Ex Vivo Antioxidant Capacities of Fruit and Vegetable Juices. Potential In Vivo Extrapolation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
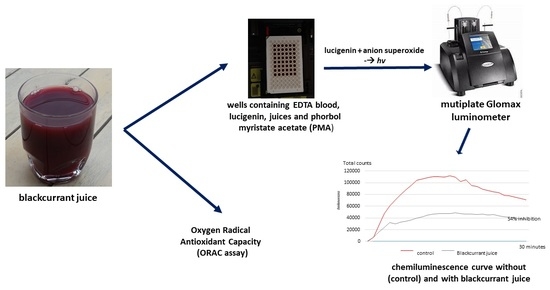
2.2.1. Inhibition of Superoxide Anion Production (Chemiluminescence Method)
2.2.2. Oxygen Radical Antioxidant Capacity (ORAC Assay)
2.3. Statistical Analyses
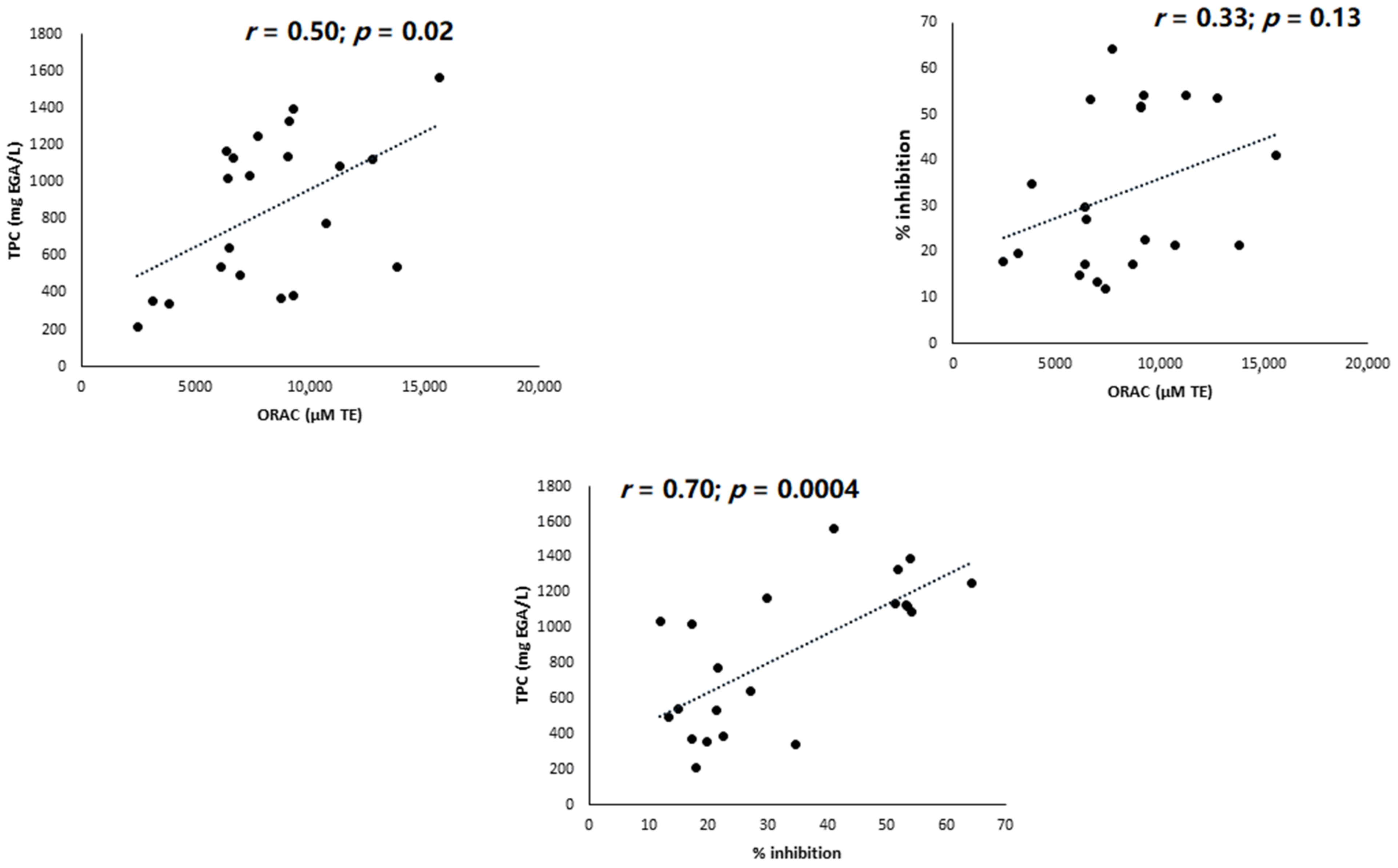
3. Results
4. Discussion
Limitations of the PMA–Whole Blood Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- Serino, A.; Salaza, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients 2019, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Renaud, J.; Martinoli, M.-G. Considerations for the use of polyphenols as therapies in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mileo, A.-M.; Miccadei, S. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxidative Med. Cell. Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: A narrative review of the evidence. Oxidative Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Hoge, A.; Guillaume, M.; Albert, A.; Tabart, J.; Dardenne, N.; Donneau, A.-F.; Kevers, C.; Defraigne, J.-O.; Pincemail, J. Validation of a food frequency questionnaire assessing dietary polyphenol exposure using the method of triads. Free Radic. Biol. Med. 2019, 130, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Jordão, J. European Fruit Juice Association. AIJN-Mark. Rep. 2018, 2018, 3–44. [Google Scholar]
- Ruxton, C.H.S.; Gardner, E.J.; Walker, D. Can pure fruit and vegetable juices protect against cancer and cardiovascular disease too? A review of the evidence. Int. J. Food Sci. Nutr. 2006, 57, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A Review and Critical Analysis of the Scientific Literature Related to 100% Fruit Juice and Human Health. Adv. Nutr. 2015, 6, 37–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonin, F.S.; Steimbach, L.M.; Wiens, A.; Perlin, C.M.; Pontarolo, R.; Kitts, D.D. Impact of natural juice consumption on plasma antioxidant status: A systematic review and meta-analysis. Molecules 2015, 20, 22146–22156. [Google Scholar] [CrossRef] [Green Version]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Chabert, P.; Auger, C.; Pincemail, J.; Schini-Kerth, V.B. Overview of Plant-Derived Antioxidants. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Malta, L.G.; Liu, R.H. Analyses of total phenolics, total flavonoids, and total antioxidant in foods and dietary supplements. Encycl. Agric. Food Syst. 2014, 305–314. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Michiels, J.-A.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Fereidoon, S.; Han Peng, J. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar]
