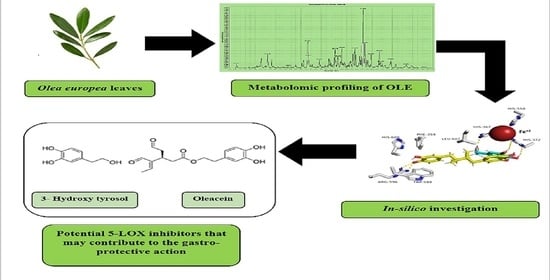
Antiulcer Potential of Olea europea L. cv. Arbequina Leaf Extract Supported by Metabolic Profiling and Molecular Docking
Abstract
1. Introduction
2. Material and Methods
2.1. Plants Material
2.2. Preparation of the Extract
2.3. Metabolic Profiling
2.4. In Vitro Antioxidant Activity
2.4.1. H2O2 Scavenging Activity
2.4.2. Superoxide Radical Scavenging Activity
2.5. In Vivo Antiulcer Potential
2.5.1. Experimental Design
2.5.2. Assessment of Gastric Mucosal Lesions
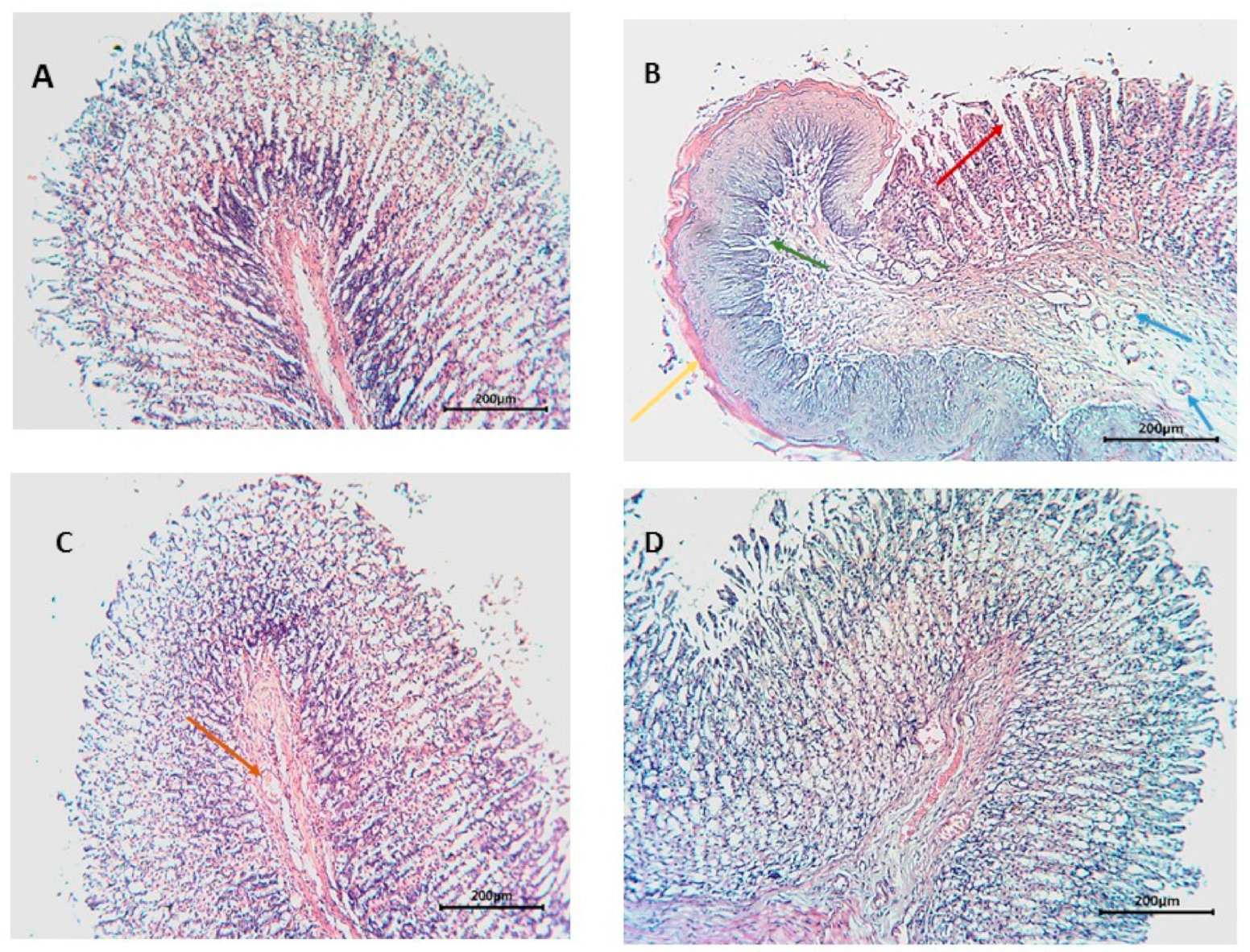
2.5.3. Histopathological Examination
2.6. Statistical Analysis
2.7. In Silico Based Molecular Target Identification
2.8. Lipoxygenase (LOXs) Inhibition Assay
2.9. Docking Analysis and Molecular Dynamic Simulation Refinement
3. Results and Discussion
3.1. Metabolic Profiling
3.2. In Vitro Antioxidant Activity
3.2.1. H2O2 Scavenging Activity
3.2.2. Superoxide Radical Scavenging Activity
3.3. In Vivo Antiulcer Potential
3.3.1. Assessment of Gastric Mucosal Lesions
3.3.2. Histopathological Examination
3.4. In Silico Based Molecular Target Identification
3.5. The 5-LOX Inhibition Assay
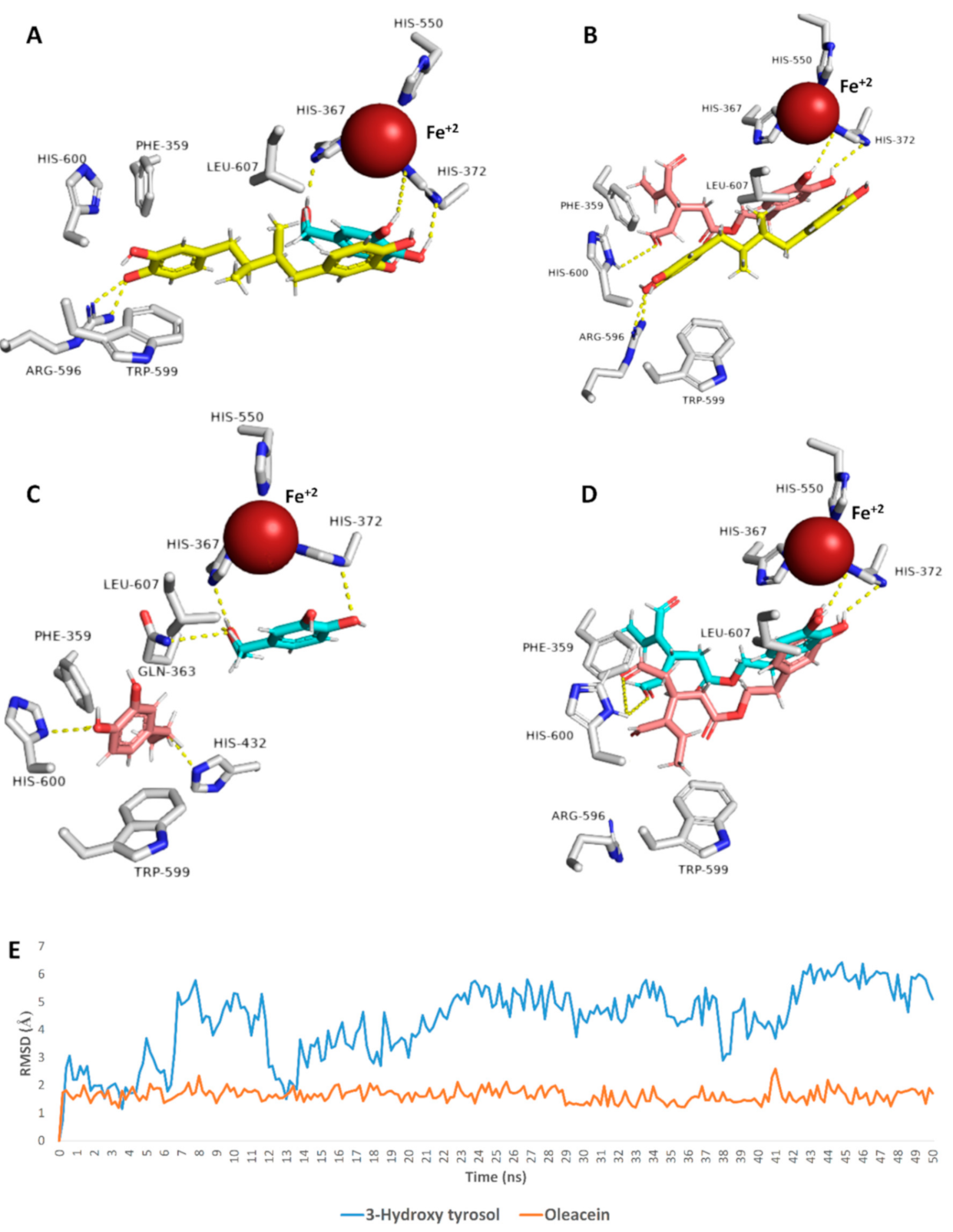
3.6. Docking Analysis and Molecular Dynamic Simulation Refinement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Castejón, M.L.; Montoya, T.; Alarcón-de-la-Lastra, C.; Sánchez-Hidalgo, M. Potential protective role exerted by secoiridoids from Olea europaea L. in cancer, cardiovascular, neurodegenerative, aging-related, and immunoinflammatory diseases. Antioxidants 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Panchal, S.; Vyas, N.; Butani, A.; Kumar, V. Olea europaea: A phyto-pharmacological review. Pharmacogn. Rev. 2007, 1, 114–118. [Google Scholar]
