Zebrafish as a Useful Model to Study Oxidative Stress-Linked Disorders: Focus on Flavonoids
Abstract
1. Introduction
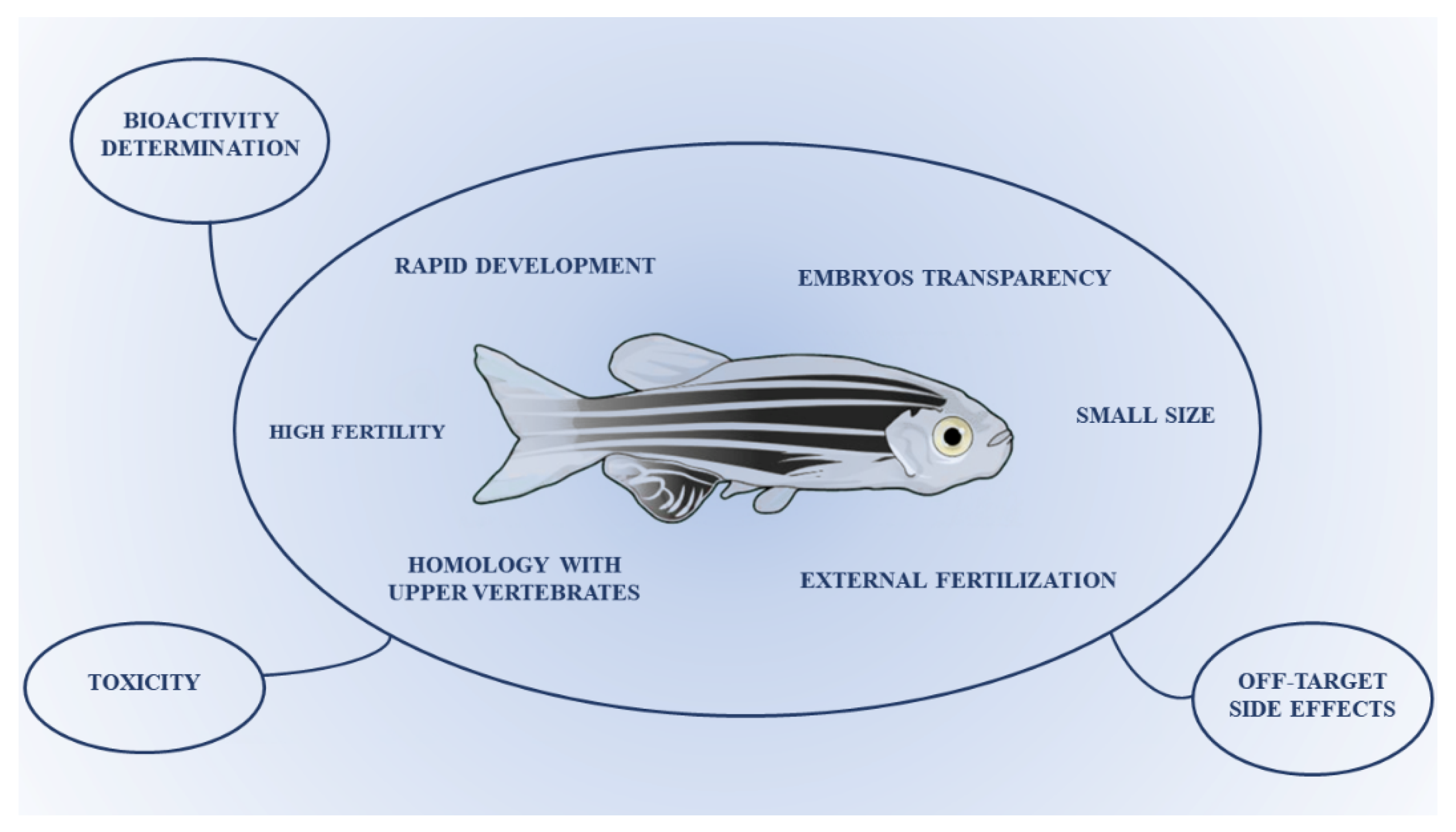
1.1. Zebrafish as Experimental Model
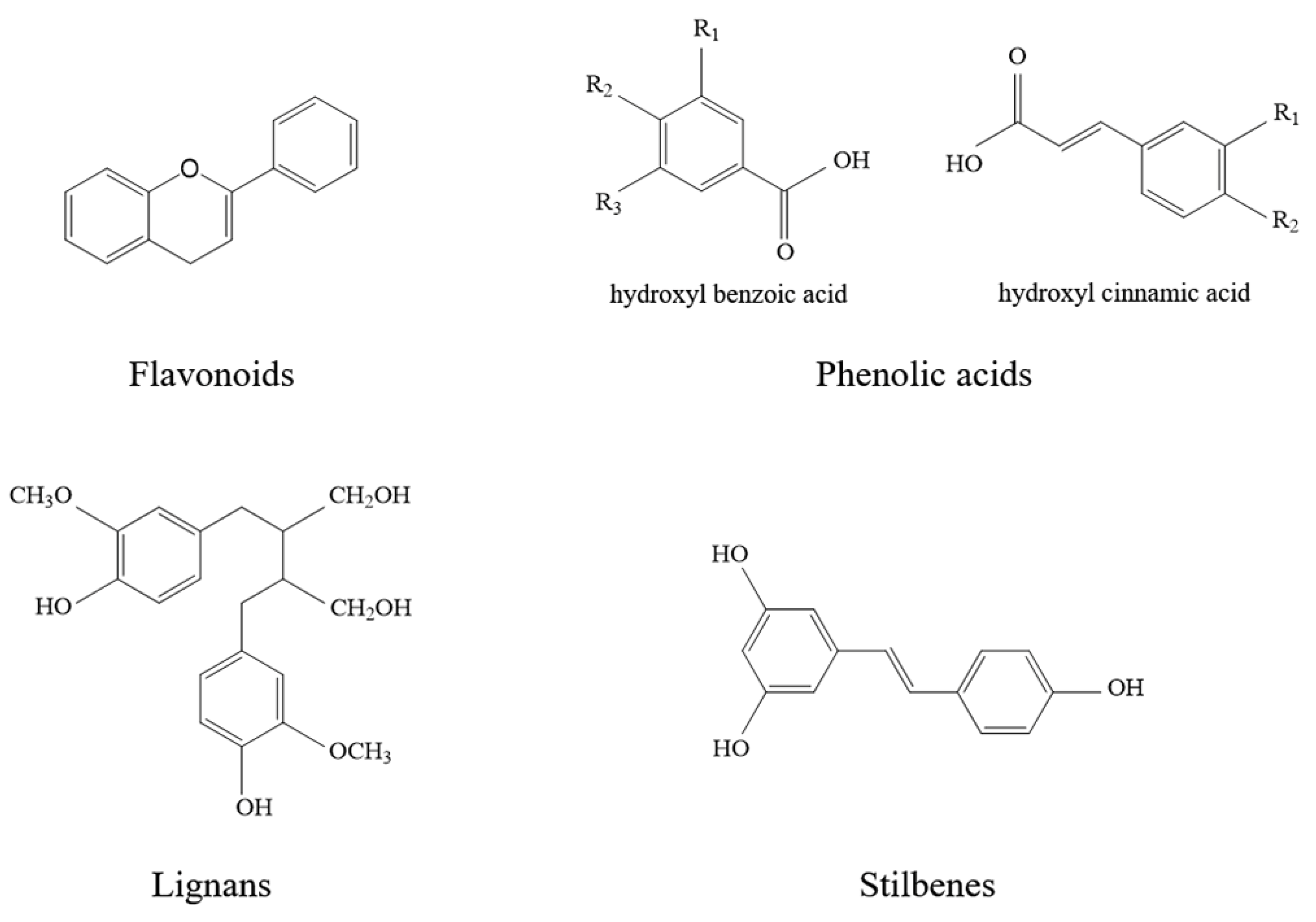
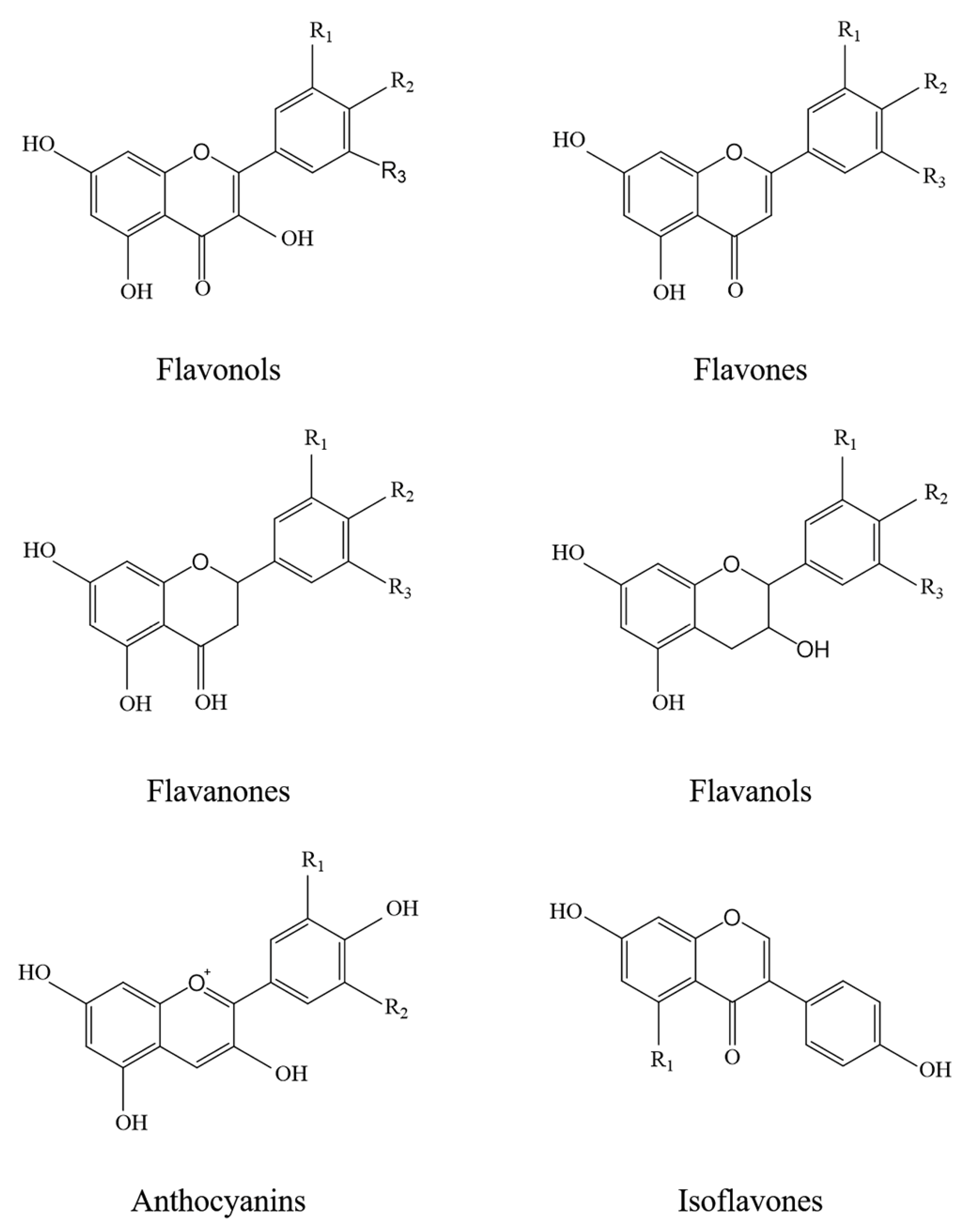
1.2. Flavonoids
2. Effects of Flavonoids Employing Zebrafish as Experimental Model
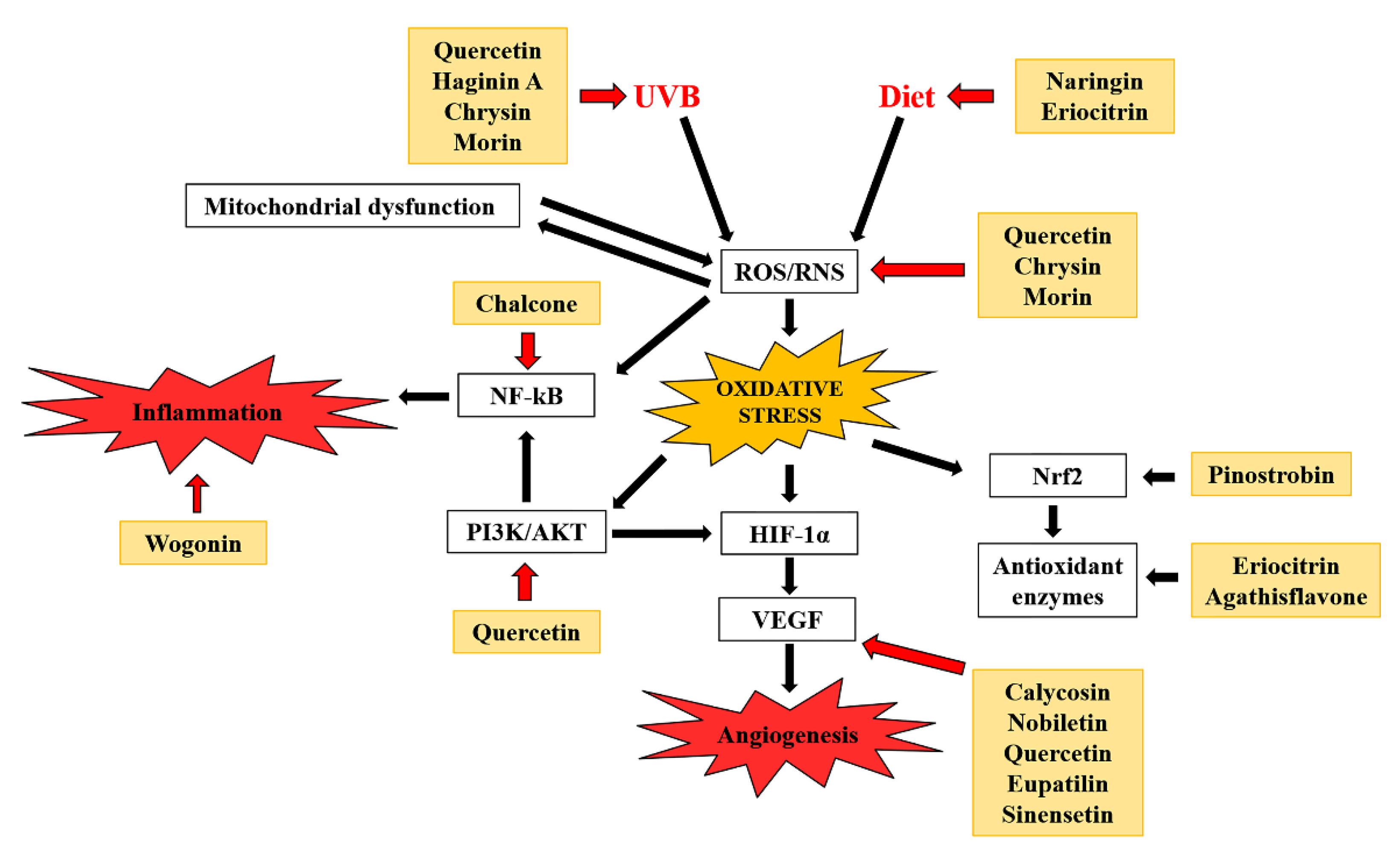
2.1. Antioxidant Effects
2.2. Antiangiogenic and Antitumor Effects
2.3. Protection from Ultraviolet (UV) Radiation
2.4. Anti-Inflammatory Effects
2.5. Toxicity of Flavonoids Employing the Zebrafish as Model
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, H.T.; Zon, L.I. Regulation of stem cells in the zebra fish hematopoietic system. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 111–118. [Google Scholar] [CrossRef]
- Parng, C.; Seng, W.L.; Semino, C.; McGrath, P. Zebrafish: A preclinical model for drug screening. Assay Drug Dev. Technol. 2002, 1, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Williams, C.H.; Webb, M.E.; Hong, C.C. Large scale zebrafish-based in vivo small molecule screen. J. Vis. Exp. 2010, 46, e2243. [Google Scholar] [CrossRef]
- Ingham, P.W. The power of the zebrafish for disease analysis. Hum. Mol. Genet. 2009, 18, R107–R112. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.R.; Noyes, P.D.; Tanguay, R.L. Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 2016, 161, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wehmas, L.C.; Tanguay, R.L.; Punnoose, A.; Greenwood, J.A. Developing a novel embryo-larval zebrafish xenograft assay to prioritize human glioblastoma therapeutics. Zebrafish 2016, 13, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.T.; Elson, D.J.; Greenwood, J.A.; Tanguay, R.L.; Kolluri, S.K. The zebrafish xenograft models for investigating cancer and cancer therapeutics. Biology 2021, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Bian, Z.P.; Lu, S.; Xu, J.D.; Gu, C.R.; Yang, D.; Zhang, J.N. Cardiac protective effect of astragalus on viral myocarditis mice: Comparison with perindopril. Am. J. Chin. Med. 2006, 34, 493–502. [Google Scholar] [CrossRef]
- Crawford, J. The evolving art of biofeedback. Beginnings 2008, 28, 14–15. [Google Scholar]
- Saleem, S.; Kannan, R.R. Zebrafish: An emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov. 2018, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Hason, M.; Bartunek, P. Zebrafish models of cancer-new insights on modeling human cancer in a non-mammalian vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Musumeci, L.; Maugeri, A.; Cirmi, S.; Lombardo, G.E.; Russo, C.; Gangemi, S.; Calapai, G.; Navarra, M. Citrus fruits and their flavonoids in inflammatory bowel disease: An overview. Nat. Prod. Res. 2020, 34, 122–136. [Google Scholar] [CrossRef]
- Cirmi, S.; Navarra, M.; Woodside, J.V.; Cantwell, M.M. Citrus fruits intake and oral cancer risk: A systematic review and meta-analysis. Pharmacol. Res. 2018, 133, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Filocamo, A.; Bisignano, C.; Ferlazzo, N.; Cirmi, S.; Mandalari, G.; Navarra, M. In Vitro effect of bergamot (Citrus bergamia) juice against caga-positive and-negative clinical isolates of helicobacter pylori. BMC Complement. Altern. Med. 2015, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; Cirmi, S.; Navarra, M. Effectiveness of citrus fruits on helicobacter pylori. Evid. Based Complement. Altern. Med. 2017, 2017, 8379262. [Google Scholar] [CrossRef] [PubMed]
