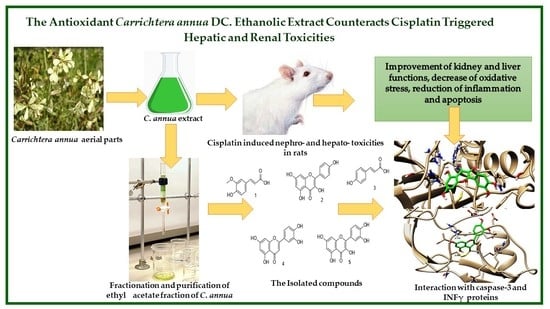
The Antioxidant Carrichtera annua DC. Ethanolic Extract Counteracts Cisplatin Triggered Hepatic and Renal Toxicities
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Plant Material Collection and Extraction
2.3. Animals
2.4. Experimental Design
2.5. Blood and Tissue Sampling and Biochemical Analysis
2.5.1. Histopathological Examination
2.5.2. Liver Function Tests
2.5.3. Kidney Function Tests
2.5.4. Estimation of Malondialdehyde (MDA) and Reduced Glutathione (GSH)
2.5.5. Determination of Interferon Gamma, Interleukin-1β and Caspase 3
2.5.6. Isolation of Mitochondria and Mitochondrial DNA
2.6. In Vitro Anticancer Assay of C. annua—Cisplatin Combination
2.7. Statistical Analysis
2.8. Fractionation of C. annua Extract and Isolation of Its Phytochemicals
2.9. Spectrometric Data of the Isolated Compounds 1–5
2.10. Virtual Screening and Molecular Docking
3. Results and Discussion
3.1. C. annua Protective Effects against Cisplatin Induced Hepato- and Nephro Toxicities
3.1.1. Histopathological Examination
3.1.2. Liver Function Tests
3.1.3. Kidney Function Tests
3.1.4. Malondialdehyde (MDA) and Reduced Glutathione Concentrations
3.1.5. Inflammatory Markers (Interferon Gamma and IL-1β) and Apoptotic Marker (Caspase 3)
3.1.6. Mitochondrial DNA (mtDNA) Quantity
3.2. In Vitro Anticancer Activity of C. annua—Cisplatin Combination
3.3. Phytochemical Investigation
3.4. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. Future perspectives on the treatment of hepatocellular carcinoma with cisplatin. World J. Hepatol. 2009, 1, 8–16. [Google Scholar] [CrossRef]
- De Oliveira, M.L.; Antunes, L.M.; Francescato, H.D.; Bianchi, M.D. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacol. Res. 2003, 47, 517–522. [Google Scholar]
- Amptoulach, S.; Tsavaris, N. Neurotoxicity caused by the treatment with platinum analogues. Chemother. Res. Pract. 2011, 2011, 843019. [Google Scholar] [CrossRef]
- Ognjanovic, B.I.; Djordjevic, N.Z.; Matic, M.M.; Obradovic, J.M.; Mladenovic, J.M.; Stajn, A.S.; Saicic, Z.S. Lipid peroxidative damage on cisplatin exposure and alterations in antioxidant defense system in rat kidneys: A possible protective effect of selenium. Int. J. Mol. Sci. 2012, 13, 1790–1803. [Google Scholar] [CrossRef]
- Soliman, A.M.; Desouky, S.; Marzouk, M.; Sayed, A.A. Origanum majorana attenuates nephrotoxicity of cisplatin anticancer drug through ameliorating oxidative stress. Nutrients 2016, 8, 264. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Abduldaium, Y.S.; Younis, N.S. Ameliorative effect of linalool in cisplatin-induced nephrotoxicity: The role of HMGB1/TLR4/NF-κB and Nrf2/HO1 pathways. Biomolecules 2020, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Hussain, A.; Hussain, A.; Abdullah, I.; Ali, M.S.; Froeyen, M.; Mirza, M.U. Quantification of berberine in Berberis vulgaris L. root extract and its curative and prophylactic role in cisplatin-induced in vivo toxicity and in vitro cytotoxicity. Antioxidants 2019, 8, 185. [Google Scholar] [CrossRef]
- Stankovic, J.S.K.; Selakovic, D.; Mihailovic, V.; Rosic, G. Antioxidant supplementation in the treatment of neurotoxicity induced by platinum-based chemotherapeutics—A review. Int. J. Mol. Sci. 2020, 21, 7753. [Google Scholar] [CrossRef]
- Ekinci Akdemir, F.N.; Albayrak, M.; Çalik, M.; Bayir, Y.; Gülçin, İ. The protective effects of p-coumaric acid on acute liver and kidney damages induced by cisplatin. Biomedicines 2017, 5, 18. [Google Scholar] [CrossRef]
