New Neuroprotective Effect of Lemon IntegroPectin on Neuronal Cellular Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Solubilization of Pectin
2.2. Cell Cultures and Treatment
2.3. Cell Viability and Cell Morphology
2.4. Analysis of Reactive Oxygen Species (ROS)
2.5. Oxidation Kinetics
2.6. Mitochondrial Analysis
2.6.1. Mitochondrial Membrane Potential Analysis
2.6.2. Mitochondrial Morphology Analysis
2.6.3. Remodeling of Isolated Mitochondria
2.7. Pectin Sructural Characterization
2.8. Statistical Analysis
3. Results
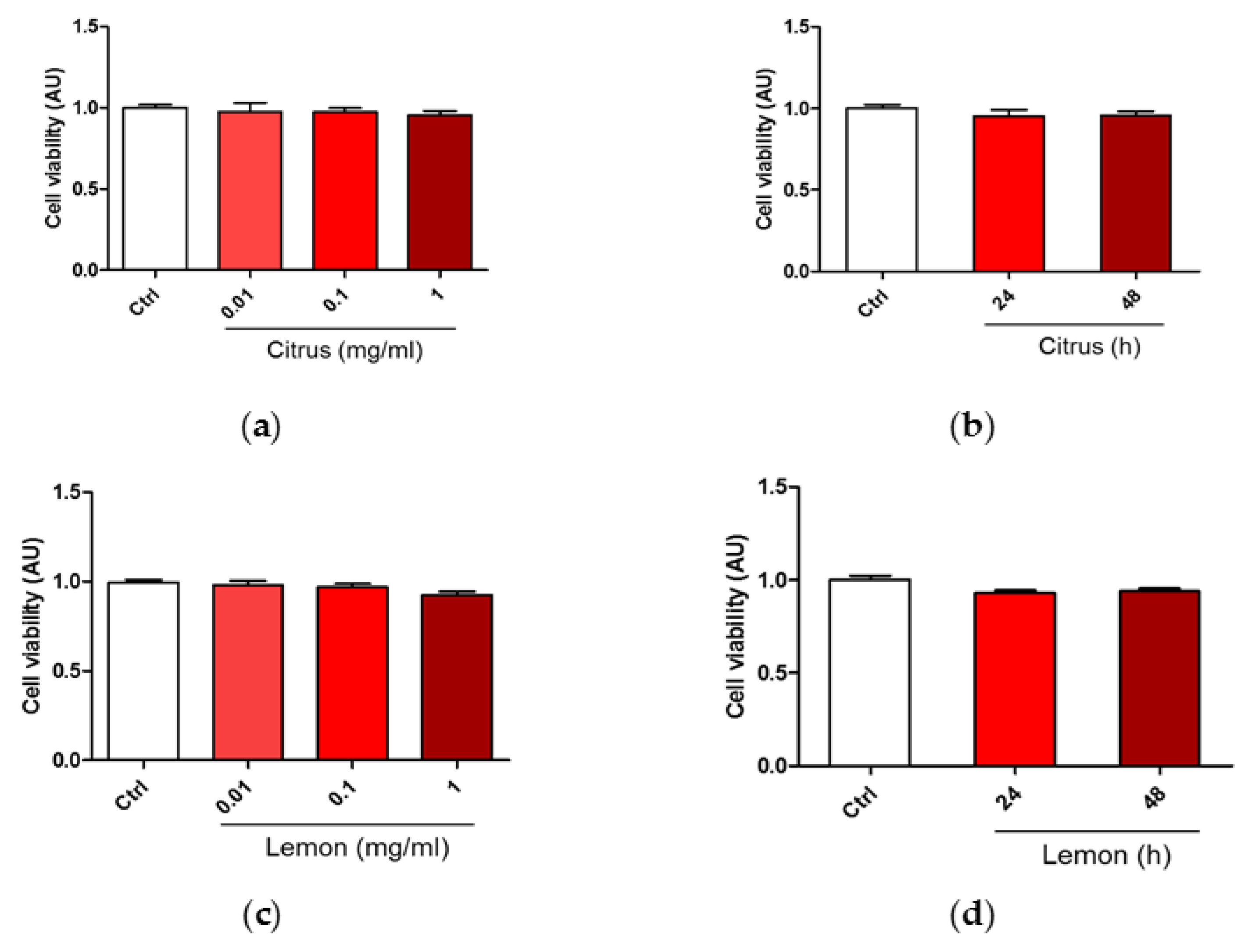
3.1. Effect of Pectins on Cell Viability upon Treatment with H2O2
3.2. Effects of Pectins on Cell Morphology Impaired by H2O2 Treatment
3.3. Effects of Pectins on ROS Production Induced by H2O2 Treatment
3.4. Effects of Pectins on Mitochondrial Membrane Potential Altered by H2O2 Treatment
3.5. Effects of Pectins on Mitochondrial Morphology Induced by H2O2 Treatment
3.6. Structural Insight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Zaitseva, O.; Khudyakov, A.; Sergushkina, M.; Solomina, O.; Polezhaeva, T. Pectins as a universal medicine. Fitoterapia 2020, 146, 104676. [Google Scholar] [CrossRef]
- Ciriminna, R.; Chavarría-Hernández, N.; Hernández, A.R.; Pagliaro, M. Pectin: A new perspective from the biorefinery standpoint. Biofuels Bioprod. Biorefin. 2015, 9, 368–377. [Google Scholar] [CrossRef]
- Willats, W.G.T.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Presentato, A.; Scurria, A.; Nuzzo, D.; Alduina, R.; Ilharco, L.M.; Pagliaro, M. Pectin: A long-neglected broad-spectrum antibacterial. ChemMedChem 2020, 15, 2228–2235. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, M.; Yang, Z.; Pan, S. Anti-diabetic effect of citrus pectin in diabetic rats and potential mechanism via PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2016, 89, 484–488. [Google Scholar] [CrossRef]
- Wu, J.; Chen, M.; Shi, S.; Wang, H.; Li, N.; Su, J.; Liu, R.; Huang, Z.; Jin, H.; Ji, X.; et al. Hypoglycemic effect and mechanism of a pectic polysaccharide with hexenuronic acid from the fruits of Ficus pumila L. in C57BL/KsJ db/db mice. Carbohydr. Polym. 2017, 178, 209–220. [Google Scholar] [CrossRef]
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef]
- Picone, P.; Di Carlo, M.; Nuzzo, D. Obesity and Alzheimer’s disease: Molecular bases. Eur. J. Neurosci. 2020, 52, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Alfos, S. Chapter 13—Fish oil supplementation prevents age-related memory decline: Involvement of nuclear hormone receptors. In Omega-3 Fatty Acids in Brain and Neurological Health; Watson, R.R., De Meester, F., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 147–161. [Google Scholar]