- Citraro, R.; Navarra, M.; Leo, A.; Donato Di Paola, E.; Santangelo, E.; Lippiello, P.; Aiello, R.; Russo, E.; De Sarro, G. The Anticonvulsant activity of a flavonoid-rich extract from orange juice involves both NMDA and GABA-benzodiazepine receptor complexes. Molecules 2016, 21, 1261. [Google Scholar] [CrossRef]
- Mannucci, C.; Calapai, F.; Cardia, L.; Inferrera, G.; D’Arena, G.; Di Pietro, M.; Navarra, M.; Gangemi, S.; Ventura Spagnolo, E.; Calapai, G. Clinical pharmacology of citrus aurantium and citrus sinensis for the treatment of anxiety. Evid. Based Complement. Altern. Med. 2018, 2018, 3624094. [Google Scholar] [CrossRef]
- Mannucci, C.; Navarra, M.; Calapai, F.; Squeri, R.; Gangemi, S.; Calapai, G. Clinical pharmacology of citrus bergamia: A systematic review. Phytother. Res. 2017, 31, 27–39. [Google Scholar] [CrossRef]
- Mannucci, C.; Casciaro, M.; Sorbara, E.E.; Calapai, F.; Di Salvo, E.; Pioggia, G.; Navarra, M.; Calapai, G.; Gangemi, S. Nutraceuticals against oxidative stress in autoimmune disorders. Antioxidants 2021, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-inflammatory activity of citrus bergamia derivatives: Where do we stand? Molecules 2016, 21, 1273. [Google Scholar] [CrossRef]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegenerative diseases: Might citrus flavonoids play a protective role? Molecules 2016, 21, 1312. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Ferlazzo, N.; De Luca, L.; Gitto, R.; Navarra, M. The link between the AMPK/SIRT1 axis and a flavonoid-rich extract of citrus bergamia juice: A cell-free, in silico, and In Vitro study. Phytother. Res. 2019, 33, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Paterniti, I.; Cordaro, M.; Morabito, R.; Campolo, M.; Navarra, M.; Esposito, E.; Cuzzocrea, S. Role of natural antioxidants and potential use of bergamot in treating rheumatoid arthritis. PharmaNutrition 2015, 3, 53–59. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Visalli, G.; Cirmi, S.; Lombardo, G.E.; Lagana, P.; Di Pietro, A.; Navarra, M. Natural iron chelators: Protective role in A549 cells of flavonoids-rich extracts of citrus juices in Fe(3+)-induced oxidative stress. Environ. Toxicol. Pharmacol. 2016, 43, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Cirmi, S.; Minciullo, P.L.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Citrus fruits and inflammaging: A systematic review. Phytochem. Rev. 2019, 18, 1025–1049. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Ferlazzo, N.; Gangemi, S.; Calapai, G.; Schumacher, U.; Navarra, M. Anticancer potential of citrus juices and their extracts: A systematic review of both preclinical and clinical studies. Front. Pharm. 2017, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Curro, M.; Risitano, R.; Ferlazzo, N.; Cirmi, S.; Gangemi, C.; Caccamo, D.; Ientile, R.; Navarra, M. Citrus bergamia juice extract attenuates beta-amyloid-induced pro-inflammatory activation of THP-1 cells through MAPK and AP-1 pathways. Sci. Rep. 2016, 6, 20809. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Cirmi, S.; Maugeri, A.; Russo, C.; Lombardo, G.E.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Neuroprotective effect of bergamot juice in 6-OHDA-induced SH-SY5Y cell death, an In Vitro model of parkinson’s disease. Pharmaceutics 2020, 12, 326. [Google Scholar] [CrossRef]
- Navarra, M.; Femia, A.P.; Romagnoli, A.; Tortora, K.; Luceri, C.; Cirmi, S.; Ferlazzo, N.; Caderni, G. A flavonoid-rich extract from bergamot juice prevents carcinogenesis in a genetic model of colorectal cancer, the Pirc rat (F344/NTac-Apc(am1137)). Eur. J. Nutr. 2020, 59, 885–894. [Google Scholar] [CrossRef]