- Naqshbandi, A.; Khan, W.; Rizwan, S.; Khan, F. Studies on the protective effect of flaxseed oil on cisplatin-induced hepatotoxicity. Hum. Exp. Toxicol. 2012, 31, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Rather, I.A. Myricetin abrogates cisplatin-induced oxidative stress, inflammatory response, and goblet cell disintegration in colon of wistar rats. Plants 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Ezz-Din, D.; Gabry, M.S.; Farrag, A.R.; Abdel Moneim, A.E. Physiological and histological impact of Azadirachta indica (neem) leaves extract in a rat model of cisplatin-induced hepato and nephrotoxicity. J. Med. Plants Res. 2011, 5, 5499–5506. [Google Scholar]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin nephrotoxicity: A review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Dachuri, V.; Song, P.H.; Kim, Y.W.; Ku, S.-K.; Song, C.-H. Protective effects of traditional polyherbs on cisplatin-induced acute kidney injury cell model by inhibiting oxidative stress and MAPK signaling pathway. Molecules 2020, 25, 5641. [Google Scholar] [CrossRef]
- Fang, C.Y.; Lou, D.Y.; Zhou, L.Q.; Wang, J.C.; Yang, B.; He, Q.J.; Wang, J.J.; Weng, Q.J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021. [Google Scholar] [CrossRef]
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 13, 25. [Google Scholar] [CrossRef]
- Morgan, K.P.; Buie, L.W.; Savage, S.W. The role of mannitol as a nephroprotectant in patients receiving cisplatin therapy. Ann. Pharmacother. 2012, 46, 276–281. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Park, J.H.; Lee, S.H.; Lee, D.E.; Kim, I.S.; Lee, K.Y.; Lee, B.M.; et al. Protective effects of Dendropanax morbifera against cisplatin-induced nephrotoxicity without altering chemotherapeutic efficacy. Antioxidants 2019, 8, 256. [Google Scholar] [CrossRef]
- Beagloo, I.E.; Valilu, M.R.; Motiei, M.; Rahbar, M.; Hejazi, A. The antioxidant and hepatoprotective effect of alcoholic extract of ginger against the cisplatin-induced oxidative stress in rats. Biomed. J. Sci. Tech. Res. 2019, 19. [Google Scholar] [CrossRef]
- Hassan, H.A.; Edrees, G.M.; El-Gamel, E.M.; El-Sayed, E.A. Amelioration of cisplatin-induced nephrotoxicity by grape seed extract and fish oil is mediated by lowering oxidative stress and DNA damage. Cytotechnology 2014, 66, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Karwasra, R.; Kalra, P.; Gupta, Y.K.; Saini, D.; Kumar, A.; Singh, S. Antioxidant and anti-inflammatory potential of pomegranate rind extract to ameliorate cisplatin-induced acute kidney injury. Food Funct. 2016, 7, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, Z.; Afsar, M.; Rizwan, S.; Khan, A.A.; Khan, F. Oral administration of Nigella sativa oil ameliorates the effect of cisplatin on membrane enzymes, carbohydrate metabolism and oxidative damage in rat liver. Toxicol. Rep. 2016, 13, 328–335. [Google Scholar] [CrossRef]
- Mazaheri, S.; Nematbakhsh, M.; Bahadorani, M.; Pezeshki, Z.; Talebi, A.; Ghannadi, A.R.; Ashrafi, F. Effects of fennel essential oil on cisplatin-induced nephrotoxicity in ovariectomized rats. Toxicol. Int. 2013, 20, 138–145. [Google Scholar]
- Lahmar, A.; Dhaouefi, Z.; Khlifi, R.; Sioud, F.; Ghedira, L.C. Pituranthos chloranthus oil as an antioxidant-based adjuvant therapy against cisplatin-induced nephrotoxicity. J. Toxicol. 2020, 2020, 7054534. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Palma, G.; Barbieri, A.; Bimonte, S.; Amruthraj, N.J.; Muzio, M.R.; Del Vecchio, V.; Rea, D.; Falco, M.; Luciano, A.; et al. Role of Nigella sativa and its constituent thymoquinone on chemotherapy-induced nephrotoxicity: Evidences from experimental animal studies. Nutrients 2017, 9, 625. [Google Scholar] [CrossRef]