- Wang, L.; Geng, C.; Jiang, L.; Gong, D.; Liu, D.; Yoshimura, H.; Zhong, L. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur. J. Nutr. 2008, 47, 235–243. [Google Scholar] [CrossRef]
- Somova, L.; Shode, F.; Mipando, M. Cardiotonic and antidysrhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine 2004, 11, 121–129. [Google Scholar] [CrossRef]
- Dekanski, D.; Ristić, S.; Mitrović, D. Antioxidant effect of dry olive (Olea europaea L.) leaf extract on ethanol-induced gastric lesions in rats. Mediterr. J. Nutr. Metab. 2009, 2, 205–211. [Google Scholar] [CrossRef]
- Alirezaei, M.; Dezfoulian, O.; Sookhtehzari, A.; Asadian, P.; Khoshdel, Z. Antioxidant effects of oleuropein versus oxidative stress induced by ethanol in the rat intestine. Comp. Clin. Pathol. 2014, 23, 1359–1365. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Othman, M.S.; Dkhil, M.A.; Moneim, A.E.A. Olive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activities. Biomed. Pharmacother. 2017, 91, 338–349. [Google Scholar] [CrossRef]
- Xiang, Z.; Si, J.-M.; Huang, H.-D. Chronic gastritis rat model and role of inducing factors. World J. Gastroenterol. 2004, 10, 3212. [Google Scholar] [CrossRef]
- Rainsford, K. The effects of 5-lipoxygenase inhibitors and leukotriene antagonists on the development of gastric lesions induced by nonsteroidal anti-inflammatory drugs in mice. Agents Actions 1987, 21, 316–319. [Google Scholar] [CrossRef]
- Konturek, S.J.; Brzozowski, T.; Drozdowicz, D.; Beck, G. Role of leukotrienes in acute gastric lesions induced by ethanol, taurocholate, aspirin, platelet-activating factor and stress in rats. Dig. Dis. Sci. 1988, 33, 806–813. [Google Scholar] [CrossRef]