- Fusco, R.; Cirmi, S.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. A flavonoid-rich extract of orange juice reduced oxidative stress in an experimental model of inflammatory bowel disease. J. Funct. Foods 2017, 30, 168–178. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; Lombardo, G.E.; Ulaszewska, M.M.; Mattivi, F.; Bramanti, P.; Mazzon, E.; Navarra, M. Neuroprotective effects of a polyphenolic white grape juice extract in a mouse model of experimental autoimmune encephalomyelitis. Fitoterapia 2015, 103, 171–186. [Google Scholar] [CrossRef]
- Celano, M.; Maggisano, V.; De Rose, R.F.; Bulotta, S.; Maiuolo, J.; Navarra, M.; Russo, D. Flavonoid fraction of citrus reticulata juice reduces proliferation and migration of anaplastic thyroid carcinoma cells. Nutr. Cancer 2015, 67, 1183–1190. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Peditto, M.; Oteri, G.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. Treatment with a flavonoid-rich fraction of bergamot juice improved lipopolysaccharide-induced periodontitis in rats. Front. Pharmacol. 2018, 9, 1563. [Google Scholar] [CrossRef]
- Samuele, A.; Mangiagalli, A.; Armentero, M.T.; Fancellu, R.; Bazzini, E.; Vairetti, M.; Ferrigno, A.; Richelmi, P.; Nappi, G.; Blandini, F. Oxidative stress and pro-apoptotic conditions in a rodent model of Wilson’s disease. Biochim. Biophys. Acta 2005, 1741, 325–330. [Google Scholar] [CrossRef]
- Tseng, H.L.; Li, C.J.; Huang, L.H.; Chen, C.Y.; Tsai, C.H.; Lin, C.N.; Hsu, H.Y. Quercetin 3-O-methyl ether protects FL83B cells from copper induced oxidative stress through the PI3K/Akt and MAPK/Erk pathway. Toxicol. Appl. Pharm. 2012, 264, 104–113. [Google Scholar] [CrossRef]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharm. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Yang, Z.S.; Wen, C.C.; Chang, Y.S.; Wang, B.C.; Hsiao, C.A.; Shih, T.L. Evaluation of the structure-activity relationship of flavonoids as antioxidants and toxicants of zebrafish larvae. Food Chem. 2012, 134, 717–724. [Google Scholar] [CrossRef]
- Hiramitsu, M.; Shimada, Y.; Kuroyanagi, J.; Inoue, T.; Katagiri, T.; Zang, L.; Nishimura, Y.; Nishimura, N.; Tanaka, T. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci. Rep. 2014, 4, 3708. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamamoto, K.; Morimitsu, Y.; Osawa, T. Characteristics of antioxidative flavonoid glycosides in lemon fruit. Food Sci. Technol. Int. Tokyo 1998, 4, 48–53. [Google Scholar] [CrossRef][Green Version]
- Miyake, Y.; Suzuki, E.; Ohya, S.; Fukumoto, S.; Hiramitsu, M.; Sakaida, K.; Osawa, T.; Furuichi, Y. Lipid-lowering effect of eriocitrin, the main flavonoid in lemon fruit, in rats on a high-fat and high-cholesterol diet. J. Food Sci. 2006, 71, S633–S637. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamamoto, K.; Tsujihara, N.; Osawa, T. Protective effects of lemon flavonoids on oxidative stress in diabetic rats. Lipids 1998, 33, 689–695. [Google Scholar] [CrossRef]
- Montalbano, G.; Maugeri, A.; Guerrera, M.C.; Miceli, N.; Navarra, M.; Barreca, D.; Cirmi, S.; Germana, A. A white grape juice extract reduces fat accumulation through the modulation of ghrelin and leptin expression in an in vivo model of overfed zebrafish. Molecules 2021, 26, 1119. [Google Scholar] [CrossRef]
