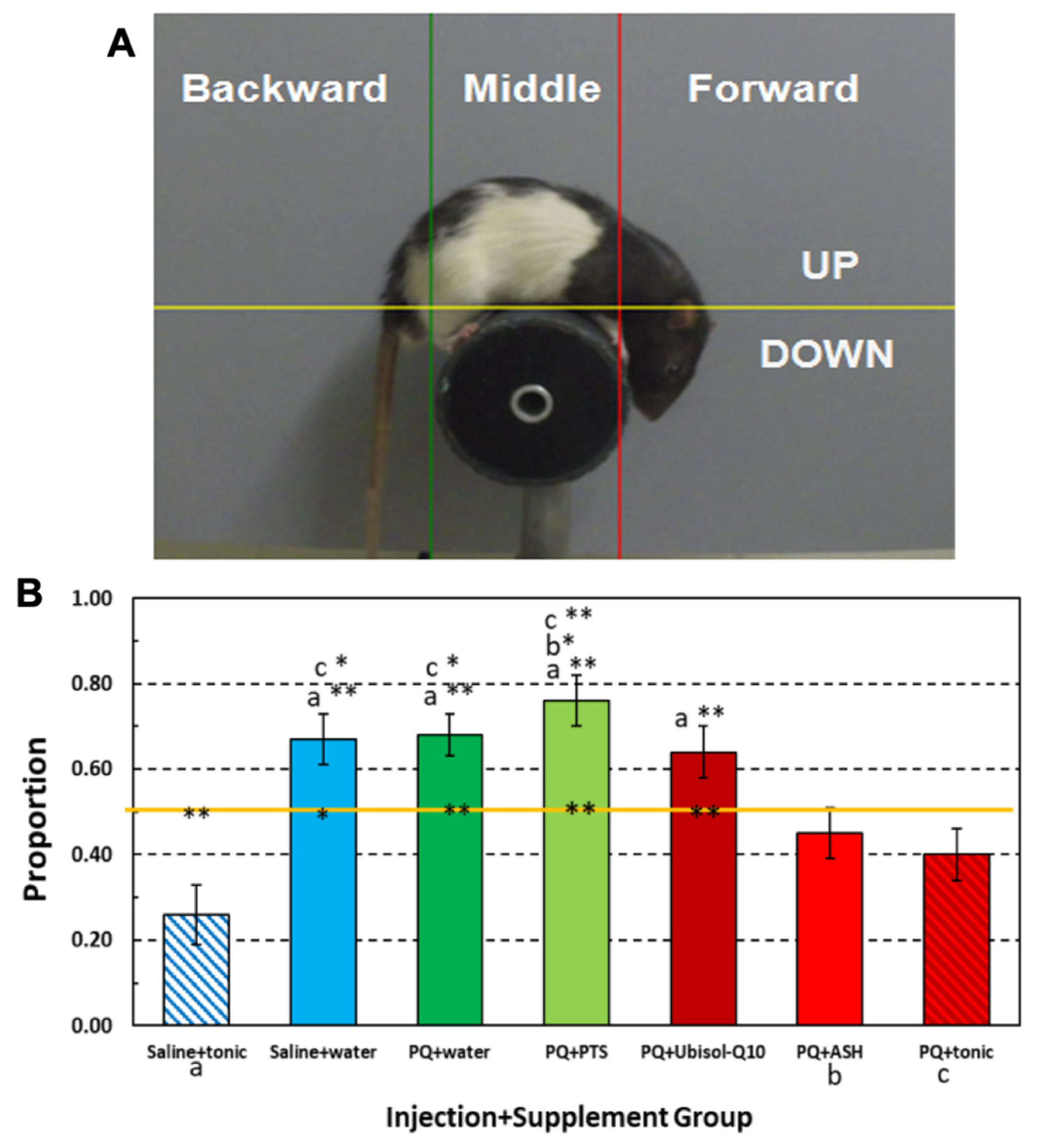
2.5. Behavioral Assessments
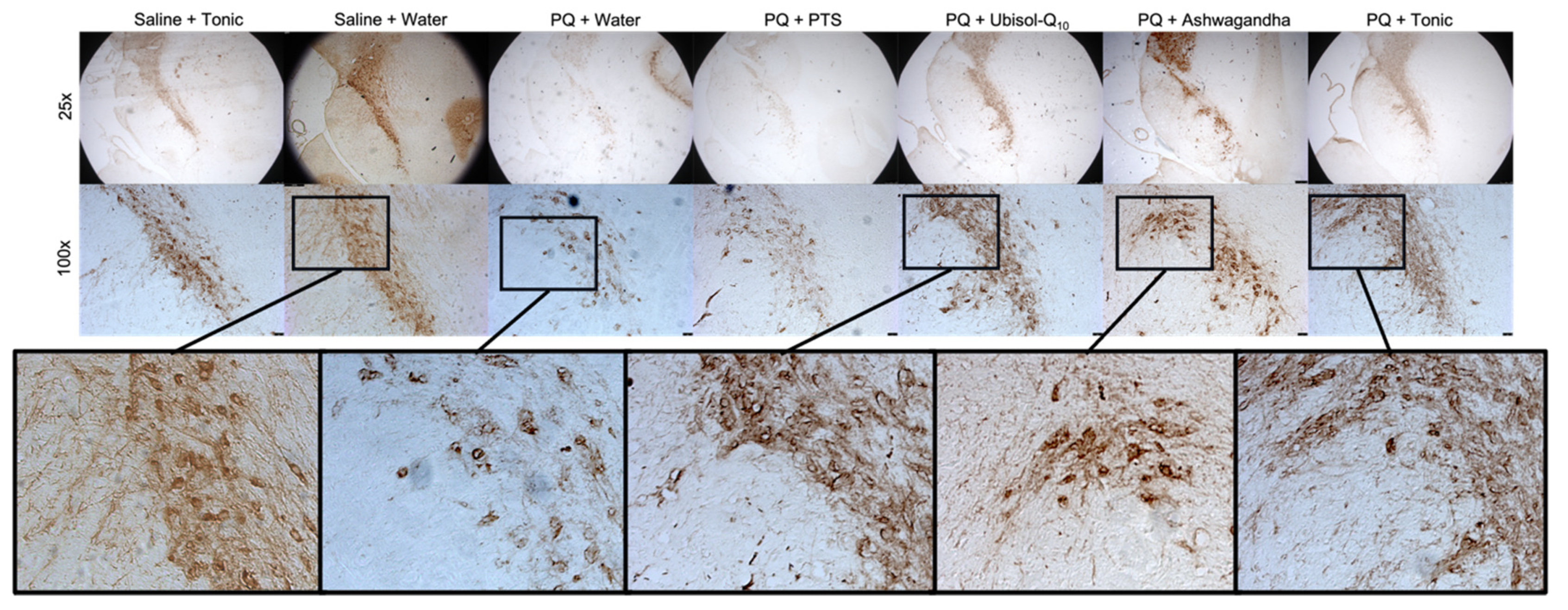
2.6. Tissue Preparation for Immunohistochemistry
2.7. Immunohistochemistry (Colorimetric)
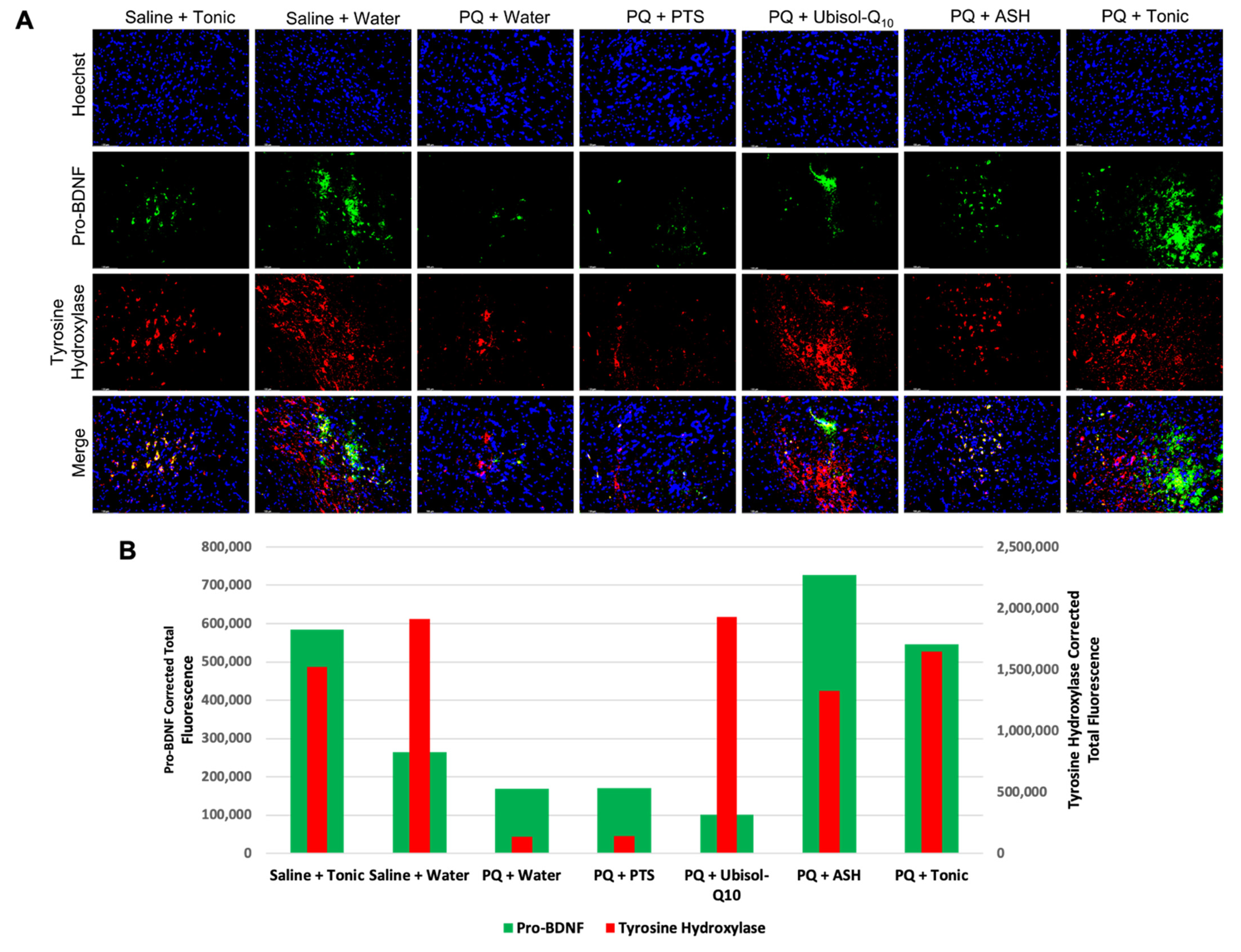
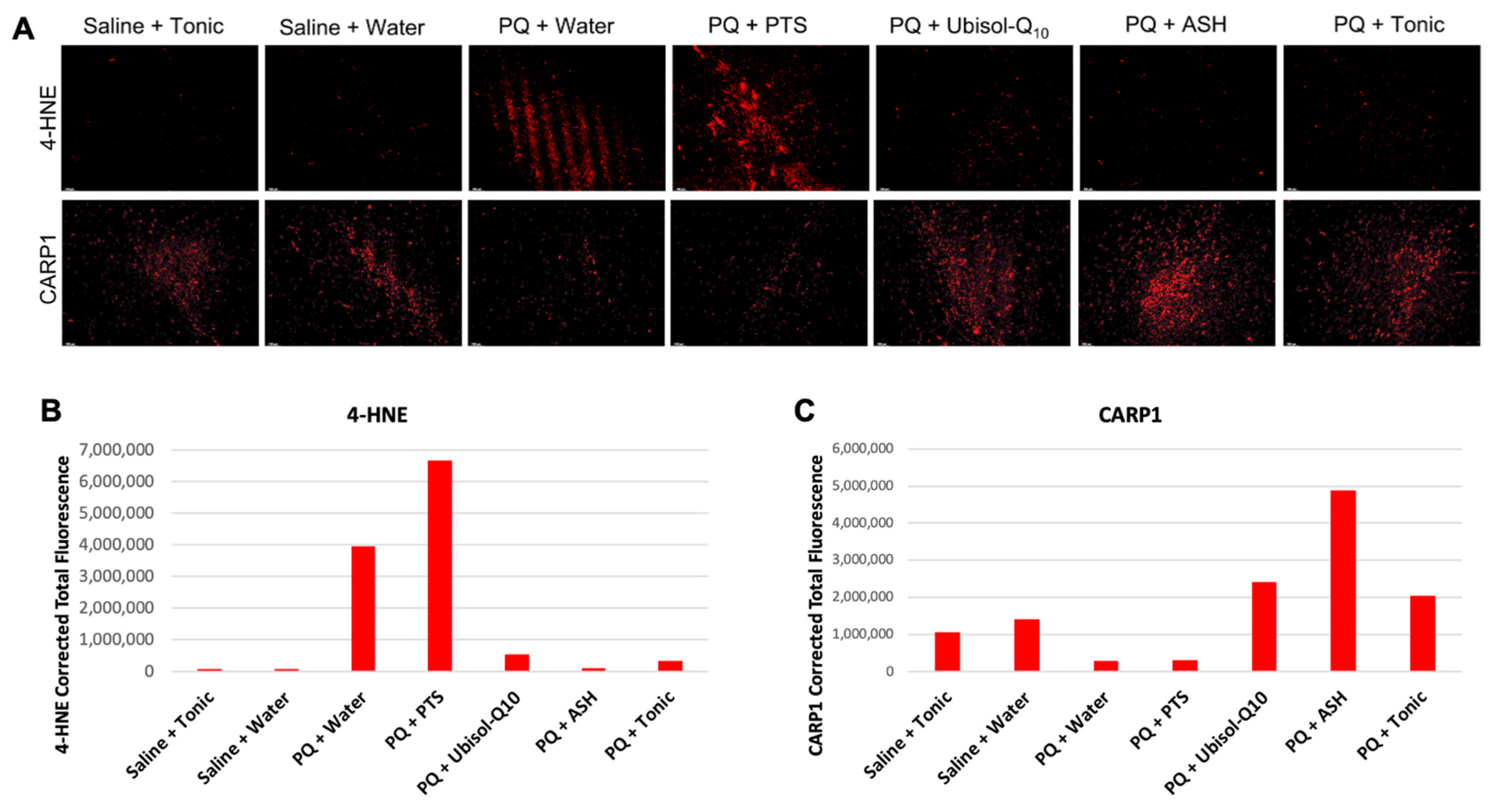