- Sener, G.; Kapucu, C.; Cetinel, S.; Cikler, E.; Ayanoğlu-Dülger, G. Gastroprotective effect of leukotriene receptor blocker montelukast in alendronat-induced lesions of the rat gastric mucosa. Prostaglandins Leukot. Essent. Fat. Acids 2005, 72, 1–11. [Google Scholar] [CrossRef]
- Dengiz, G.O.; Odabasoglu, F.; Halici, Z.; Cadirci, E.; Suleyman, H. Gastroprotective and antioxidant effects of montelukast on indomethacin-induced gastric ulcer in rats. J. Pharmacol. Sci. 2007, 105, 94–102. [Google Scholar] [CrossRef]
- Mahdavi, F.S.; Mardi, P.; Mahdavi, S.S.; Kamalinejad, M.; Hashemi, S.A.; Khodaii, Z.; Mehrabani-Natanzi, M. Therapeutic and Preventive Effects of Olea europaea Extract on Indomethacin-Induced Small Intestinal Injury Model in Rats. Evid. Based Complement. Altern. Med. 2020, 2020. [Google Scholar] [CrossRef]
- Althaiban, M. Antiulcer potential of olive leaves extract in gastric ulcer induced by indomethacin in male rats: Antioxidant and anti-inflammatory effects. Pharmacophore 2018, 9, 57–64. [Google Scholar]
- Motawea, M.H.; Abd Elmaksoud, H.A.; Elharrif, M.G.; Desoky, A.A.E.; Ibrahimi, A. Evaluation of Anti-inflammatory and Antioxidant Profile of Oleuropein in Experimentally Induced Ulcerative Colitis. Int. J. Mol. Cell. Med. 2020, 9, 224. [Google Scholar]
- Hassan, H.A.; Allam, A.E.; Abu-Baih, D.H.; Mohamed, M.F.; Abdelmohsen, U.R.; Shimizu, K.; Desoukey, S.Y.; Hayallah, A.M.; Elrehany, M.A.; Mohamed, K.M. Isolation and characterization of novel acetylcholinesterase inhibitors from Ficus benghalensis L. leaves. Rsc. Adv. 2020, 10, 36920–36929. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chandrasekar, M.; Nanjan, M.; Suresh, B. Antioxidant activity of Caesalpinia digyna root. J. Ethnopharmacol. 2007, 113, 284–291. [Google Scholar] [CrossRef]