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Laura, R.; Abbate, F.; Levanti, M.; Maugeri, A.; Germana, A.; Navarra, M. Effects of a flavonoid-rich extract from citrus sinensis juice on a diet-induced obese zebrafish. Int. J. Mol. Sci. 2019, 20, 5116. [Google Scholar] [CrossRef]
- Li, C.; Tang, B.; Feng, Y.; Tang, F.; Pui-Man Hoi, M.; Su, Z.; Ming-Yuen Lee, S. Pinostrobin exerts neuroprotective actions in neurotoxin-induced parkinson’s disease models through Nrf2 induction. J. Agric. Food Chem. 2018, 66, 8307–8318. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, G.; El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Boiangiu, R.S.; Todirascu-Ciornea, E.; Hritcu, L.; Singab, A.N.B. Agathisflavone isolated from Schinus polygamus (Cav.) Cabrera leaves prevents scopolamine-induced memory impairment and brain oxidative stress in zebrafish (Danio rerio). Phytomedicine 2019, 58, 152889. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, Y.; Huang, P.; Xie, L.; Lin, H.; Zhou, Z.; Mo, C.; Deng, G.; Yan, W.; Gao, Z.; et al. Naringin attenuates alcoholic liver injury by reducing lipid accumulation and oxidative stress. Life Sci. 2019, 216, 305–312. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kang, M.C.; Lee, H.H.L.; Cho, C.H.; Choi, I.; Park, Y.; Lee, S.H. Antioxidant effects of turmeric leaf extract against hydrogen peroxide-induced oxidative stress In Vitro in vero cells and In Vivo in zebrafish. Antioxidants 2021, 10, 112. [Google Scholar] [CrossRef]
- Larrivee, B.; Freitas, C.; Suchting, S.; Brunet, I.; Eichmann, A. Guidance of vascular development: Lessons from the nervous system. Circ. Res. 2009, 104, 428–441. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Quesada, A.R.; Munoz-Chapuli, R.; Medina, M.A. Anti-angiogenic drugs: From bench to clinical trials. Med. Res. Rev. 2006, 26, 483–530. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Carrasco, E.; Garcia-Ramirez, M.; Hernandez, C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr. Diabetes Rev. 2006, 2, 71–98. [Google Scholar] [CrossRef]
- Huang, S.T.; Yang, R.C.; Lee, P.N.; Yang, S.H.; Liao, S.K.; Chen, T.Y.; Pang, J.H. Anti-tumor and anti-angiogenic effects of phyllanthus urinaria in mice bearing lewis lung carcinoma. Int. Immunopharmacol. 2006, 6, 870–879. [Google Scholar] [CrossRef]
- Oh, S.H.; Woo, J.K.; Jin, Q.; Kang, H.J.; Jeong, J.W.; Kim, K.W.; Hong, W.K.; Lee, H.Y. Identification of novel antiangiogenic anticancer activities of deguelin targeting hypoxia-inducible factor-1 alpha. Int. J. Cancer 2008, 122, 5–14. [Google Scholar] [CrossRef]
- Tuli, H.S.; Mittal, S.; Loka, M.; Aggarwal, V.; Aggarwal, D.; Masurkar, A.; Kaur, G.; Varol, M.; Sak, K.; Kumar, M.; et al. Deguelin targets multiple oncogenic signaling pathways to combat human malignancies. Pharmacol. Res. 2021, 166, 105487. [Google Scholar] [CrossRef]
- Tang, J.Y.; Li, S.; Li, Z.H.; Zhang, Z.J.; Hu, G.; Cheang, L.C.; Alex, D.; Hoi, M.P.; Kwan, Y.W.; Chan, S.W.; et al. Calycosin promotes angiogenesis involving estrogen receptor and mitogen-activated protein kinase (MAPK) signaling pathway in zebrafish and HUVEC. PLoS ONE 2010, 5, e11822. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yang, X.; Wei, J.R.; Chen, N.M.; Xu, J.P.; Bi, Y.Q.; Yang, M.; Gong, X.; Li, Z.Y.; Ren, K.; et al. Ethnopharmacology, Phytochemistry, Pharmacology, Toxicology and Clinical Applications of Radix Astragali. Chin. J. Integr. Med. 2021, 27, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.C.; Gao, Q.T.; Cheung, A.W.; Zhu, J.T.; Lau, F.T.; Li, J.; Li, W.Z.; Chu, G.K.; Duan, R.; Cheung, J.K.; et al. A chinese herbal decoction, danggui buxue tang, stimulates proliferation, differentiation and gene expression of cultured osteosarcoma cells: Genomic approach to reveal specific gene activation. Evid. Based Complement. Altern. Med. 2011, 2011, 307548. [Google Scholar] [CrossRef]