- Nuzzo, D.; Picone, P. Potential neurological effects of severe COVID-19 infection. Neurosci. Res. 2020, 158, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Nuzzo, D.; Caruana, L.; Scafidi, V.; Di Carlo, M. Mitochondrial dysfunction: Different routes to Alzheimer’s disease therapy. Oxid. Med. Cell. Longev. 2014, 2014, 780179. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, C.; Gao, X.; Wang, F.; Li, X.; Kassim, R.M.; Tai, G.; Zhou, Y. Neuroprotective effects of ginseng pectin through the activation of ERK/MAPK and Akt survival signaling pathways. Mol. Med. Rep. 2012, 5, 1185–1190. [Google Scholar] [CrossRef][Green Version]
- Leonoudakis, D.; Rane, A.; Angeli, S.; Lithgow, G.J.; Andersen, J.K.; Chinta, S.J. Anti-inflammatory and neuroprotective role of natural product securinine in activated glial cells: Implications for Parkinson’s disease. Mediat. Inflamm. 2017, 2017, 8302636. [Google Scholar] [CrossRef] [PubMed]
- Magalingam, K.B.; Radhakrishnan, A.K.; Haleagrahara, N. Protective mechanisms of flavonoids in Parkinson’s disease. Oxid. Med. Cell. Longev. 2015, 2015, 314560. [Google Scholar] [CrossRef]
- Bordoni, L.; Gabbianelli, R. Mitochondrial DNA and neurodegeneration: Any role for dietary antioxidants? Antioxidants 2020, 9, 764. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible neuroprotective mechanisms of physical exercise in neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive compounds of edible fruits with their anti-aging properties: A comprehensive review to prolong human life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; dos Santos Nascimento, L.B.; De Carlo, A.; et al. Real-scale integral valorization of waste orange peel via hydrodynamic cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Presentato, A.; Piacenza, E.; Scurria, A.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; Martino, D.C.; Alduina, R.; et al. A new water-soluble bactericidal agent for the treatment of infections caused by Gram-positive and Gram-negative bacterial strains. Antibiotics 2020, 9, 586. [Google Scholar] [CrossRef]
- Nuzzo, D.; Cristaldi, L.; Sciortino, M.; Albanese, L.; Scurria, A.; Zabini, F.; Lino, C.; Pagliaro, M.; Meneguzzo, F.; Di Carlo, M.; et al. Exceptional antioxidant, non-cytotoxic activity of integral lemon pectin from hydrodynamic cavitation. ChemistrySelect 2020, 5, 5066–5071. [Google Scholar] [CrossRef]
- Miyazono, Y.; Hirashima, S.; Ishihara, N.; Kusukawa, J.; Nakamura, K.I.; Ohta, K. Uncoupled mitochondria quickly shorten along their long axis to form indented spheroids, instead of rings, in a fission-independent manner. Sci. Rep. 2018, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Chapa-Dubocq, X.; Makarov, V.; Javadov, S. Simple kinetic model of mitochondrial swelling in cardiac cells. J. Cell. Physiol. 2018, 233, 5310–5321. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (Δψ m) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Perelman, A.; Wachtel, C.; Cohen, M.; Haupt, S.; Shapiro, H.; Tzur, A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012, 3, e430. [Google Scholar] [CrossRef] [PubMed]