2.8. Immunohistochemistry (Fluorescent)
3. Results
3.1. Phytochemical Content of Ashwagandha Extract
3.2. Effect of Ubisol-Q10 and Ashwagandha Combined Compared to Agents Alone in Protecting DA Neurons from PQ-Induced Neurotoxicity
3.3. Ubisol-Q10 and ASH Antioxidant Effects against PQ-Induced Neurotoxicity
3.4. CARP1 Expression in Response to PQ Insult and Treatment with Ubisol-Q10 and Ashwagandha Extract
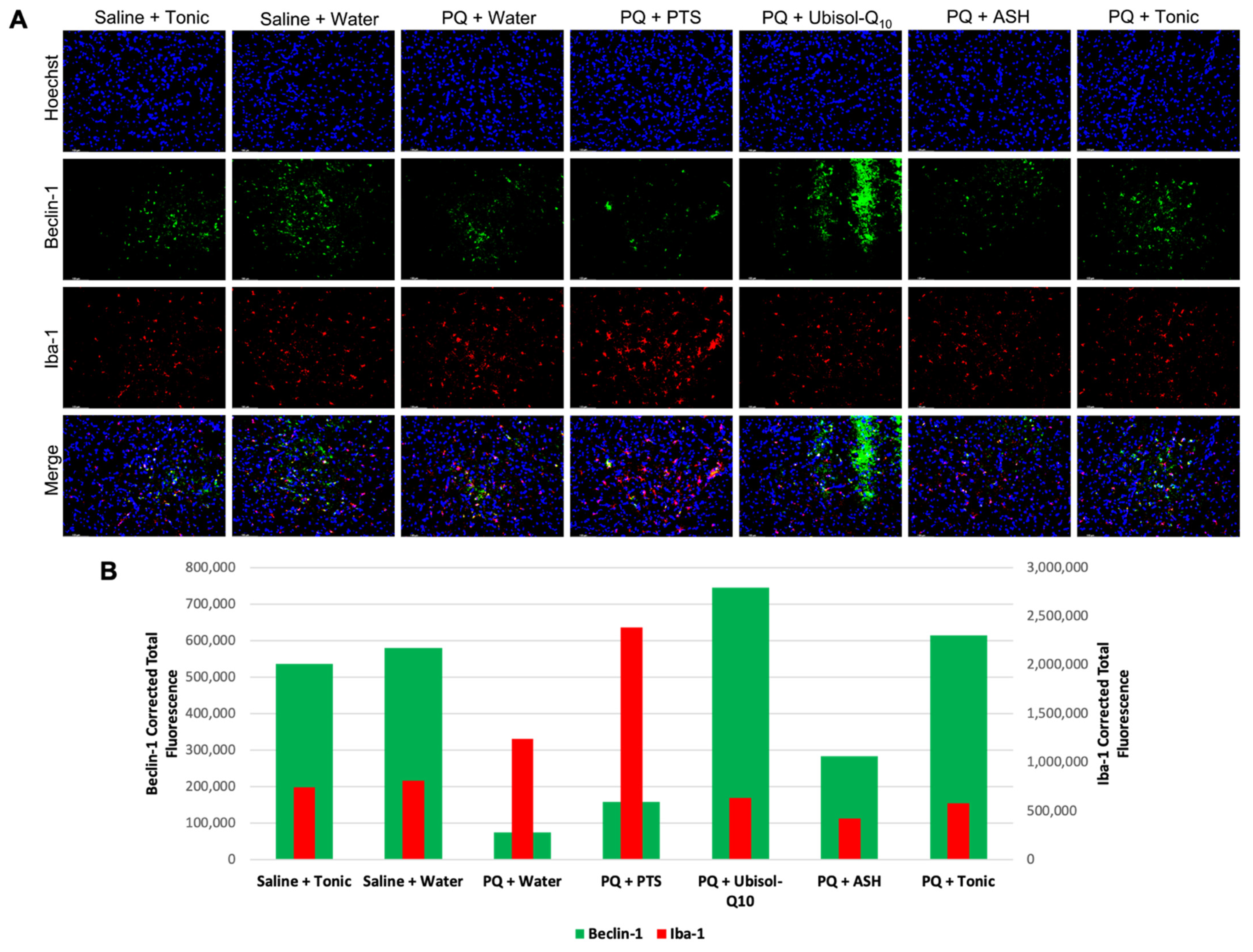
3.5. Effect of Ubisol-Q10 and Ashwagandha Treatment on Beclin-1 Expression
