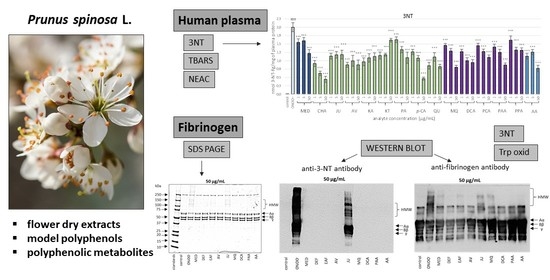
The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extracts Preparation
2.2. Reference Standards of Model Polyphenols and Phenolic Metabolites
2.3. Synthesis of ONOO−
2.4. Antioxidant Activity in Human Plasma Model
2.4.1. Isolation of Blood Plasma and Preparation of Samples
2.4.2. Determination of 3-NT in Plasma Proteins
2.4.3. Determination of TBARS in Plasma
2.4.4. Determination of the NEAC of Plasma
2.5. Activity against Oxidative/Nitrative Modifications of Human Fibrinogen
2.5.1. Isolation of Fibrinogen from Blood Plasma
2.5.2. SDS PAGE Analysis
2.5.3. Western Blot Analysis
2.5.4. C-ELISA of the ONOO−-Induced 3-NT Formation
2.5.5. Fluorometric Analysis of the ONOO−-Induced Tryptophan Residue Modifications
2.6. Statistical Analysis
3. Results
3.1. Protective Effects on Human Plasma Components against the ONOO−-Induced Oxidative Stress
3.2. Protective Effects against Oxidative/Nitrative Modifications of Fibrinogen
3.2.1. SDS-PAGE Analysis of the ONOO−-Induced Changes in the Isolated Fibrinogen
3.2.2. Western Blot Analysis of the Isolated Fibrinogen with Anti-3-NT Antibody
3.2.3. Determination of the ONOO−-Induced 3-NT Formation in the Isolated Fibrinogen by C-ELISA
3.2.4. Determination of the ONOO−-Induced Modifications of Tryptophan Residues in the Isolated Fibrinogen
3.2.5. Influence of the Analytes on the ONOO−-Induced Modifications of Fibrinogen in Blood Plasma Matrix—Western Blot Analysis with Anti-Fibrinogen Antibody
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-NT | 3-nitrotyrosine |
| AA | ascorbic acid |
| AV | avicularin (quercetin 3-O-α-L-arabinofuranoside |
| C-ELISA | the competitive enzyme-linked immunosorbent assay |
| CHA | chlorogenic acid |
| CVDs | cardiovascular diseases |
| CYE | Cyanidin chloride equivalents |
| DCA | 3-(3′,4′-dihydroxyphenyl)propionic acid (dihydrocaffeic acid) |
| DEF | diethyl ether fraction |
| dw | dry weight |
| EAF | ethyl acetate fraction |
| GAE | Gallic acid equivalents |
| H2O2 | hydrogen peroxide |
| HOCl | hypochlorous acid |
| HMW | high molecular weight |
| JU | juglanin (kaempferol 3-O-α-L-arabinofuranoside) |
| KA | kaempferol |
| KT | kaempferitrin (kaempferol 3,7-di-O-α-L-rhamnopyranoside) |
| LOOH | lipid hydroperoxides |
| MED | defatted methanol-water (7:3, v/v) extract |
| MQ | miquelianin (quercetin 3-O-β-D-glucuronopyranoside) |
| MW | molecular weight |
| NEAC | non-enzymatic antioxidant capacity of plasma |
| NO• | nitric oxide |
| ONOO− | peroxynitrite |
| O2•− | superoxide anion |
| OH• | hydroxyl radical |
| PA2 | proanthocyanidin A2 |
| PAA | 2-(3′,4′-dihydroxyphenyl)acetic acid |
| p-CA | p-coumaric acid |
| PCA | protocatechuic acid |
| PPA | 3-(4′-hydroxyphenyl)propionic acid |
| QU | quercetin |
| ROS | reactive oxygen species |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TAC | total content of phenolic acids (HPLC-PDA) |
| TBARS | thiobarbituric acid-reactive substances |
| TFC | total flavonoid content (HPLC-PDA) |
| TPA | total proanthocyanidin content (n-butanol/HCl assay) |
| TPC | total phenolic content (Folin-Ciocalteu assay) |
| TPH | total phenolic content (HPLC-PDA-fingerprint) |
References