- Baloyannis, S.J. Mitochondrial alterations in Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 119–126. [Google Scholar] [CrossRef]
- Vila, M.; Ramonet, D.; Perier, C. Mitochondrial alterations in Parkinson’s disease: New clues. J. Neurochem. 2008, 107, 317–328. [Google Scholar] [CrossRef]
- Chung, M.J.; Suh, Y.L. Ultrastructural changes of mitochondria in the skeletal muscle of patients with amyotrophic lateral sclerosis. Ultrastruct. Pathol. 2002, 26, 3–7. [Google Scholar] [CrossRef]
- Filippov, M.P. Practical infrared spectroscopy of pectic substances. Food Hydrocoll. 1992, 6, 115–142. [Google Scholar] [CrossRef]
- Coenen, G.J.; Bakx, E.J.; Verhoef, R.P.; Schols, H.A.; Voragen, A.G.J. Identification of the connecting linkage between homo- or xylogalacturonan and rhamnogalacturonan type I. Carbohydr. Polym. 2007, 70, 224–235. [Google Scholar] [CrossRef]
- Morris, G.A.; Foster, T.J.; Harding, S.E. Further observations on the size, shape, and hydration of casein micelles from novel analytical ultracentrifuge and capillary viscometry approaches. Biomacromolecules 2000, 1, 764–767. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Carnaroglio, D.; Grillo, G.; Cravotto, G.; Tamburino, A.; Ilharco, L.M.; Pagliaro, M. High-quality essential oils extracted by an eco-friendly process from different citrus fruits and fruit regions. ACS Sustain. Chem. Eng. 2017, 5, 5578–5587. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Sousa, A.G.; Crepeau, M.J.; Sorensen, S.O.; Ralet, M.C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Presentato, A.; Lino, C.; Piacenza, E.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; et al. Volatile compounds of lemon and grapefruit integropectin. Molecules 2021, 26, 51. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Ilharco, L.; Pagliaro, M. Pectin production and global market. Agro Food Ind. Hi-Tech 2016, 27, 17–20. [Google Scholar]
- Moghimi, M.; Parvardeh, S.; Zanjani, T.M.; Ghafghazi, S. Protective effect of alpha-terpineol against impairment of hippocampal synaptic plasticity and spatial memory following transient cerebral ischemia in rats. Iran. J. Basic Med. Sci. 2016, 19, 960–969. [Google Scholar]
- Burcul, F.; Blazevic, I.; Radan, M.; Politeo, O. Terpenes, phenylpropanoids, sulfur and other essential oil constituents as inhibitors of cholinesterases. Curr. Med. Chem. 2020, 27, 4297–4343. [Google Scholar] [CrossRef]