- Bami, E.; Ozakpınar, O.B.; Ozdemir-Kumral, Z.N.; Köroglu, K.; Ercan, F.; Cirakli, Z.; Sekerler, T.; Izzettin, F.V.; Sancar, M.; Okuyan, B. Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2017, 54, 105–111. [Google Scholar] [CrossRef]
- Abd El-Raouf, O.M.; El-Sayed, E.M.; Manie, M.F. Cinnamic acid and cinnamaldehyde ameliorate cisplatin-induced splenotoxicity in rats. J. Biochem. Mol. Toxicol. 2015, 29, 426–431. [Google Scholar] [CrossRef]
- Kart, A.; Cigremis, Y.; Karaman, M.; Ozen, H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp. Toxicol. Pathol. 2010, 62, 45–52. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, P.D.; Lopez-Hernandez, F.J.; Perez-Barriocanal, F.; Morales, A.I.; Lopez-Novoa, J.M. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol. Dial. Transpl. 2011, 26, 3484–3495. [Google Scholar] [CrossRef]
- Domitrović, R.; Cvijanović, O.; Pugel, E.P.; Zagorac, G.B.; Mahmutefendić, H.; Škoda, M. Luteolin ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of platinum accumulation, inflammation and apoptosis in the kidney. Toxicology 2013, 310, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, W.; Sun, X.; Wang, Y.; Zhou, M. Kaempferol ameliorates cisplatin induced nephrotoxicity by modulating oxidative stress, inflammation and apoptosis via ERK and NF-κB pathways. AMB Express 2020, 10, 58. [Google Scholar] [CrossRef]
- Miceli, N.; Cavò, E.; Ragusa, M.; Cacciola, F.; Mondello, L.; Dugo, L.; Acquaviva, R.; Malfa, G.A.; Marino, A.; D’Arrigo, M.; et al. Brassica incana Ten. (Brassicaceae): Phenolic constituents, antioxidant and cytotoxic properties of the leaf and flowering top extracts. Molecules 2020, 25, 1461. [Google Scholar] [CrossRef] [PubMed]
- Middleditch, B.S.; Amer, A.M. Kuwaiti Plants: Distribution, Traditional Medicine, Phytochemistry, Pharmacology and Economic Value, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; p. 19. [Google Scholar]
- Abdel-Shafeek, K.A.; El-Messiry, M.M.; Shahat, A.A.; Apers, S.; Pieters, L.; Seif-El Nasr, M.M. A new acylated flavonol triglycoside from Carrichtera annua. J. Nat. Prod. 2000, 63, 845–847. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Elhady, S.S.; Ahmed, H.A.; Badr, J.M.; Noor, A.O.; Ahmed, S.A.; Nafie, M.S. Chemical profiling, antioxidant, cytotoxic activities and molecular docking simulation of Carrichtera annua DC. (Cruciferae). Antioxidants 2020, 9, 1286. [Google Scholar] [CrossRef]
- Abdel-Shafeek, K.; Elmissiry, M.M.; Hussiny, H.A.; Elnasr, M.M. The flavonoids and anticomplement activity of two cruciferous plants growing in Egypt. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 223–227. [Google Scholar]
- Shahat, A.A.; Abdelshafeek, K.; Husseiny, H.A. Isolation and identification of a new flavonoid glycoside from Carrichtera annua L. seeds. Pharmacogn. Res. 2011, 3, 151–154. [Google Scholar] [CrossRef]
- Shahat, A.A.; Abdel-Shafeek, K.A.; Claeys, M.; Apers, S.; Pieters, L.; Vlietinck, A.J. A new flavonoid from Carrichtera annua. Nat. Prod. Sci. 2006, 12, 122–124. [Google Scholar]
- Cuyckens, F.; Shahat, A.A.; Heuvel, H.V.D.; Abdel-Shafeek, K.A.; El-Messiry, M.M.; Elnasr, M.S.; Pieters, L.; Vlietinck, A.J.; Claeys, M. The application of liquid chromatography-electrospray ionization mass spectrometry and collision-induced dissociation in the structural characterization of acylated flavonol O-Glycosides from the seeds of Carrichtera annua. Eur. J. Mass Spectrom. 2003, 9, 409–420. [Google Scholar] [CrossRef]