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The role of oxidative stress in cardiac disease: From physiological response to injury factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956:1–5732956:29. [Google Scholar] [CrossRef] [PubMed]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and oxidative stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046:1–6501046:32. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Parastatidis, I.; Thomson, L.; Burke, A.; Chernysh, I.; Nagaswami, C.; Visser, J.; Stamer, S.; Liebler, D.C.; Koliakos, G.; Ischiropoulos, H.; et al. Fibrinogen beta-chain tyrosine nitration is a prothrombotic risk factor. J. Biol. Chem. 2008, 283, 33846–33853. [Google Scholar] [CrossRef]
- Nowak, P.; Zbikowska, H.M.; Ponczek, M.; Kolodziejczyk, J.; Wachowicz, B. Different vulnerability of fibrinogen subunits to oxidative/nitrative modifications induced by peroxynitrite: Functional consequences. Thromb. Res. 2007, 121, 163–174. [Google Scholar] [CrossRef]
- Martinez, M.; Cuker, A.; Mills, A.; Lightfoot, R.; Fan, Y.; Tang, W.H.; Hazen, S.L.; Ischiropoulos, H. Nitrated fibrinogen is a biomarker of oxidative stress in venous thromboembolism. Free Radic. Biol. Med. 2012, 53, 230–236. [Google Scholar] [CrossRef]
- Ariëns, R.A. Fibrin(ogen) and thrombotic disease. J. Thromb. Haemost. 2013, 11, 294–305. [Google Scholar] [CrossRef]
- Medeiros, R.; Sousa, B.; Rossi, S.; Afonso, C.; Bonino, L.; Pitt, A.; López, E.; Spickett, C.; Borthagaray, G. Identification and relative quantification of 3-nitrotyrosine residues in fibrinogen nitrated in vitro and fibrinogen from ischemic stroke patient plasma using LC-MS/MS. Free Radic. Biol. Med. 2021, 165, 334–347. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Koch, W. Dietary polyphenols-important non-nutrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef]
- Bijak, M.; Saluk, J.; Antosik, A.; Ponczek, M.B.; Żbikowska, H.M.; Borowiecka, M.; Nowak, P. Aronia melanocarpa as a protector against nitration of fibrinogen. Int. J. Biol. Macromol. 2013, 55, 264–268. [Google Scholar] [CrossRef]
- Bijak, M.; Nowak, P.; Borowiecka, M.; Ponczek, M.B.; Żbikowska, H.M.; Wachowicz, B. Protective effects of (-)-epicatechin against nitrative modifications of fibrinogen. Thromb. Res. 2012, 130, 123–128. [Google Scholar] [CrossRef]
- Hoppe, H.A. Taschenbuch der Drogenkunde; De Gruyter: New York, NY, USA, 1981. [Google Scholar]
- Berger, F. Handbuch der Drogenkunde, 1st ed.; Maudrich: Wien, Austria, 1949. [Google Scholar]
- Wawrzyniak, E. Leczenie Ziołami: Kompendium Fitoterapii; Contrast: Warszawa, Poland, 1992. [Google Scholar]
- Blumenthal, M.; Busse, W.R. The Complete German Commission E monographs: Therapeutic Guide to Herbal Medicines; The American Botanical Council: Austin, TX, USA, 1998. [Google Scholar]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity potential of Prunus spinosa flower extracts: Phytochemical profiling, cellular safety, pro-inflammatory enzymes inhibition and protective effects against oxidative stress in vitro. Front. Pharmacol. 2017, 8, 680:1–680:15. [Google Scholar] [CrossRef]
- Marchelak, A.; Owczarek, A.; Rutkowska, M.; Michel, P.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. New insights into antioxidant activity of Prunus spinosa flowers: Extracts, model polyphenols and their phenolic metabolites in plasma towards multiple in vivo-relevant oxidants. Phytochem. Lett. 2019, 30, 288–295. [Google Scholar] [CrossRef]
- Marchelak, A.; Olszewska, M.A.; Owczarek, A. Simultaneous quantification of thirty polyphenols in blackthorn flowers and dry extracts prepared thereof: HPLC-PDA method development and validation for quality control. J. Pharm. Biomed. 2020, 184, 113121:1–113121:8. [Google Scholar] [CrossRef] [PubMed]