- Vieira, G.; Cavalli, J.; Goncalves, E.C.D.; Braga, S.F.P.; Ferreira, R.S.; Santos, A.R.S.; Cola, M.; Raposo, N.R.B.; Capasso, R.; Dutra, R.C. Antidepressant-like effect of terpineol in an inflammatory model of depression: Involvement of the cannabinoid system and D2 dopamine receptor. Biomolecules 2020, 10, 792. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverria, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sanchez, E. Hesperidin as a neuroprotective agent: A review of animal and clinical evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef]
- Karim, N.; Shishir, M.R.I.; Gowd, V.; Chen, W. Hesperidin-an emerging bioactive compound against metabolic diseases and its potential biosynthesis pathway in microorganism. Food Rev. Int. 2021, 1–23. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Spolidorio, L.C.; Manthey, J.A.; Cesar, T.B. Citrus flavanones prevent systemic inflammation and ameliorate oxidative stress in C57BL/6J mice fed high-fat diet. Food Funct. 2016, 7, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.B.; Ramos, F.M.; Manthey, J.A.; Cesar, T.B. Effectiveness of Eriomin® in managing hyperglycemia and reversal of prediabetes condition: A double-blind, randomized, controlled study. Phytother. Res. 2019, 33, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Kuwabara, R.; Beukema, M.; Ferrari, M.; de Haan, B.J.; Walvoort, M.T.C.; de Vos, P.; Smink, A.M. Low methyl-esterified pectin protects pancreatic beta-cells against diabetes-induced oxidative and inflammatory stress via galectin-3. Carbohydr. Polym. 2020, 249, 116863. [Google Scholar] [CrossRef] [PubMed]
- Siew, J.J.; Chen, H.M.; Chen, H.Y.; Chen, H.L.; Chen, C.M.; Soong, B.W.; Wu, Y.R.; Chang, C.P.; Chan, Y.C.; Lin, C.H.; et al. Galectin-3 is required for the microglia-mediated brain inflammation in a model of Huntington’s disease. Nat. Commun. 2019, 10, 3473. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Zhao, D.; Yan, J.; Sun, C.; Zhou, Y.; Tai, G. Multiple approaches to assess pectin binding to galectin-3. Int. J. Biol. Macromol. 2016, 91, 994–1001. [Google Scholar] [CrossRef]
- Wang, W.; Wu, X.; Chantapakul, T.; Wang, D.; Zhang, S.; Ma, X.; Ding, T.; Ye, X.; Liu, D. Acoustic cavitation assisted extraction of pectin from waste grapefruit peels: A green two-stage approach and its general mechanism. Food Res. Int. 2017, 102, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Tamburino, A.; Carnaroglio, D.; Cravotto, G.; Ilharco, L.M.; Pagliaro, M. Controlling the degree of esterification of citrus pectin for demanding applications by selection of the source. ACS Omega 2017, 2, 7991–7995. [Google Scholar] [CrossRef]
- Nuzzo, D.; Galizzi, G.; Amato, A.; Terzo, S.; Picone, P.; Cristaldi, L.; Mulè, F.; Di Carlo, M. Regular intake of pistachio mitigates the deleterious effects of a high fat-diet in the brain of obese mice. Antioxidants 2020, 9, 317. [Google Scholar] [CrossRef]