- Song, Z.H.; Ji, Z.N.; Lo, C.K.; Dong, T.T.; Zhao, K.J.; Li, O.T.; Haines, C.J.; Kung, S.D.; Tsim, K.W. Chemical and biological assessment of a traditional chinese herbal decoction prepared from radix astragali and radix angelicae sinensis: Orthogonal array design to optimize the extraction of chemical constituents. Planta Med. 2004, 70, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.T.X.; Zhao, K.J.; Gao, Q.T.; Ji, Z.N.; Zhu, T.T.; Li, J.; Duan, R.; Cheung, A.W.H.; Tsim, K.W.K. Chemical and biological assessment of a chinese herbal decoction containing radix astragali and radix angelicae sinensis: Determination of drug ratio in having optimized properties. J. Agric. Food Chem. 2006, 54, 2767–2774. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.P.; Yeh, J.C.; Leung, K.W.; Yue, P.Y.; Wong, R.N. Angiogenesis: From plants to blood vessels. Trends Pharmacol. Sci. 2006, 27, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lou, S.; Lei, B.U.; Chan, T.F.; Kwan, Y.W.; Chan, S.W.; Leung, G.P.; Tsui, S.K.; Lee, S.M. Transcriptional profiling of angiogenesis activities of calycosin in zebrafish. Mol. Biosyst. 2011, 7, 3112–3121. [Google Scholar] [CrossRef]
- Lam, K.H.; Alex, D.; Lam, I.K.; Tsui, S.K.W.; Yang, Z.F.; Lee, S.M.Y. Nobiletin, a polymethoxylated flavonoid from citrus, shows anti-angiogenic activity in a zebrafish In Vivo model and HUVEC In Vitro model. J. Cell Biochem. 2011, 112, 3313–3321. [Google Scholar] [CrossRef]
- Seng, W.L.; Eng, K.; Lee, J.; McGrath, P. Use of a monoclonal antibody specific for activated endothelial cells to quantitate angiogenesis in vivo in zebrafish after drug treatment. Angiogenesis 2004, 7, 243–253. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Zhou, C.H.; Tao, J.; Li, S.N. Antagonistic effects of nobiletin, a polymethoxyflavonoid, on eosinophilic airway inflammation of asthmatic rats and relevant mechanisms. Life Sci. 2006, 78, 2689–2696. [Google Scholar] [CrossRef]
- Choi, S.Y.; Hwang, J.H.; Ko, H.C.; Park, J.G.; Kim, S.J. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-kappaB and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Guan, X.; Zhou, L. Apoptotic effect of citrus fruit extract nobiletin on lung cancer cell line A549 In Vitro and In Vivo. Cancer Biol. Ther. 2008, 7, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Yasui, Y.; Tanaka, T.; Ohigashi, H.; Murakami, A. Suppressive effects of nobiletin on hyperleptinemia and colitis-related colon carcinogenesis in male ICR mice. Carcinogenesis 2008, 29, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Yoshimizu, N.; Otani, Y.; Saikawa, Y.; Kubota, T.; Yoshida, M.; Furukawa, T.; Kumai, K.; Kameyama, K.; Fujii, M.; Yano, M.; et al. Anti-tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: Antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment. Pharmacol. Ther. 2004, 20, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.W.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, improves memory impairment and abeta pathology in a transgenic mouse model of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Yamakuni, T.; Hashimoto, M.; Haque, A.M.; Shido, O.; Mimaki, Y.; Sashida, Y.; Ohizumi, Y. Nobiletin restoring beta-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer’s disease model rats. Neurosci. Lett. 2006, 400, 230–234. [Google Scholar] [CrossRef]