3.6. Inflammatory Status in the Brains of Rats in Response to PQ Neurotoxicity and Treatment with Ubisol-Q10 and Ashwagandha Extract
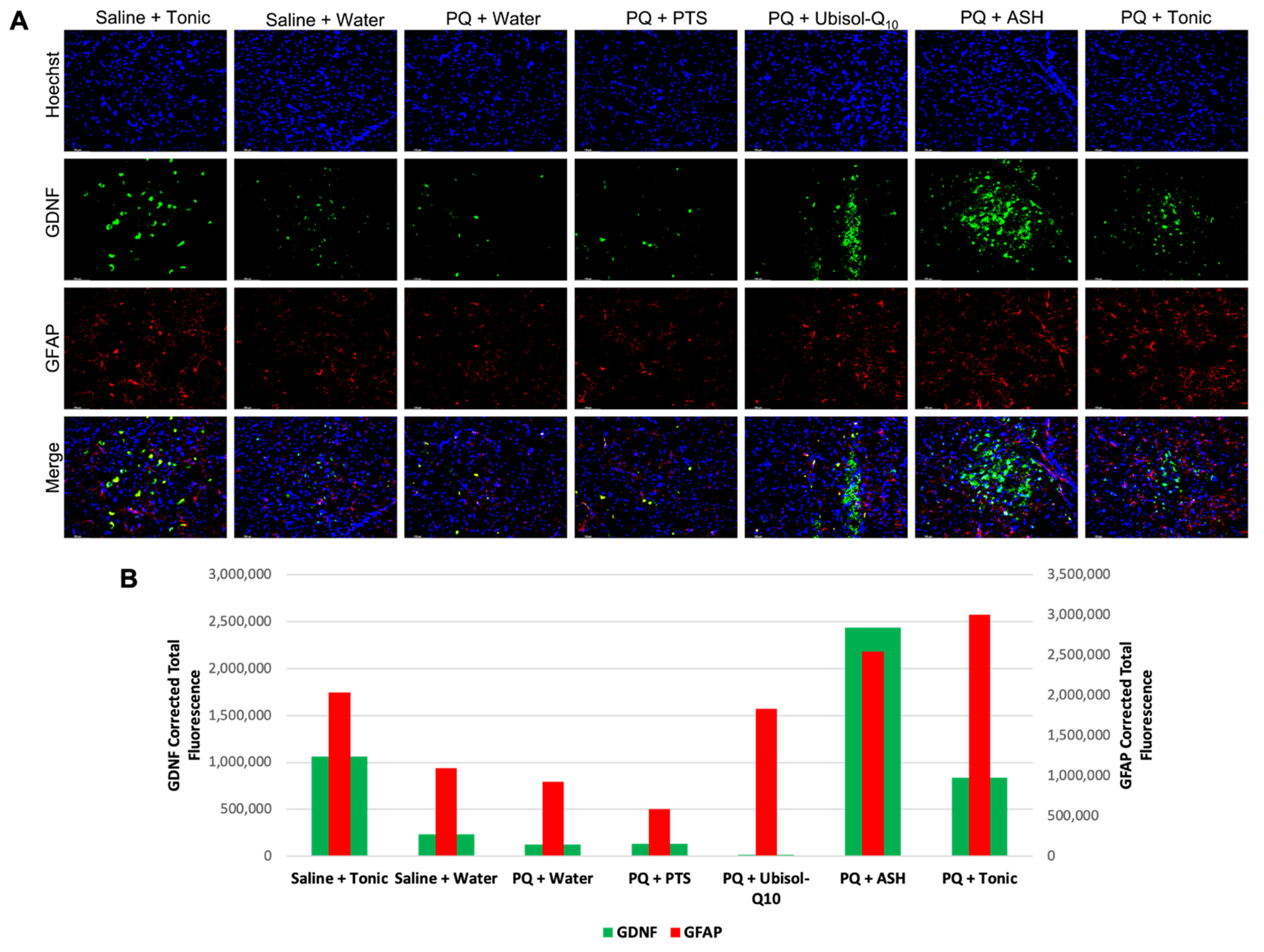
3.7. Modulation of Levels of Neurotrophic Factors, GDNF and Pro-BDNF in Response to PQ Insult and Treatment with Ubisol-Q10 and Ashwagandha Extract
3.8. Effect of Ubisol-Q10 and Ashwagandha Extract on PQ-Induced Motor Deficits
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parkinson′s Disease Information Page; NINDS: Bethesda, MD, USA, 2016.