- Nuzzo, D.; Contardi, M.; Kossyvaki, D.; Picone, P.; Cristaldi, L.; Galizzi, G.; Bosco, G.; Scoglio, S.; Athanassiou, A.; Di Carlo, M. Heat-resistant aphanizomenon flos-aquae (AFA) extract (Klamin®) as a functional ingredient in food strategy for prevention of oxidative stress. Oxid. Med. Cell. Longev. 2019, 9481390. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.; Presti, G.; Picone, P.; Galizzi, G.; Gulotta, E.; Giuliano, S.; Mannino, C.; Gambino, V.; Scoglio, S.; Di Carlo, M. Effects of the aphanizomenon flos-aquae extract (Klamin®) on a neurodegeneration cellular model. Oxid. Med. Cell. Longev. 2018, 9089016. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.; Amato, A.; Picone, P.; Terzo, S.; Galizzi, G.; Bonina, F.P.; Mulè, F.; Di Carlo, M. A natural dietary supplement with a combination of nutrients prevents neurodegeneration induced by a high fat diet in mice. Nutrients 2018, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Scurria, A.; Sciortino, M.; Albanese, L.; Nuzzo, D.; Zabini, F.; Meneguzzo, F.; Alduina, R.V.; Presentato, A.; Pagliaro, M.; Avellone, G.; et al. Flavonoids in Lemon and Grapefruit IntegroPectin. Preprints 2021, 2021020620. [Google Scholar] [CrossRef]







| Infrared Component | /cm−1 | FWHM/cm−1 | Area % |
|---|---|---|---|
| ν(C=O)Methyl Ester | 1743.7 ± 0.7 | 44 ± 3 | 20 ± 2 |
| ν(C=O)Methyl Ester, H.bonded | 1715.7 ± 0.8 | 32 ± 2 | 6 ± 2 |
| ν(C=O)Acid | 1692 ± 3 | 61 ± 6 | 21 ± 4 |
| νas(COO−) | 1600 ± 2 | 101 ± 5 | 52 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, D.; Picone, P.; Giardina, C.; Scordino, M.; Mudò, G.; Pagliaro, M.; Scurria, A.; Meneguzzo, F.; Ilharco, L.M.; Fidalgo, A.; et al. New Neuroprotective Effect of Lemon IntegroPectin on Neuronal Cellular Model. Antioxidants 2021, 10, 669. https://doi.org/10.3390/antiox10050669
Nuzzo D, Picone P, Giardina C, Scordino M, Mudò G, Pagliaro M, Scurria A, Meneguzzo F, Ilharco LM, Fidalgo A, et al. New Neuroprotective Effect of Lemon IntegroPectin on Neuronal Cellular Model. Antioxidants. 2021; 10(5):669. https://doi.org/10.3390/antiox10050669
Chicago/Turabian StyleNuzzo, Domenico, Pasquale Picone, Costanza Giardina, Miriana Scordino, Giuseppa Mudò, Mario Pagliaro, Antonino Scurria, Francesco Meneguzzo, Laura M. Ilharco, Alexandra Fidalgo, and et al. 2021. "New Neuroprotective Effect of Lemon IntegroPectin on Neuronal Cellular Model" Antioxidants 10, no. 5: 669. https://doi.org/10.3390/antiox10050669
APA StyleNuzzo, D., Picone, P., Giardina, C., Scordino, M., Mudò, G., Pagliaro, M., Scurria, A., Meneguzzo, F., Ilharco, L. M., Fidalgo, A., Alduina, R., Presentato, A., Ciriminna, R., & Di Liberto, V. (2021). New Neuroprotective Effect of Lemon IntegroPectin on Neuronal Cellular Model. Antioxidants, 10(5), 669. https://doi.org/10.3390/antiox10050669
