- Mohamed, M.T.; Zaitone, S.A.; Ahmed, A.; Mehanna, E.T.; El-Sayed, N.M. Raspberry ketones attenuate cyclophosphamide-induced pulmonary toxicity in mice through inhibition of oxidative stress and NF-ΚB pathway. Antioxidants 2020, 9, 1168. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.L. Creatinine. In Clinical Chemistry; Theory, Analysis and Correlation; Kaplan, L.A., Pesce, A.J., Eds.; The C.V. Mosby Co.: St. Louis, MO, USA, 1984; pp. 1247–1253. [Google Scholar]
- Kaplen, L.A. Urea. In Clinical Chemistry; Kaplan, L.A., Pesce, A.J., Eds.; The C.V. Mosby Co.: St. Louis, MO, USA, 1984; pp. 1257–1260. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Pradhita, A.; Sahar, N.; Natadisastra, M.; Dzulhulaifah, W.; Thuffi, R. GnRH receptor expression and endometrial cell proliferation of Macaca nemestrina after administration of GnRH agonist in controlled ovarian stimulation procedures. AIP Conf. Proc. 2019, 2193. [Google Scholar] [CrossRef]
- Elgawish, R.A.R.; Rahman, H.G.A.; Abdelrazek, H.M.A. Green tea extract attenuates CCl4-induced hepatic injury in male hamsters via inhibition of lipid peroxidation and p53-mediated apoptosis. Toxicol. Rep. 2015, 10, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Chappel, J.B.; Hansford, R.G. Subcellular Components, 2nd ed.; Butterworths: London, UK, 1969. [Google Scholar]
- Birnboim, H.C.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979, 7, 1513–1523. [Google Scholar] [CrossRef]
- Freshney, R.I. Culture of tumor cells. In Culture of Animal Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 463–479. ISBN 978-0-470-64936-7. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nafie, M.S.; Tantawy, M.A.; Elmgeed, G.A. Screening of different drug design tools to predict the mode of action of steroidal derivatives as anti-cancer agents. Steroids 2019, 152, 108485. [Google Scholar] [CrossRef]
- Aly, A.A.; Sayed, S.M.; Abdelhafez, E.-S.M.N.; Abdelhafez, S.M.N.; Abdelzaher, W.Y.; Raslan, M.A.; Ahmed, A.E.; Thabet, K.; El-Reedy, A.A.M.; Brown, A.B.; et al. New quinoline-2-one/pyrazole derivatives; design, synthesis, molecular docking, anti-apoptotic evaluation, and caspase-3 inhibition assay. Bioorg. Chem. 2020, 94, 103348. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, X.; Li, L.; Xu, Z.; Huang, H.; Zhao, J. Pu-Erh tea powder preventive effects on cisplatin-induced liver oxidative damage in Wistar rats. Asian Pac. J. Cancer Prev. 2013, 15, 7389–7394. [Google Scholar] [CrossRef]
- Bentli, R.; Parlakpinar, H.; Polat, A.; Samdanci, E.; Sarihan, M.; Sagir, M. Molsidomine prevents cisplatin-induced hepatotoxicity. Arch. Med. Res. 2013, 44, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Arab, M.; Shahraki, M.; Mashhadi, M.; Salar, M.; Aval, F.; Karimfar, M. Toxic effects of cisplatin on hepatocytes and liver enzymes of rats. J. Iran. Anat. Sci. 2015, 12, 171–175. [Google Scholar]
- Shimeda, Y.; Hirotani, Y.; Akimoto, Y.; Shindou, K.; Ijiri, Y.; Nishihori, T.; Tanaka, K. Protective effects of capsaicin against cisplatin-induced nephrotoxicity in rats. Biol. Pharm. Bull. 2005, 28, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Palipoch, S.; Punsawad, C.; Koomhin, P.; Suwannalert, P. Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatininduced oxidative stress. BMC Complement. Altern. Med. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, J.; Singh, M.; Sharma, S.; Sharma, S. Antioxidant and nephroprotective potential of Aegle marmelos leaves extract. J. Herbs Spices Med. Plants 2017, 23, 363–377. [Google Scholar] [CrossRef]