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef]
- Klein, C.; Schlossmacher, M.G. Parkinson disease, 10 years after its genetic revolution: Multiple clues to a complex disorder. Neurology 2007, 69, 2093–2104. [Google Scholar] [CrossRef]
- Davie, C.A. A review of Parkinson’s disease. Br. Med. Bull. 2008, 86, 109–127. [Google Scholar] [CrossRef]
- Langston, J.W.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–983. [Google Scholar] [CrossRef]
- Riachi, N.J.; Arora, P.K.; Sayre, L.M.; Harik, S.I. Potent neurotoxic fluorinated 1-Methyl-4-Phenyl-1,2,3,6-tetrahydropyridine analogs as potential probes in models of Parkinson disease. J. Neurochem. 1988, 50, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Sundstorm, M.E.; Stromberg, I.; Tsutsumi, T.; Olson, L.; Jonsson, G. Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Res. 1987, 405, 26–38. [Google Scholar]
- Heikkila, R.E.; Sonsalla, P.K. The MPTP-treated mouse as a model of Parkinsonism: How good is it? Neurochem. Int. 1992, 20, 299S–303S. [Google Scholar] [CrossRef]
- Gerecke, K.M.; Jiao, Y.; Pani, A.; Pagala, V.; Smeyne, R.J. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010, 1341, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; la Vecchia, C.; Nicotera, P. Paraquat and Parkinson’s disease. Cell. Death Differ. 2010, 17, 1115–1125. [Google Scholar] [CrossRef]
- Rappold, P.; Cui, M.; Chesser, A.S.; Tibbett, J.; Grima, J.C.; Duan, L.; Sen, N.; Javitch, J.A.; Tieu, K. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc. Natl. Acad. Sci. USA 2011, 108, 20766–20771. [Google Scholar] [CrossRef]
- Liou, H.H.; Tsai, M.C.; Chen, C.J.; Jeng, J.S.; Chang, Y.C.; Chen, S.Y.; Chen, R.C. Environmental risk factors and Parkinson’s disease: A case–control study in Taiwan. Neurology 1997, 48, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Gatto, N.M.; Cockburn, M.; Bronstein, J.; Manthripragada, A.D.; Ritz, M. Well-water consumption and Parkinson’s disease in rural California. Environ. Health Perspect. 2009, 117, 1912–1918. [Google Scholar] [CrossRef]
- Somayajulu-Niţu, M.; Sandhu, J.; Cohen, J.; Sikorska, M.; Sridhar, T.; Matei, A.; Borowy-Borowski, H.; Pandey, S. Paraquat induces oxidative stress, neuronal loss in substantia nigra region and Parkinsonism in adult rats: Neuropro- tection and amelioration of symptoms by water-soluble formulation of Coenzyme Q10. BMC Neurosci. 2009, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.J.; Essa, M.M.; Akbar, M.; Akbar, M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A mini review. Oxid. Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.M.; Dawson, V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science 2003, 302, 819–822. [Google Scholar] [CrossRef]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef]
- Stojkovska, I.; Wagner, B.M.; Morrison, B.E. Parkinson’s disease and enhanced inflammatory response. Exp. Biol. Med. 2015, 240, 1387–1395. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Mirza, B.; Hadberg, H.; Thomsen, P.; Moos, T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience 2000, 95, 425–432. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Zhang, P.; Agid, Y.; Javoy-Agid, F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 1993, 52, 1–6. [Google Scholar] [CrossRef]
- Neurotrophic Factors; Nature Publishing Group: London, UK, 2020.
- Malenka, R.C.; Nestler, E.J.; Hyman, S.E. Chapter 8: Atypical neurotransmitters. In Molecular Neuropharmacology: A Foundation for Clinical Neuroscience; Sydor, A., Brown, R.Y., Eds.; McGraw-Hill Medical: New York, NY, USA, 2009; pp. 211–221. [Google Scholar]
- Zigmond, M.J.; Cameron, J.L.; Hoffer, B.J.; Smeyne, R.J. Neurorestoration by physical exercise: Moving forward. Parkinsonism Relat. Disord. 2012, 18, S147–S150. [Google Scholar] [CrossRef]
- Sikorska, M.; Borowy-Borowski, H.; Zurakowski, B.; Walker, P.R. Derivatised alpha- tocopherol as a CoQ10 carrier in a novel water-soluble formulation. Biofactors 2003, 18, 173–183. [Google Scholar] [CrossRef] [PubMed]