- Lam, I.K.; Alex, D.; Wang, Y.H.; Liu, P.; Liu, A.L.; Du, G.H.; Lee, S.M.Y. In Vitro and In Vivo structure and activity relationship analysis of polymethoxylated flavonoids: Identifying sinensetin as a novel antiangiogenesis agent. Mol. Nutr. Food Res. 2012, 56, 945–956. [Google Scholar] [CrossRef]
- Lin, C.; Wu, M.; Dong, J. Quercetin-4′-O-beta-D-glucopyranoside (QODG) inhibits angiogenesis by suppressing VEGFR2-mediated signaling in zebrafish and endothelial cells. PLoS ONE 2012, 7, e31708. [Google Scholar] [CrossRef]
- Zhao, D.; Qin, C.; Fan, X.; Li, Y.; Gu, B. Inhibitory effects of quercetin on angiogenesis in larval zebrafish and human umbilical vein endothelial cells. Eur. J. Pharmacol. 2014, 723, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Zhou, D.; Ruan, J.; Cai, Y.; Xiong, C.; Wu, G. Anti-tumor and anti-angiogenic effects of macrothelypteris viridifrons and its constituents by HPLC-DAD/MS analysis. J. Ethnopharmacol. 2012, 139, 373–380. [Google Scholar] [CrossRef]
- Fu, W.; Fang, W.; Ruan, J.L. Two new flavanone glycosides from Macrothelypteris torresiana (Gaud.) Ching. Chin. Chem. Lett. 2009, 20, 579–581. [Google Scholar] [CrossRef]
- Tang, Y.F.; Fang, W.; Ma, Y.T.; Cai, Y.L.; Ruan, J.L. A novel flavonoid from the root of Macrothelypteris torresiana (Gaud.) Ching. Chin. Chem. Lett. 2009, 20, 815–816. [Google Scholar] [CrossRef]
- Fang, W.; Ruan, J.; Cai, Y.; Wei, A.; Zhou, D.; Zhang, W. Flavonoids from the aerial parts of Macrothelypteris torresiana. Nat. Prod. Res. 2011, 25, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.S.; Chang, F.R.; Yen, H.F.; Bjorkeborn, H.; Norlen, P.; Wu, Y.C. Novel flavonoids of Thelypteris torresiana. Chem. Pharm. Bull. 2007, 55, 635–637. [Google Scholar] [CrossRef][Green Version]
- Lee, J.Y.; Bae, H.; Yang, C.; Park, S.; Youn, B.S.; Kim, H.S.; Song, G.; Lim, W. Eupatilin promotes cell death by calcium influx through ER-Mitochondria axis with SERPINB11 inhibition in epithelial ovarian cancer. Cancers 2020, 12, 1459. [Google Scholar] [CrossRef] [PubMed]
- de Gruijl, F.R.; Ananthaswamy, H.N. Ultraviolet Radiation as a Carcinogen; Elsevier: Oxford, UK, 2010. [Google Scholar] [CrossRef]
- Guinea, M.; Franco, V.; Araujo-Bazan, L.; Rodriguez-Martin, I.; Gonzalez, S. In vivo UVB-photoprotective activity of extracts from commercial marine macroalgae. Food Chem. Toxicol. 2012, 50, 1109–1117. [Google Scholar] [CrossRef]
- Sambandan, D.R.; Ratner, D. Sunscreens: An overview and update. J. Am. Acad. Dermatol. 2011, 64, 748–758. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Fernandez-Lorente, M.; Gilaberte-Calzada, Y. The latest on skin photoprotection. Clin. Dermatol. 2008, 26, 614–626. [Google Scholar] [CrossRef]
- Halliday, G.M.; Norval, M.; Byrne, S.N.; Huang, X.X.; Wolf, P. The effects of sunlight on the skin. Drug Discov. Today Dis. Mech. 2008, 5, e201–e209. [Google Scholar] [CrossRef]
- Marrot, L.; Meunier, J.R. Skin DNA photodamage and its biological consequences. J. Am. Acad. Dermatol. 2008, 58, S139–S148. [Google Scholar] [CrossRef]