- Aydogan, S.; Yapislar, H.; Artis, S.; Aydogan, B. Impaired erythrocytes deformability in H2O2-induced oxidative stress: Protective effect of L-carnosine. Clin. Hemorheol. Microcirc. 2008, 39, 93–98. [Google Scholar] [CrossRef]
- Yilmaz, H.R.; Sogut, S.; Ozyurt, B.; Ozugurlu, F.; Sahin, S.; Isik, B.; Uz, E.; Ozyurt, H. The activities of liver adenosine deaminase, xanthine oxidase, catalase, superoxide dismutase enzymes and the levels of malondialdehyde and nitric oxide after cisplatin toxicity in rats: Protective effect of caffeic acid phenethyl ester. Toxicol. Ind. Health 2005, 21, 67–73. [Google Scholar] [CrossRef]
- Chirino, Y.I.; Sánchez-González, D.J.; Martínez-Martínez, C.M.; Cruz, C.; Pedraza-Chaverri, J. Protective effects of apocynin against cisplatin-induced oxidative stress and nephrotoxicity. Toxicology 2008, 245, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef]
- El-Gizawy, M.M.; Hosny, E.N.; Mourad, H.H.; Abd-El Razik, A.N. Curcumin nanoparticles ameliorate hepatotoxicity and nephrotoxicity induced by cisplatin in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.M.; Busselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Huang, H.; Shen, Z.; Geng, Q.; Wu, Z.; Shi, P.; Miao, X. Protective effect of Schisandra chinensis bee pollen extract on liver and kidney injury induced by cisplatin in rats. Biomed. Pharmacother. 2017, 95, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Ortaldo, J.R.; Mason, A.; Rehberg, E.; Moschera, J.; Kelder, B.; Pestka, S.; Herberman, R.B. Effects of recombinant and hybrid recombinant human leukocyte interferons on cytotoxic activity of natural killer cells. J. Biol. Chem. 1983, 258, 15011–15015. [Google Scholar] [CrossRef]
- Sarhan, A.A.M.; Boraei, A.T.A.; Barakat, A.; Nafie, M.S. Discovery of hydrazide-based pyridazino[4,5-b] indole scaffold as a new phosphoinositide 3-kinase (PI3K) inhibitor for breast cancer therapy. RSC Adv. 2020, 10, 19534–19541. [Google Scholar] [CrossRef]
- Nafie, M.S.; Amer, A.M.; Mohamed, A.K.; Tantawy, E.S. Discovery of novel pyrazolo[3,4-b] pyridine scaffold-based derivatives as potential PIM-1 kinase inhibitors in breast cancer MCF-7 cells. Bioorg. Med. Chem. 2020, 28, 115828. [Google Scholar] [CrossRef] [PubMed]
- Khodair, A.I.; Alsafi, M.A.; Nafie, M.S. Synthesis, molecular modeling and anti-cancer evaluation of a series of quinazoline derivatives. Carbohydr. Res. 2019, 486, 107832. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, C.Y.; Huang, S.; Yang, T.; Dong, Z. Cisplatin-Induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am. J. Physiol. Ren. Physiol. 2009, 296, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Sioud, F.; Toumia, I.B.; Lahmer, A.; Khlifi, R.; Dhaouefi, Z.; Maatouk, M.; Ghedira, K.; Chekir-Ghedira, L. Methanolic extract of Ephedra alata ameliorates cisplatin-induced nephrotoxicity and hepatotoxicity through reducing oxidative stress and genotoxicity. Environ. Sci. Pollut. Res. 2020, 27, 12792–12801. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. Cisplatin induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol. Sci. 2006, 89, 515–523. [Google Scholar] [CrossRef]
- Neamatallah, T.; El-Shitany, N.A.; Abbas, A.T.; Ali, S.S.; Eid, B.G. Honey protects against cisplatin-induced hepatic and renal toxicity through inhibition of NF-κB-mediated COX-2 expression and the oxidative stress dependent BAX/Bcl-2/caspase-3 apoptotic pathway. Food Funct. 2018, 9, 3743. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Chemotherapy for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/chemotherapy-for-breast-cancer.html (accessed on 5 January 2021).