- The Parkinson Study Group QE3 Investigators. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: No evidence of benefit. JAMA Neurol. 2014, 71, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Naderi, J.; Somayajulu-Nitu, M.; Mukerji, A.; Sharda, P.; Sikorska, M.; Borowy-Borowski, H.; Antonsson, B.; Pandey, S. Water-soluble formulation of Coenzyme Q10 inhibits Bax-induced destabilization of mitochondria in mammalian cells. Apoptosis 2006, 11, 1359–1369. [Google Scholar] [CrossRef]
- Somayajulu, M.; McCarthy, S.; Hung, M.; Sikorska, M.; Borowy-Borowski, H.; Pandey, S. Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by Coenzyme Q10. Neurobiol. Dis. 2005, 18, 618–625. [Google Scholar] [CrossRef]
- Muthukumaran, K.; Leahy, S.; Harrison, K.; Sikorska, M.; Sandhu, J.; Cohen, J.; Keshan, C.; Lopatin, D.; Miller, H.; Borowy-Borowski, H.; et al. Orally delivered water soluble Coenzyme Q10 (Ubisol-Q10) blocks on-going neurodegeneration in rats exposed to paraquat: Potential for therapeutic application in Parkinson’s disease. BMC Neurosci. 2014, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, K.; Smith, J.; Jasra, H.; Sikorska, M.; Sandhu, J.; Cohen, J.; Lopatin, D.; Pandey, S. Genetic susceptibility model of Parkinson’s disease resulting from exposure of DJ-1 deficient mice to MPTP: Evaluation of neuroprotection by Ubisol-Q10. J. Parkinsons Dis. 2014, 4, 523–530. [Google Scholar] [CrossRef]
- Ma, D.; Stokes, K.; Mahngar, K.; Domazet-Damjanov, D.; Sikorska, M.; Pandey, S. Inhibition of stress induced premature senescence in presenilin-1 mutated cells with water soluble Coenzyme Q10. Mitochondrion 2014, 17, 106–115. [Google Scholar] [CrossRef]
- Muthukumaran, K.; Kanwar, A.; Vegh, C.; Marginean, A.; Elliott, A.; Guilbeault, N.; Badour, A.; Sikorska, M.; Cohen, J.; Pandey, S. Ubisol-Q10 (a nanomicellar water-soluble formulation of CoQ10) treatment inhibits Alzheimer-type behavioral and pathological symptoms in a double transgenic mouse (TgAPEswe, PSEN1dE9) model of Alzheimer’s disease. J. Alzheimer Dis. 2018, 61, 221–236. [Google Scholar] [CrossRef]
- Vegh, C.; Pupulin, S.; Wear, D.; Culmone, L.; Huggard, R.; Ma, D.; Pandey, S. Resumption of autophagy by Ubisol-Q10 in presenilin-1 mutated fibroblasts and transgenic AD mice: Implications for inhibition of senescence and neuroprotection. Oxid. Med. Cell. Longev. 2019, 2019, 7404815. [Google Scholar] [CrossRef]
- Vegh, C.; Stokes, K.; Ma, D.; Wear, D.; Cohen, J.; Ray, S.D.; Pandey, S. A bird’s-eye view of the multiple biochemical mechanisms that propel pathology of Alzheimer’s disease: Recent advances and mechanistic perspectives on how to halt the disease progression targeting multiple pathways. J. Alzheimer Dis. 2019, 69, 631–649. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Effects of Ashwagandha (Roots of Withania somnifera) on neurodegenerative diseases. Biol. Pharm. Bull. 2014, 37, 892–897. [Google Scholar] [CrossRef]
- Prakash, J.; Yadav, S.K.; Chouhan, S.; Singh, S.P. Neuroprotective role of withania somnifera root extract in maneb-paraquat induced mouse model of parkinsonism. Neurochem. Res. 2013, 38, 972–980. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Goel, I.; Roy, T.; Shukla, S.D.; Khurana, S. Complete Comparison Display (CCD) evaluation of ethanol extracts of Centella asiatica and Withania somnifera shows that they can non-synergistically ameliorate biochemical and behavioural damages in MPTP induced Parkinson’s model of mice. PLoS ONE 2017, 12, e0177254. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, N.; Gupta, A.; Valli, R.K.; Joshi, S.D.; Mills, J.T.; Hamel, E.; Khanna, P.; Jain, S.C.; Thakur, S.S.; Ravindranath, V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA 2012, 109, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Whishaw, I.Q.; Li, K.; Whishaw, P.A.; Gorny, B.; Metz, G.A. Distinct forelimb and hind limb stepping impairments in unilateral dopamine-depleted rats: Use of the rotorod as a method for the qualitative analysis of skilled walking. J. Neurosci. Methods 2003, 126, 13–23. [Google Scholar] [CrossRef]