- Kim, J.H.B.; Baek, S.H.; Kim, D.H.; Choi, T.Y.; Yoon, T.J.; Hwang, J.S.; Kim, M.R.; Kwon, H.J.; Lee, C.W. Downregulation of melanin synthesis by haginin A and its application to in vivo light-ening model. J. Investig. Dermatol. 2008, 128, 1227–1235. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wen, C.C.; Yang, Z.S.; Cheng, C.C.; Tsai, J.N.; Ku, C.C.; Wu, H.J.; Chen, Y.H. Development of a whole-organism model to screen new compounds for sun protection. Mar. Biotechnol. 2009, 11, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Svoboda, K.; Tiersch, T.R.; Monroe, W.T. Photobiological effects of UVA and UVB light in zebrafish embryos: Evidence for a competent photorepair system. J. Photochem. Photobiol. B Biol. 2007, 88, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Ham, Y.M.; Yoon, W.J.; Roh, S.W.; Jeon, Y.J.; Oda, T.; Kang, S.M.; Kang, M.C.; Kim, E.A.; Kim, D.; et al. Quercitrin protects against ultraviolet B-induced cell death in vitro and in an in vivo zebrafish model. J. Photochem. Photobiol. B Biol. 2012, 114, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Hashimoto, T. Ultraviolet B-induced cell death in four cutaneous cell lines exhibiting different enzymatic antioxidant defences: Involvement of apoptosis. J. Dermatol. Sci. 1998, 17, 140–150. [Google Scholar] [CrossRef]
- Tyrrell, R.M. Ultraviolet radiation and free radical damage to skin. Biochem. Soc. Symp. 1995, 61, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; D’Angelo, V.; Denaro, M.; Trombetta, D.; Raimondo, F.M.; Germano, M.P. Polyphenol Characterization, Antioxidant and Skin Whitening Properties of Alnus cordata Stem Bark. Chem. Biodivers. 2019, 16, e1900314. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, W.H.; Wang, Y.H.; Lin, Z.Y.; Wen, C.C.; Chern, C.Y. Evaluation of the anti-inflammatory effect of chalcone and chalcone analogues in a zebrafish model. Molecules 2013, 18, 2052–2060. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Jantan, I.; Jasamai, M. Anti-inflammatory trends of 1, 3-diphenyl-2-propen-1-one derivatives. Mini Rev. Med. Chem. 2013, 13, 87–94. [Google Scholar] [CrossRef]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005, 12, 481–499. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar] [CrossRef]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.; Elworthy, S.; Ingham, P.W.; Whyte, M.K. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–3978. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.D.; Allen, K.C.; Dorward, D.A.; Hoodless, L.J.; Melrose, L.A.; Marwick, J.A.; Tucker, C.S.; Haslett, C.; Duffin, R.; Rossi, A.G. Flavones induce neutrophil apoptosis by down-regulation of Mcl-1 via a proteasomal-dependent pathway. FASEB J. 2013, 27, 1084–1094. [Google Scholar] [CrossRef]
- Rishitha, N.; Muthuraman, A. Therapeutic evaluation of solid lipid nanoparticle of quercetin in pentylenetetrazole induced cognitive impairment of zebrafish. Life Sci. 2018, 199, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Randazzo, B.; Russo, C.; Musumeci, L.; Maugeri, A.; Montalbano, G.; Guerrera, M.C.; Lombardo, G.E.; Levanti, M. Anti-inflammatory effect of a flavonoid-rich extract of orange juice in adult zebrafish subjected to Vibrio anguillarum-induced enteritis. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef]
- Bugel, S.M.; Bonventre, J.A.; Tanguay, R.L. Comparative developmental toxicity of flavonoids using an integrative zebrafish system. Toxicol. Sci. 2016, 154, 55–68. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Flavonoid | Model | Effects |
|---|---|---|---|
| [36] | quercetin 3-O-methyl ether | zebrafish exposed to 5μM Cu2+ | protective role against oxidative damage |
| [39] | 15 flavonoids | UVB-exposed embryos zebrafish | reduction of ROS |
| [40] | eriocitrin | DIO-zebrafish | improved dyslipidaemia and decreased lipid droplets in the liver; increased mRNA of mitochondria transcription factor, nuclear respiratory factor 1, cytochrome c oxidase subunit 4 and ATP synthase |