- Shamseddine, A.I.; Farhat, F.S. Platinum-Based compounds for the treatment of metastatic breast cancer. Chemotherapy 2011, 57, 468–487. [Google Scholar] [CrossRef]
- Cataldo, A.; Romero-Cordoba, S.; Plantamura, I.; Cosentino, G.; Hidalgo-Miranda, A.; Tagliabue, E.; Iorio, M.V. MiR-302b as a combinatorial therapeutic approach to improve cisplatin chemotherapy efficacy in human triple-negative breast cancer. Cancers 2020, 12, 2261. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Luszczki, J.J.; Grabarska, A.; Gumbarewicz, E.; Dmoszynska-Graniczka, M.; Polberg, K.; Stepulak, A. Assessment of interactions between cisplatin and two histone deacetylase inhibitors in MCF7, T47D and MDA-MB-231 human breast cancer cell lines—An isobolographic analysis. PLoS ONE 2015, 10, e0143013. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional ingredients from Brassicaceae species: Overview and perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.R.; Kuo, Y.H.; Ho, Y.L.; Wang, C.Y.; Yang, C.S.; Lin, C.W.; Chang, Y.S. Studies on cytotoxic constituents from the leaves of Elaeagnus oldhamii Maxim. in non-small cell lung cancer A549 cells. Molecules 2014, 19, 9515–9534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Wang, C.F.; Shen, S.M.; Wang, G.L.; Liu, P.; Liu, Z.M.; Wang, Y.Y.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Antioxidant phenolic compounds from Pu-erh tea. Molecules 2012, 17, 14037–14045. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Mou, Y.; Zhao, J.; Wang, J.; Zhou, L.; Wang, M.; Wang, D.; Han, J.; Yu, Z.; Yang, F. Flavonoids from Halostachys caspica and their antimicrobial and antioxidant activities. Molecules 2010, 15, 7933–7945. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.; Leyva-López, N.; Gutiérrez-Grijalva, E.; Heredia, J. Phenolic compounds: Natural alternative in inflammation treatment. A review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and mitochondria: Activation of cytoprotective pathways? Molecules 2020, 25, 3060. [Google Scholar] [CrossRef]
- Tantawy, E.S.; Amer, A.M.; Mohamed, E.K.; Abd Alla, M.M.; Nafie, M.S. Synthesis, characterization of some pyrazine derivatives as anti-cancer agents: In vitro and in silico approaches. J. Mol. Struct. 2020, 1210, 128013. [Google Scholar] [CrossRef]
- Youssef, E.; El-Moneim, M.A.; Fathalla, W.; Nafie, M.S. Design, synthesis and antiproliferative activity of new amine, amino acid and dipeptide-coupled benzamides as potential sigma-1 receptor. J. Iran. Chem. Soc. 2020, 17, 2515–2532. [Google Scholar] [CrossRef]










| Groups | Liver Enzymes | Kidney Parameters | ||
|---|---|---|---|---|
| AST (U/L) | ALT (U/L) | Creatinine (mg/dL) | BUN (mg/dL) | |
| Normal control group | 43.7 ± 0.83 | 14.5 ± 0.75 | 0.84 ± 0.018 | 21.79 ± 0.92 |
| Cisplatin group (8 mg/kg) | 124.6 ± 2.16 a | 68.5 ± 0.96 a | 5.24 ± 0.17 a | 210.1 ± 2.6 a |
| Prophylactic C. annua- treated group (250 mg/kg) | 69.7 ± 0.97 a,b | 32 ± 0.68 a,b | 1.81 ± 0.06 a,b | 79.78 ± 1.84 a,b |
| Groups | Liver | Kidney | ||
|---|---|---|---|---|