- Gonçalves, J.; Ramos, R.; Luís, A.; Rocha, S.; Rosado, T.; Gallardo, E.; Duarte, A.P. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef] [Green Version]
- Dabulici, C.M.; Sârbu, I.; Vamanu, E. Bioactive Potential of Functional Products and Bioavailability of Phenolic Compounds. Foods 2020, 9, 953. [Google Scholar] [CrossRef]
- Gerardi, G.; Mónica Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. The dose-response effect on polyphenol bioavailability after intake of white and red wine pomace products by Wistar rats. Food Funct. 2020, 11, 1661–1671. [Google Scholar] [CrossRef]
- Pincemail, J.; Cillard, J.; Neve, J.; Defraigne, J.O. Determination of the plasma global antioxidant capacity: A critical review. Ann. Biol. Clin. 2014, 72, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Curti, C.; Zaccaria, V.; Tsetegho Sokeng, A.J.; Dacrema, M.; Masiello, I.; Mascaro, A.; D’Antona, G.; Daglia, M. Bioavailability and in vivo antioxidant activity of a standardized polyphenol mixture extracted from brown propolis. Int. J. Mol. Sci. 2019, 20, 1250. [Google Scholar] [CrossRef] [Green Version]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Lenaz, G. The mitochondrial production of reactive oxygen species: Mechanisms and implications in human pathology. Life 2001, 52, 159–164. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase-derived reactive species: Physiological and pathological effects. Oxidative Med. Cell. Longev. 2016, 2016, 3527579. [Google Scholar] [CrossRef] [Green Version]
- Bedard, K.; Krause, K.-H. NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauseef, N.M. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim. Biophys. Acta 2014, 1840, 757–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista, G.; Dupuy, A.-M.; Jaussent, A.; Durant, R.; Ventura, E.; Sauguet, P.; Picot, M.-C.; Jeandel, C.; Cristol, J.P. Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Radic. Res. 2012, 46, 1108–1114. [Google Scholar] [CrossRef]
- Matute, A.; Tabart, J.; Cheramy-Bien, J.P.; Pirotte, B.; Kevers, C.; Auger, C.; Schini-Kerth, V.; Dommes, J.; Defraigne, J.O.; Pincemail, J. Compared phenolic compound contents of 22 commercial fruit and vegetable juices: Relationship to ex-vivo vascular reactivity and potential in vivo projection. Antioxidants 2020, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Caboni, E.; Tonelli, M.G.; Lauri, P.; Iacovacci, P.; Kevers, C.; Damiano, C.; Gaspar, T. Biochemical aspects of almond microcuttings related to in vitro rooting ability. Biol. Plant. 1997, 39, 91–97. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA). Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods—2007. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 12 May 2007).