- Gürsan, N. Effects of Momordica charantia L.(Cucurbitaceae) on indomethacin-induced ulcer model in rats. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2005, 16, 85–88. [Google Scholar]
- Arun, M.; Asha, V. Gastroprotective effect of Dodonaea viscosa on various experimental ulcer models. J. Ethnopharmacol. 2008, 118, 460–465. [Google Scholar] [CrossRef]
- Batista, L.M.; De Almeida, A.B.A.; de Pietro Magri, L.; Toma, W.; Calvo, T.R.; Vilegas, W.; Brito, A.R.M.S. Gastric Antiulcer Activity of Syngonanthus arthrotrichus SILVEIRA. Biol. Pharm. Bull. 2004, 27, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Inas, Z.; Hala, A.; Gehan, H.H. Gastroprotective effect of Cordia myxa L. fruit extract against indomethacin-induced gastric ulceration in rats. Life Sci. J. 2011, 8, 433–445. [Google Scholar]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Lill, M.A.; Danielson, M.L. Computer-aided drug design platform using PyMOL. J. Comput.-Aided Mol. Des. 2011, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- MacKerell, A.D., Jr.; Bashford, D.; Bellott, M.; Dunbrack, R.L., Jr.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Jo, S.; Jiang, W.; Lee, H.S.; Roux, B.T.; Im, W. CHARMM-GUI Ligand Binder for Absolute Binding Free Energy Calculations and Its Application; ACS Publications: Washington, DC, USA, 2013. [Google Scholar]
- Langlois, A. Extractive from Seven African Medicinal Plants. Ph.D. Thesis, University of Natal, Natal, South African, 2003. [Google Scholar]