- Venkatesh, J.; Sekhar, S.C.; Cheriyan, V.T.; Muthu, M.; Meister, P.; Levi, E.; Dzinic, S.; Gauld, J.W.; Polin, L.A.; Rishi, A.K. Antagonizing binding of cell cycle and apoptosis regulatory protein 1 (CARP-1) to the NEMO/IKKγ protein enhances the anticancer effect of chemotherapy. J. Biol. Chem. 2020, 295, 3532–3552. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.I.; Chadwick, C.A.; Gelbard, H.A.; Cory-Slechta, D.A.; Federoff, H.J. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999, 823, 1–10. [Google Scholar] [CrossRef]
- Eriksson, M.; Nilsson, A.; Samuelsson, H.; Samuelsson, E.B.; Mo, L.; Akesson, E.; Benedikz, E.; Sundström, E. On the role of NR3A in human NMDA receptors. Physiol. Behav. 2007, 92, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.Y.; Kim, J.H.; Yang, C.K.; Stallcup, M.R. Requirement of cell cycle and apoptosis regulator 1 for target gene activation by Wnt and β-catenin and for anchorage-independent growth of human colon carcinoma cells. J. Biol. Chem. 2009, 284, 20629–20637. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.Y.; Chen, T.C.; Lee, J.V.; Wang, J.C.; Stallcup, M.R. Coregulator CCAR1 positively regulates adipocyte differentiation through the glucocorticoid signaling pathway. J. Biol. Chem. 2014, 289, 17078–17086. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.K.; Lai, Y.C.; Lin, Y.F.; Chen, H.R.; Chiang, M.K. CCAR1 is required for Ngn3-mediated endocrine differentiation. Biochem. Biophys. Res. Commun. 2012, 418, 307–312. [Google Scholar] [CrossRef]
- Francois, S.; D’Orlando, C.; Fatone, T.; Touvier, T.; Pessina, P.; Meneveri, R.; Brunell, S. Necdin enhances myoblast survival by facilitating the degradation of the mediator of apoptosis CCAR1/CARP1. PLoS ONE 2012, 7, e43335. [Google Scholar] [CrossRef]
- Puliyappadamba, V.T.; Wu, W.; Bevis, D.; Zhang, L.; Polin, L.; Kilkuskie, R.; Finley, R.L., Jr.; Larsen, S.D.; Levi, E.; Miller, F.R.; et al. Antagonists of anaphase promoting complex [APC]-2-cell cycle and apoptosis regulatory protein (CARP)-1 interaction are novel regulators of cell growth and apoptosis. J. Biol. Chem. 2011, 286, 38000–38017. [Google Scholar] [CrossRef]
- National Institute on Aging. Available online: https://www.nia.nih.gov/health/parkinsons-disease (accessed on 22 December 2020).
- Thoppil, H.; Riabowol, K. Senolytics: A translational bridge between cellular senescence and organismal aging. Front. Cell Dev. Biol. 2020, 7, 367. [Google Scholar] [CrossRef]

| Withanolides | Flavonoids | ||||
|---|---|---|---|---|---|
| Content (mg/mL) | Content (mg/100 mL) | ||||
| Withaferin A | 12-Deoxy-withastramonolide | Withanolide A | Withanolide B | Quercetin | |
| Assay 1 | 13.0 | 3.7 | 5.3 | 1.8 | Mean 1.63 ± 0.07 |
| Assay 2 | 13.9 | 4.0 | 5.6 | 2.0 | |
| Assay 3 | 13.8 | 3.8 | 5.6 | 1.7 | |
| Assay 4 | 13.6 | 3.9 | 5.5 | 1.8 | |
| Mean ± standard deviation | 13.6 ± 0.4 | 3.8 ± 0.1 | 5.5 ± 0.1 | 1.9 ± 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vegh, C.; Wear, D.; Okaj, I.; Huggard, R.; Culmone, L.; Eren, S.; Cohen, J.; Rishi, A.K.; Pandey, S. Combined Ubisol-Q10 and Ashwagandha Root Extract Target Multiple Biochemical Mechanisms and Reduces Neurodegeneration in a Paraquat-Induced Rat Model of Parkinson’s Disease. Antioxidants 2021, 10, 563. https://doi.org/10.3390/antiox10040563
Vegh C, Wear D, Okaj I, Huggard R, Culmone L, Eren S, Cohen J, Rishi AK, Pandey S. Combined Ubisol-Q10 and Ashwagandha Root Extract Target Multiple Biochemical Mechanisms and Reduces Neurodegeneration in a Paraquat-Induced Rat Model of Parkinson’s Disease. Antioxidants. 2021; 10(4):563. https://doi.org/10.3390/antiox10040563
Chicago/Turabian StyleVegh, Caleb, Darcy Wear, Iva Okaj, Rachel Huggard, Lauren Culmone, Sezen Eren, Jerome Cohen, Arun K. Rishi, and Siyaram Pandey. 2021. "Combined Ubisol-Q10 and Ashwagandha Root Extract Target Multiple Biochemical Mechanisms and Reduces Neurodegeneration in a Paraquat-Induced Rat Model of Parkinson’s Disease" Antioxidants 10, no. 4: 563. https://doi.org/10.3390/antiox10040563
APA StyleVegh, C., Wear, D., Okaj, I., Huggard, R., Culmone, L., Eren, S., Cohen, J., Rishi, A. K., & Pandey, S. (2021). Combined Ubisol-Q10 and Ashwagandha Root Extract Target Multiple Biochemical Mechanisms and Reduces Neurodegeneration in a Paraquat-Induced Rat Model of Parkinson’s Disease. Antioxidants, 10(4), 563. https://doi.org/10.3390/antiox10040563