- Haytowitz, D.B.; Bhagwat, S. USDA database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2. 2010. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 10 May 2010).
- Daniells, S. ORAC Has “Ongoing Value” Says as Expert, as USDA Removes Online Database. 2012. Available online: www.nutraingredients-USA.com/article2012/06/13 (accessed on 13 June 2012).
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1489. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Guidance on the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health. EFSA J. 2017, 16, e05136. [Google Scholar] [CrossRef] [Green Version]
- Pagonis, C.; Tauber, A.I.; Pavlotsky, N.; Simons, E.R. Flavonoid impairment of neutrophil response. Biochem. Pharmacol. 1986, 35, 237–245. [Google Scholar] [CrossRef]
- Maraldi, T. Natural compounds as modulators of NADPH oxidases. Oxidative Med. Cell. Longev. 2013, 2013, 271602. [Google Scholar] [CrossRef] [Green Version]
- Ciz, M.; Denev, P.; Kratchanova, M. Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxidative Med. Cell. Longev. 2012, 2012, 181295. [Google Scholar] [CrossRef] [PubMed]
- Zieliñska, M.; Kostrzewa, A.; Ignatowicz, E.; Budzianowski, J. The flavonoids, quercetin and isorhamnetin 3-O-acylglucosides diminish neutrophil oxidative metabolism and lipid peroxidation. Acta Biochem. Pol. 2001, 48, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Tauber, A.I.; Fay, J.R.; Marletta, M.A. Flavonoid inhibition of the human neutrophil NADPH-oxidase. Biochem. Pharmacol. 1984, 33, 1367–1369. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Lecour, S.; Lamont, K.T. Natural polyphenols and cardioprotection. Mini Rev. Med. Chem. 2011, 11, 1191–1199. [Google Scholar]
- Tressera-Rimbau, A.; Arraz, S.; Vallverder-Queralt, A. New insights into the benefits of polyphenols in chronic diseases. Oxidative Med. Cell. Longev. 2017, 2017, 1432071. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noroozi, M.; Burns, J.; Crozier, A.; Kelly, I.E.; Lean, M.E.J. Prediction of dietary flavonol consumption from fasting plasma concentration or urinary excretion. Eur. J. Clin. Nutr. 2000, 54, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Song, J.; Huang, K.; Michel, D.; Fang, J. HPLC-MS/MS analysis of anthocyanins in human plasma and urine using protein precipitation and dilute-and-shoot sample preparation methods, respectively. Biomed. Chromatogr. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Giordano, L.; Coletta, W.; Rapisarda, P.; Donati, M.B.; Rotilio, D. Development and validation of an LC-MS/MS analysis for simultaneous determination of delphinidin-3-glucoside, cyanidin-3-glucoside and cyanidin-3-(6-malonylglucoside) in human plasma and urine after blood orange juice administration. J. Sep. Sci. 2007, 30, 3127–3136. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kuhnle, G.; Hu, H.; Roedig-Penman, A.; van den Braak, M.H.; Moore, K.P.; Rice-Evans, C.A. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic. Res. 2002, 36, 1229–1241. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. Neuromol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surh, Y.-J. Xenohormesis mechanisms underlying chemopreventive effects of some dietary phytochemicals. Ann. N. Y. Acad. Sci. 2011, 1229, 1–6. [Google Scholar] [CrossRef]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Number | List of Juices | TPC | ORAC (µM TE) | % Superoxide Anion Inhibition |
|---|---|---|---|---|
| (mg GAE/L) | (PMA-Whole Blood) | |||
| 1 | Tomato (Carrefour) | 213.6 ± 68.1 | 2428.7 ± 68.2 (21) | 18.0 ± 5.9 (16) |
| 2 | Tomato (Biotta) | 358.1 ± 26.0 | 3117.8 ± 290.1 (20) | 19.8 ± 19.0 (15) |
| 3 | Carrot (Biotta) | 338.9 ± 5.6 | 3825.9 ± 840.0 (19) | 34.7 ± 6.5 (9) |
| 4 | Orange d’Espagne (Carrefour) | 541.6 ± 90.1 | 6074.3 ± 745.2 (18) | 14.9 ± 4.6 (19) |
| 5 | Pure orange (Vitamont) | 385.1 ± 155.8 | 9259.3 ± 288.2 (6) | 22.6 ± 7.7 (12) |
| 6 | Lemon (Bonneterre) | 1167.5 ± 217.3 | 6346.3 ± 628.6 (17) | 29.8 ± 17.4 (10) |
| 7 | Grapefruit | 496.8 ± 13.1 | 6936.5 ± 1482.1 (13) | 13.3 ± 3.7 (20) |
| 8 | Pure grapefruit (Vitamont) | 537.6 ± 45.8 | 13,805.2 ± 591.3 (2) | 21.5 ± 9.1 (14) |
| 9 | Grape (Materne) | 1564.0 ± 588.3 | 15,603.6 ± 458.5 (1) | 41.2 ± 3.3 (8) |
| 10 | Pure grape (Vitamont) | 643.4 ± 56.6 | 6461.7 ± 808.7 (15) | 27.1 ± 2.7 (11) |
| 11 | Pomegranate (Biotta) | 1331.9 ± 183.2 | 9046.1 ± 176.8 (8) | 51.9 ± 5.4 (6) |
| 12 | Blackcurrant (Biotta) | 1088.8 ± 80.8 | 11,256.7 ± 380.1 (4) | 54.3 ± 7.4 (2) |
| 13 | Blackcurrant (Zimmers) | 1392.2 ± 202.7 | 9219.4 ± 466.0 (7) | 54.1 ± 4.9 (3) |
| 14 | Blackcurrant (Jacoby Bio) | 1135.6 ± 93.4 | 9035.1 ± 90.6 (9) | 51.5 ± 2.5 (7) |
| 15 | Blackcurrant (Van Nahmen) | 1250.9 ± 186.8 | 7681.7 ± 422.9 (11) | 64.4 ± 12.4 (1) |
| 16 | Blackcurrant (Gut & Günstig) | 1131.1 ± 210.8 | 6636.8 ± 577.5 (14) | 53.2 ± 8.5 (5) |