- Reyes, F.J.; Centelles, J.J.; Lupiáñez, J.A.; Cascante, M. (2α, 3β)-2, 3-Dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett. 2006, 580, 6302–6310. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a bioactive compound from Olea europaea L. as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and hydrolyzable compounds in virgin olive oil. 3. Spectroscopic characterizations of the secoiridoid derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Ha, L.D.; Hansen, P.E.; Duus, F.; Pham, H.D.; Nguyen, L.H.D. Oliveridepsidones A–D, antioxidant depsidones from Garcinia oliveri. Magn. Reson. Chem. 2012, 50, 242–245. [Google Scholar] [CrossRef]
- Mousouri, E.; Melliou, E.; Magiatis, P. Isolation of megaritolactones and other bioactive metabolites from ‘megaritiki’table olives and debittering water. J. Agric. Food Chem. 2014, 62, 660–667. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (olive). Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Hettiarachchi, D.; Liu, Y.; Fox, J.; Sunderland, B. Western Australian sandalwood seed oil: New opportunities. Lipid Technol. 2010, 22, 27–29. [Google Scholar] [CrossRef]
- Goossens, J.P.A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar]
- Paiva-Martins, F.; Gordon, M.H. Isolation and characterization of the antioxidant component 3,4-dihydroxyphenylethyl 4-formyl-3-formylmethyl-4-hexenoate from olive (Olea europaea) leaves. J. Agric. Food Chem. 2001, 49, 4214–4219. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Pan, Q.-M.; Zhang, G.-J.; Liang, D. Study on chemical constituents of stems and leaves of Sapium discolor. Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2019, 44, 3738–3744. [Google Scholar]
- Romani, A.; Mulinacci, N.; Pinelli, P.; Vincieri, F.F.; Cimato, A. Polyphenolic content in five tuscany cultivars of Olea europaea L. J. Agric. Food Chem. 1999, 47, 964–967. [Google Scholar] [CrossRef]