- Marchelak, A.; Olszewska, M.A.; Owczarek, A. Data on the optimization and validation of HPLC-PDA method for quantification of thirty polyphenols in blackthorn flowers and dry extracts prepared thereof. Data Brief 2020, 29, 105319:1–105319:12. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, bioavailability, and metabolism of flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2019, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct 2019, 10, 6999–7021. [Google Scholar] [CrossRef]
- Olszewska, M.; Wolbiś, M. Flavonoids from the flowers of Prunus spinosa L. Acta Pol. Pharm. 2001, 58, 367–372. [Google Scholar]
- Olszewska, M.; Wolbiś, M. Further flavonoids from the flowers of Prunus spinosa L. Acta Pol. Pharm. 2002, 59, 133–137. [Google Scholar]
- Pryor, W.A.; Cueto, R.; Jin, X.; Koppenol, W.H.; Ngu-Schwemlein, M.; Squadrito, G.L.; Uppu, P.L.; Uppu, R.M. A practical method for preparing peroxynitrite solutions of low ionic strength and free of hydrogen peroxide. Free Radic. Biol. Med. 1995, 18, 75–83. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Wachowicz, B.; Moniuszko-Szajwaj, B.; Kowalska, I.; Oleszek, W.; Stochmal, A. Antioxidative effects of extracts from Trifolium species on blood platelets exposed to oxidative stress. J. Physiol. Biochem. 2013, 69, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Ghisaidoobe, A.B.T.; Chung, S.J. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: A focus on Förster Resonance Energy Transfer techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef] [PubMed]
- Lettieri-Barbato, D.; Tomei, F.; Sancini, A.; Morabito, G.; Serafini, M. Effect of plant foods and beverages on plasma non-enzymatic antioxidant capacity in human subjects: A meta-analysis. Br. J. Nutr. 2013, 109, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L. 3-nitrotyrosine modified proteins in atherosclerosis. Dis. Markers 2015, 2015, 708282:1–708282:8. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.S.; Chen, J.; Ischiropoulos, H.; Crow, J.P. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994, 233, 229–240. [Google Scholar]
- Miao, J.L.; Wang, W.F.; Pan, J.X.; Lu, C.Y.; Li, R.Q.; Yao, S.D. The scavenging reactions of nitrogen dioxide radical and carbonate radical by tea polyphenol derivatives: A pulse radiolysis study. Radiat. Phys. Chem. 2001, 60, 163–168. [Google Scholar] [CrossRef]
- Koenig, W. Haemostatic risk factors for cardiovascular diseases. Eur. Heart J. 1998, 19, 39–43. [Google Scholar]
- Lefevre, M.; Kris-Etherton, P.M.; Zhao, G.; Tracy, R.P. Dietary fatty acids, hemostasis, and cardiovascular disease risk. J. Am. Diet. Assoc. 2004, 104, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Stikarová, J.; Suttnar, J.; Sovova, Z.; Ceznerova, E.; Novak, J.; Louzil, J.; Kotlín, R.; Roselova, P.; Kaufmanova, J.; Chrastinova, L.; et al. Oxidative modified fibrinogen in cardiovascular diseases. Blood 2018, 132, 5011. [Google Scholar] [CrossRef]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Marcucci, R.; Bruschi, G.; Taddei, N.; Bani, D.; Gori, A.M.; Giusti, B.; Gensini, G.F.; Abbate, R.; Fiorillo, C. Oxidative modification of fibrinogen is associated with altered function and structure in the subacute phase of myocardial infarction. Arter. Thromb. Vasc. Biol. 2014, 34, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Ehrenshaft, M.; Deterding, L.J.; Mason, R.P. Tripping up Trp: Modification of protein tryptophan residues by reactive oxygen species, modes of detection, and biological consequences. Free Radic. Biol. Med. 2015, 89, 220–228. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Rutkowska, M.; Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Owczarek, A. Sorbus domestica leaf extracts and their activity markers: Antioxidant potential and synergy effects in scavenging assays of multiple oxidants. Molecules 2019, 24, 2289. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A.; Owczarek, A.; Kapusta, P.; Kolodziejczyk-Czepas, J.; Olszewska, M.A. Contribution of individual polyphenols to antioxidant activity of Cotoneaster bullatus and Cotoneaster zabelii leaves-structural relationships, synergy effects and application for quality control. Antioxidants 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of organic acids, amino acids and phenolic compounds on antioxidant characteristic of Zhenjiang aromatic vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Vree, T.B.; Katan, M.B. Bioavailabilities of quercetin-3-glucoside and quercetin-4’-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015, 2015, 905215:1–905215:18. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Gu, L. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.; García-Villalba, R.; Quartieri, A.; Raimondi, S.; Amaretti, A.; Leonardi, A.; Rossi, M. In vitro transformation of chlorogenic acid by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 1122–1131. [Google Scholar] [CrossRef]
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737. [Google Scholar] [CrossRef]
- Barrita, J.L.S.; Sánchez, M.S.S. Antioxidant role of ascorbic acid and his protective effects on chronic diseases. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Morales-Gonzalez, J.A., Ed.; IntTech Publisher: London, UK, 2013; pp. 449–484. [Google Scholar]
- German Nutrition Society (DGE). New reference values for vitamin C intake. Ann. Nutr. Metab. 2015, 67, 13–20. [Google Scholar] [CrossRef] [PubMed]









| Phytochemical Content | MED | DEF | EAF | References |
|---|---|---|---|---|
| TPC (mg GAE/g dw) | 206.07 ± 10.86 a | 464.57 ± 20.57 b | 584.07 ± 12.98 c | [18] |
| TFC (mg/g dw) | 125.12 ± 0.55 a | 490.63 ± 8.16 c | 325.53 ± 4.23 b | [18] |
| TPA (mg CYE/g dw) | 45.13 ± 2.38 a | 49.5 ± 2.23 a | 109.43 ± 3.71 b | [18] |
| TAC (mg/g dw) | 29.24 ± 0.76 c | 8.76 ± 0.27 a | 17.20 ± 0.47 b | [18] |
| TPH (mg/g dw) | 157.47 a | 491.69 c | 353.07 b | [20,21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchelak, A.; Kolodziejczyk-Czepas, J.; Wasielewska, P.; Nowak, P.; Olszewska, M.A. The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro. Antioxidants 2021, 10, 581. https://doi.org/10.3390/antiox10040581
Marchelak A, Kolodziejczyk-Czepas J, Wasielewska P, Nowak P, Olszewska MA. The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro. Antioxidants. 2021; 10(4):581. https://doi.org/10.3390/antiox10040581
Chicago/Turabian StyleMarchelak, Anna, Joanna Kolodziejczyk-Czepas, Paulina Wasielewska, Pawel Nowak, and Monika A. Olszewska. 2021. "The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro" Antioxidants 10, no. 4: 581. https://doi.org/10.3390/antiox10040581
APA StyleMarchelak, A., Kolodziejczyk-Czepas, J., Wasielewska, P., Nowak, P., & Olszewska, M. A. (2021). The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro. Antioxidants, 10(4), 581. https://doi.org/10.3390/antiox10040581