| MDA (nmol/g tissue) | Reduced-GSH (μg/g tissue) | MDA (nmol/g tissue) | Reduced-GSH (μg/tissue) | |
| Normal control group | 24.59 ± 0.18 | 43.04 ± 0.2 | 16.03 ± 0.67 | 39.62 ± 0.13 |
| Cisplatin group (8 mg/kg) | 50.86 ± 1.07 a | 23.26 ± 0.37 a | 34.10 ± 0.12 a | 24.54 ± 0.2 a |
| Prophylactic C. annua- treated group (250 mg/kg) | 35.48 ± 0.36 a,b | 36.88 ± 0.24 a,b | 24.39 ± 0.23 a,b | 30.12 ± 0.33 a,b |
| Sample | MCF-7 (μg/mL) |
|---|---|
| Cisplatin | 6.4 ± 0.34 |
| C. annua extract+ cisplatin | 1.94 ± 0.11 |
| Compound | Caspase-3 (PDB: 6CKZ) * | IFN-γ (PDB: 2R3Z) # | ||||
|---|---|---|---|---|---|---|
| 1 | Arg 207 His 121 | 2 HB-acceptor and donor 1 HB-acceptor | 1 arene-cation with His 121 | Ile 12 His 13 Asp 15 | 1 HB-acceptor 1 HB-acceptor 1 HB-donor | - |
 |  | |||||
| Caspase-3 (PDB: 6CKZ) * | IFN-γ (PDB: 2R3Z) # | |||||
| 2 | Arg 207 His 121 | 1 HB-acceptor 1 HB-acceptor | 1 arene-cation with Arg 207 | Ile 1 His 13 Asp 15 Gln 51 | 1 HB-acceptor 1 HB-acceptor 1 HB-donor 1 HB-donor | 1 arene-cation with Ile 1 |
 |  | |||||
| Caspase-3 (PDB: 6CKZ) * | IFN-γ (PDB: 2R3Z) # | |||||
| 3 | Arg 64 | 1 HB-acceptor | 1 arene-cation with Arg 207 | Gln 51 Cys 53 | 1 HB-donor 1HB-acceptor | 1 arene-cation with Ile 1 |
 |  | |||||
| Caspase-3 (PDB: 6CKZ) * | IFN-γ (PDB: 2R3Z) # | |||||
| 4 | Arg 207 His 121 | 1 HB-donor I HB-acceptor | 1 arene-cation with Arg 207 | Ile 1 Cys 53 | 1 HB-acceptor I HB-acceptor | 1 arene-arene with His 13 |
 |  | |||||
| Caspase-3 (PDB: 6CKZ) * | IFN-γ (PDB: 2R3Z) # | |||||
| 5 | Arg 207 Arg 64 Gly 122 | 1 HB-acceptor I HB-acceptor I HB-acceptor | 1 arene-cation with His 121 | Asp 15 Glu 57 | 1 HB-donor I HB-donor | 1 arene-cation with His 13 1 arene-cation with Ile 1 |
 |  | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eltamany, E.E.; Elhady, S.S.; Nafie, M.S.; Ahmed, H.A.; Abo-Elmatty, D.M.; Ahmed, S.A.; Badr, J.M.; Abdel-Hamed, A.R. The Antioxidant Carrichtera annua DC. Ethanolic Extract Counteracts Cisplatin Triggered Hepatic and Renal Toxicities. Antioxidants 2021, 10, 825. https://doi.org/10.3390/antiox10060825
Eltamany EE, Elhady SS, Nafie MS, Ahmed HA, Abo-Elmatty DM, Ahmed SA, Badr JM, Abdel-Hamed AR. The Antioxidant Carrichtera annua DC. Ethanolic Extract Counteracts Cisplatin Triggered Hepatic and Renal Toxicities. Antioxidants. 2021; 10(6):825. https://doi.org/10.3390/antiox10060825
Chicago/Turabian StyleEltamany, Enas E., Sameh S. Elhady, Mohamed S. Nafie, Haidy A. Ahmed, Dina M. Abo-Elmatty, Safwat A. Ahmed, Jihan M. Badr, and Asmaa R. Abdel-Hamed. 2021. "The Antioxidant Carrichtera annua DC. Ethanolic Extract Counteracts Cisplatin Triggered Hepatic and Renal Toxicities" Antioxidants 10, no. 6: 825. https://doi.org/10.3390/antiox10060825
APA StyleEltamany, E. E., Elhady, S. S., Nafie, M. S., Ahmed, H. A., Abo-Elmatty, D. M., Ahmed, S. A., Badr, J. M., & Abdel-Hamed, A. R. (2021). The Antioxidant Carrichtera annua DC. Ethanolic Extract Counteracts Cisplatin Triggered Hepatic and Renal Toxicities. Antioxidants, 10(6), 825. https://doi.org/10.3390/antiox10060825