- Pieroni, A.; Heimler, D.; Pieters, L.; Van Poel, B.; Vlietinck, A. In vitro anti-complementary activity of flavonoids from oliva (Olea europaea L.) leaves. Pharmazie 1996, 51, 765–767. [Google Scholar]
- Damak, N.; Allouche, N.; Hamdi, B.; Litaudon, M.; Damak, M. New secoiridoid from olive mill wastewater. Nat. Prod. Res. 2012, 26, 125–131. [Google Scholar] [CrossRef]
- Toyota, M.; Koyama, H.; Asakawa, Y. Volatile components of the liverworts Archilejeunea olivacea, Cheilolejeunea imbricata and Leptolejeunea elliptica. Phytochemistry 1997, 44, 1261–1264. [Google Scholar] [CrossRef]
- Czerwińska, M.; Kiss, A.K.; Naruszewicz, M. A comparison of antioxidant activities of oleuropein and its dialdehydic derivative from olive oil, oleacein. Food Chem. 2012, 131, 940–947. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Toaldo, C.; Pettazzoni, P.; Dianzani, M.U.; Barrera, G. The “two-faced” effects of reactive oxygen species and the lipid peroxidation product 4-hydroxynonenal in the hallmarks of cancer. Cancers 2010, 2, 338–363. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Parminder, N.; Sidana, J. Oxidative stress induced ulcer protected by natural antioxidant: A review. Int. Res. J. Pharm. 2012, 3, 5. [Google Scholar]
- Dekanski, D.; Janićijević-Hudomal, S.; Tadić, V.; Marković, G.; Arsić, I.; Mitrović, D.M. Phytochemical analysis and gastroprotective activity of an olive leaf extracts. J. Serb. Chem. Soc. 2009, 74, 367–377. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Hussein, A.S.; Salem, M.A.; Khalil, H.E.; Yehia Desoukey, S.; Fouad, M.A.; Kamel, M.S. Antiulcer potential and molecular docking of flavonoids from Ocimum forskolei Benth., family Lamiaceae. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Koscielny, G.; An, P.; Carvalho-Silva, D.; Cham, J.A.; Fumis, L.; Gasparyan, R.; Hasan, S.; Karamanis, N.; Maguire, M.; Papa, E. Open Targets: A platform for therapeutic target identification and validation. Nucleic Acids Res. 2017, 45, D985–D994. [Google Scholar] [CrossRef]
- Wang, L.; Ma, C.; Wipf, P.; Liu, H.; Su, W.; Xie, X.-Q. TargetHunter: An in silico target identification tool for predicting therapeutic potential of small organic molecules based on chemogenomic database. AAPS J. 2013, 15, 395–406. [Google Scholar] [CrossRef]
- de la Puerta, R.; Gutierrez, V.R.; Hoult, J.R.S. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Vougogiannopoulou, K.; Lemus, C.; Halabalaki, M.; Pergola, C.; Werz, O.; Smith, A.B., III; Michel, S.; Skaltsounis, L.; Deguin, B. One-step semisynthesis of oleacein and the determination as a 5-lipoxygenase inhibitor. J. Nat. Prod. 2014, 77, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat. Chem. Biol. 2020, 16, 783–790. [Google Scholar] [CrossRef]
- Kemal, C.; Louis-Flamberg, P.; Krupinski-Olsen, R.; Shorter, A.L. Reductive inactivation of soybean lipoxygenase 1 by catechols: A possible mechanism for regulation of lipoxygenase activity. Biochemistry 1987, 26, 7064–7072. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]


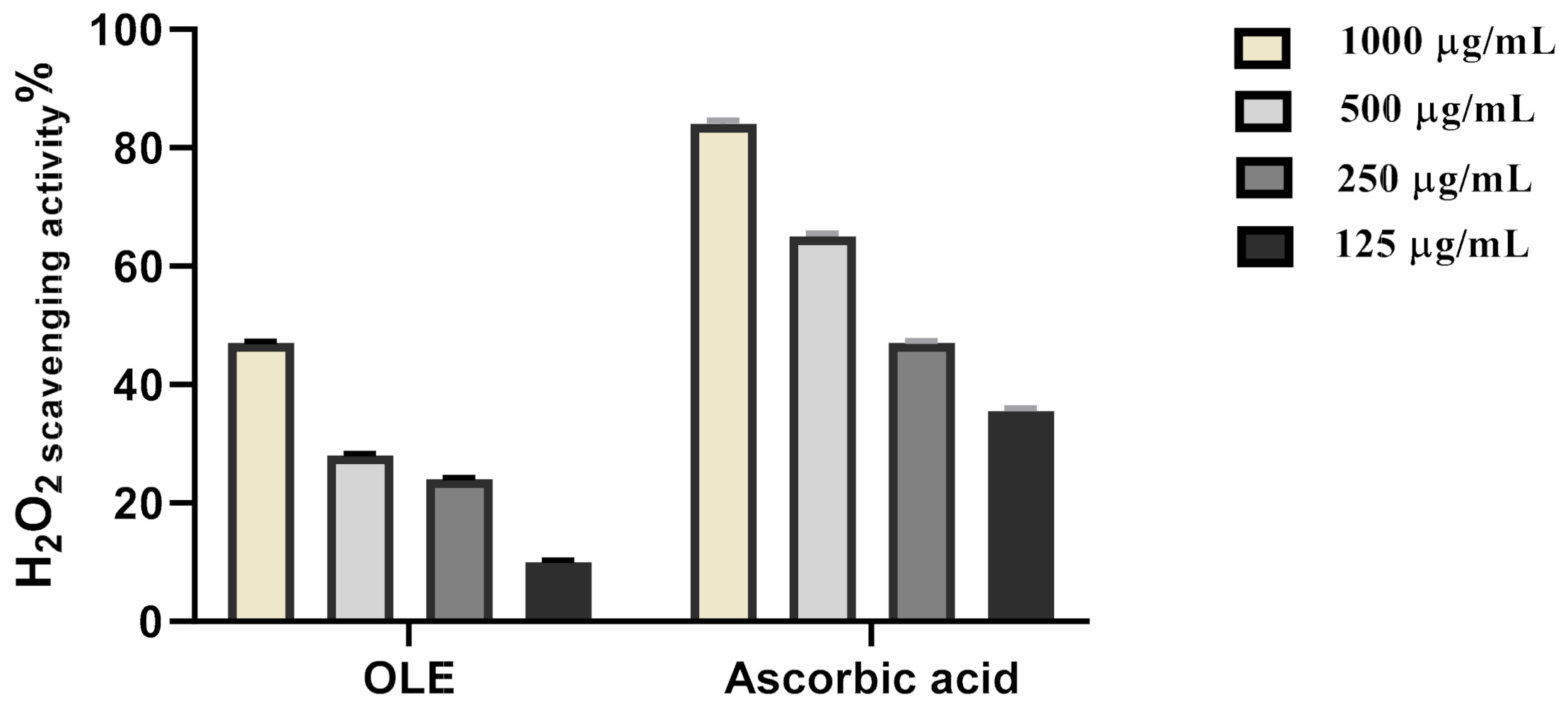
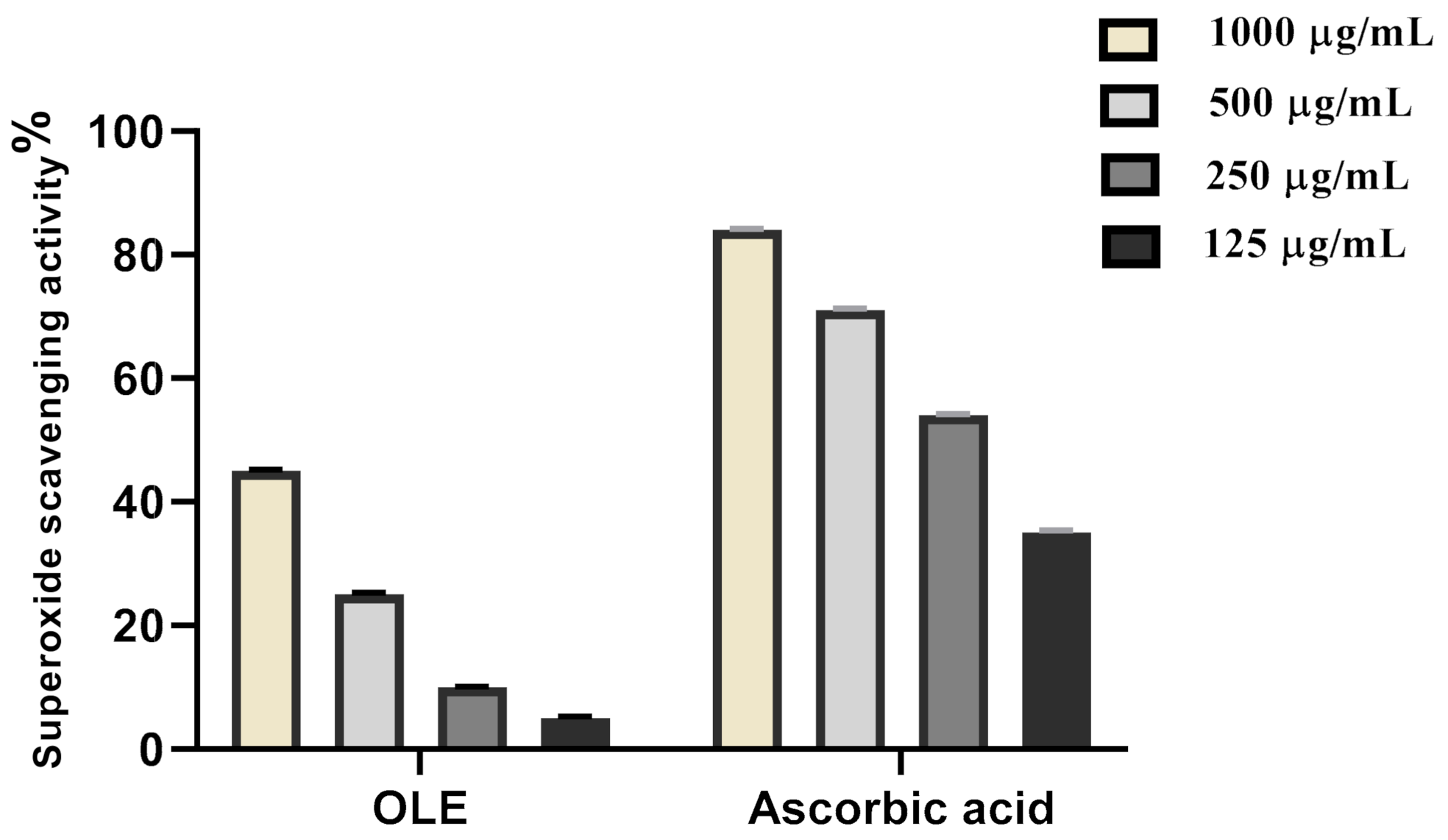


| Sample | IC50 |
|---|---|
| OLE extract | 279.2 µg/mL |
| Ascorbic acid | 178.7 µg/mL |
| Sample | IC50 |
|---|---|
| OLE extract | 419.5 µg/mL |
| Ascorbic acid | 161.4 µg/mL |
| Group | Level 1 | Level II | Level III | UI (mm) | PI (%) |
|---|---|---|---|---|---|
| Normal | - | - | - | - | - |
| Indomethacin | 22 ± 3.04 | 26.67 ± 2.17 | 14 ± 5.28 | 117.32 ± 23.4 | - |