| 17 | Blackcurrant (Albi) | 1121.6 ± 166.2 | 12,722.9 ± 622.4 (3) | 53.5 ± 6.9 (4) |
| 18 | Pineapple juice (Carrefour) | 1018.6 ± 60.8 | 6363.1 ± 1141.8 (16) | 17.2 ± 4.5 (17) |
| 19 | Pineapple juice (De Drie Wilgen) | 369.6 ± 21.6 | 8700.1 ± 213.6 (10) | 17.2 ± 3.4 (18) |
| 20 | Apple (Carrefour) | 1035.3 ± 8.7 | 7351.3 ± 2924.7 (12) | 11.9 ± 6.8 (21) |
| 21 | Pure apple (Vitamont) | 776.4 ± 121.0 | 10,682.4 ± 1965.4 (5) | 21.6 ± 2.8 (13) |
| Flavonols (µM) | |
| Myr | 0.060 ± 0.072 |
| Quer | 0.039 ± 0.019 |
| Kaemp | 0.011 ± 0.009 |
| Flavanols (µM) | |
| EGC | 0.949 ± 3.266 |
| EGCG | 0.332 ± 0.472 |
| ECG | 0.043 ± 0.040 |
| C | 0.008 ± 0.014 |
| ECG | 0.043 ± 0.033 |
| Anthocyanins (µM) | |
| DG | 0.745 ± 1.531 |
| DR | 1.633 ± 3.540 |
| CyG | 0.196 ± 0.308 |
| CyR | 1.424 ± 2.720 |
| PG | 0.005 ± 0.005 |
| GC | 0.367 ± 1.141 |
| Phenolic Compounds | ORAC | % Inhibition | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| DG | 0.32 | 0.40 | 0.17 | 0.66 |
| DR | 0.37 | 0.32 | 0.19 | 0.63 |
| CyG | 0.36 | 0.34 | 0.22 | 0.57 |
| CyR | 0.29 | 0.44 | 0.18 | 0.65 |
| PG | 0.25 | 0.52 | 0.87 | 0.010 |
| GC | 0.04 | 0.87 | 0.21 | 0.33 |
| EGC | 0.11 | 0.62 | 0.28 | 0.20 |
| EGCG | 0.30 | 0.19 | 0.67 | 0.0009 |
| ECG | 0.45 | 0.043 | 0.26 | 0.24 |
| C | 0.47 | 0.032 | 0.47 | 0.02 |
| EC | 0.28 | 0.21 | 0.04 | 0.99 |
| Myr | 0.48 | 0.027 | 0.33 | 0.14 |
| Quer | 0.21 | 0.36 | 0.46 | 0.036 |
| Kaemp | 0.13 | 0.57 | 0.24 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matute, A.; Tabart, J.; Cheramy-Bien, J.-P.; Kevers, C.; Dommes, J.; Defraigne, J.-O.; Pincemail, J. Ex Vivo Antioxidant Capacities of Fruit and Vegetable Juices. Potential In Vivo Extrapolation. Antioxidants 2021, 10, 770. https://doi.org/10.3390/antiox10050770
Matute A, Tabart J, Cheramy-Bien J-P, Kevers C, Dommes J, Defraigne J-O, Pincemail J. Ex Vivo Antioxidant Capacities of Fruit and Vegetable Juices. Potential In Vivo Extrapolation. Antioxidants. 2021; 10(5):770. https://doi.org/10.3390/antiox10050770
Chicago/Turabian StyleMatute, Alexis, Jessica Tabart, Jean-Paul Cheramy-Bien, Claire Kevers, Jacques Dommes, Jean-Olivier Defraigne, and Joël Pincemail. 2021. "Ex Vivo Antioxidant Capacities of Fruit and Vegetable Juices. Potential In Vivo Extrapolation" Antioxidants 10, no. 5: 770. https://doi.org/10.3390/antiox10050770
APA StyleMatute, A., Tabart, J., Cheramy-Bien, J.-P., Kevers, C., Dommes, J., Defraigne, J.-O., & Pincemail, J. (2021). Ex Vivo Antioxidant Capacities of Fruit and Vegetable Juices. Potential In Vivo Extrapolation. Antioxidants, 10(5), 770. https://doi.org/10.3390/antiox10050770