| [46] | pinostrobin | MPTP-exposed zebrafish | increase in anti-oxidant enzymes such as GSH-Px, SOD and CAT; suppression of mitochondria-mediated neural apoptosis by Nrf2 pathway |
| [47] | agathisflavone | Scopolamine-treated zebrafish | restoration of SOD, CAT, GSH-Px activities and MDA levels, decreased after scopolamine administration |
| [48] | naringin | Ethanol-exposed zebrafish larvae | reduction of lipid accumulation and superoxide radical levels |
| Ref. | Flavonoid | Model | Effects |
|---|---|---|---|
| [65] | calycosin | zebrafish embryos | modulation of VEGF, FGF and ErbB signaling pathways |
| [66] | nobiletin | transgenic zebrafish embryos | inhibited formation of ISVs; induced G0/G1 phase accumulation in FLI1-positive endothelial cells; induced VEGF-A mRNA expression |
| [75] | 7 polymethoxylated flavonoids | zebrafish | downregulation of the mRNA expressions of angiogenesis genes flt1, kdrl, and hras |
| [76] | quercetin-4′-O-β-D-glucopyranoside | transgenic zebrafish | suppressed VEGF-induced phosphorylation of VEGFR2 through the involvement of c-Src, FAK, ERK, AKT, mTOR and S6K |
| [77] | quercetin | transgenic zebrafish embryos | VEGFR2 inhibition |
| [77] | quercetin | transgenic zebrafish embryos | inhibited the formation of ISVs, dorsal aorta and posterior cardinal vein |
| [80] | calycosin | transgenic zebrafish | promotion of angiogenesis through estrogen receptor and MAPK |
| [83] | eupatilin | zebrafish xenograft model | inhibited tumorigenesis; suppressed both VEGF and its receptor expression |
| Ref. | Flavonoid | Model | Effects |
|---|---|---|---|
| [91] | haginin A | UVB-exposed zebrafish | decreased tyrosinase production |
| [94] | quercitrin-3-O-rhamnoside | UVB-exposed zebrafish | reduced UVB-induced ROS generation and cell death |
| Ref. | Flavonoid | Model | Effects |
|---|---|---|---|
| [98] | chalcone | transgenic zebrafish | affected wound-induced neutrophil recruitment, Mpx enzymatic activity, protein expression levels of Mpx, NF-κB, and TNF-α |
| [103] | wogonin | zebrafish model of sterile tissue injury | neutrophil apoptosis blocked by caspase inhibition |
| [104] | quercetin | pentylenetetrazole-treated zebrafish | decrease in lipid peroxidation together with an antioxidant role, and reduced glutathione |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbate, F.; Maugeri, A.; Laurà, R.; Levanti, M.; Navarra, M.; Cirmi, S.; Germanà, A. Zebrafish as a Useful Model to Study Oxidative Stress-Linked Disorders: Focus on Flavonoids. Antioxidants 2021, 10, 668. https://doi.org/10.3390/antiox10050668
Abbate F, Maugeri A, Laurà R, Levanti M, Navarra M, Cirmi S, Germanà A. Zebrafish as a Useful Model to Study Oxidative Stress-Linked Disorders: Focus on Flavonoids. Antioxidants. 2021; 10(5):668. https://doi.org/10.3390/antiox10050668
Chicago/Turabian StyleAbbate, Francesco, Alessandro Maugeri, Rosaria Laurà, Maria Levanti, Michele Navarra, Santa Cirmi, and Antonino Germanà. 2021. "Zebrafish as a Useful Model to Study Oxidative Stress-Linked Disorders: Focus on Flavonoids" Antioxidants 10, no. 5: 668. https://doi.org/10.3390/antiox10050668
APA StyleAbbate, F., Maugeri, A., Laurà, R., Levanti, M., Navarra, M., Cirmi, S., & Germanà, A. (2021). Zebrafish as a Useful Model to Study Oxidative Stress-Linked Disorders: Focus on Flavonoids. Antioxidants, 10(5), 668. https://doi.org/10.3390/antiox10050668