| Indomethacin + cimetidine | 1.31 ± 0.32 | 0.3 ± 0.31 | 0 | 2.0 ± 1.01 *** | 98.2 |
| Indomethacin + total extract | 2.31 ± 0.88 | 0 | 0 | 2.31 ± 2.17 *** | 98.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, A.; Shady, N.H.; Ahmed, S.R.; Alnusaire, T.S.; Sayed, A.M.; Alowaiesh, B.F.; Sabouni, I.; Al-Sanea, M.M.; Mostafa, E.M.; Youssif, K.A.; et al. Antiulcer Potential of Olea europea L. cv. Arbequina Leaf Extract Supported by Metabolic Profiling and Molecular Docking. Antioxidants 2021, 10, 644. https://doi.org/10.3390/antiox10050644
Musa A, Shady NH, Ahmed SR, Alnusaire TS, Sayed AM, Alowaiesh BF, Sabouni I, Al-Sanea MM, Mostafa EM, Youssif KA, et al. Antiulcer Potential of Olea europea L. cv. Arbequina Leaf Extract Supported by Metabolic Profiling and Molecular Docking. Antioxidants. 2021; 10(5):644. https://doi.org/10.3390/antiox10050644
Chicago/Turabian StyleMusa, Arafa, Nourhan Hisham Shady, Shaimaa R. Ahmed, Taghreed S. Alnusaire, Ahmed M. Sayed, Bassam F. Alowaiesh, Ibrahim Sabouni, Mohammad M. Al-Sanea, Ehab M. Mostafa, Khayrya A. Youssif, and et al. 2021. "Antiulcer Potential of Olea europea L. cv. Arbequina Leaf Extract Supported by Metabolic Profiling and Molecular Docking" Antioxidants 10, no. 5: 644. https://doi.org/10.3390/antiox10050644
APA StyleMusa, A., Shady, N. H., Ahmed, S. R., Alnusaire, T. S., Sayed, A. M., Alowaiesh, B. F., Sabouni, I., Al-Sanea, M. M., Mostafa, E. M., Youssif, K. A., Abu-Baih, D. H., Elrehany, M. A., & Abdelmohsen, U. R. (2021). Antiulcer Potential of Olea europea L. cv. Arbequina Leaf Extract Supported by Metabolic Profiling and Molecular Docking. Antioxidants, 10(5), 644. https://doi.org/10.3390/antiox10050644