Hydrogen Peroxide Increases during Endodormancy and Decreases during Budbreak in Grapevine (Vitis vinifera L.) Buds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Dormancy Depth
2.3. Chemical Treatments
2.4. H2O2 Measurements
2.5. Peroxidase and Catalase Activity
2.6. RNA Purification, and cDNA Synthesis
2.7. Gene Expression Analysis
2.8. Data Analysis
3. Results
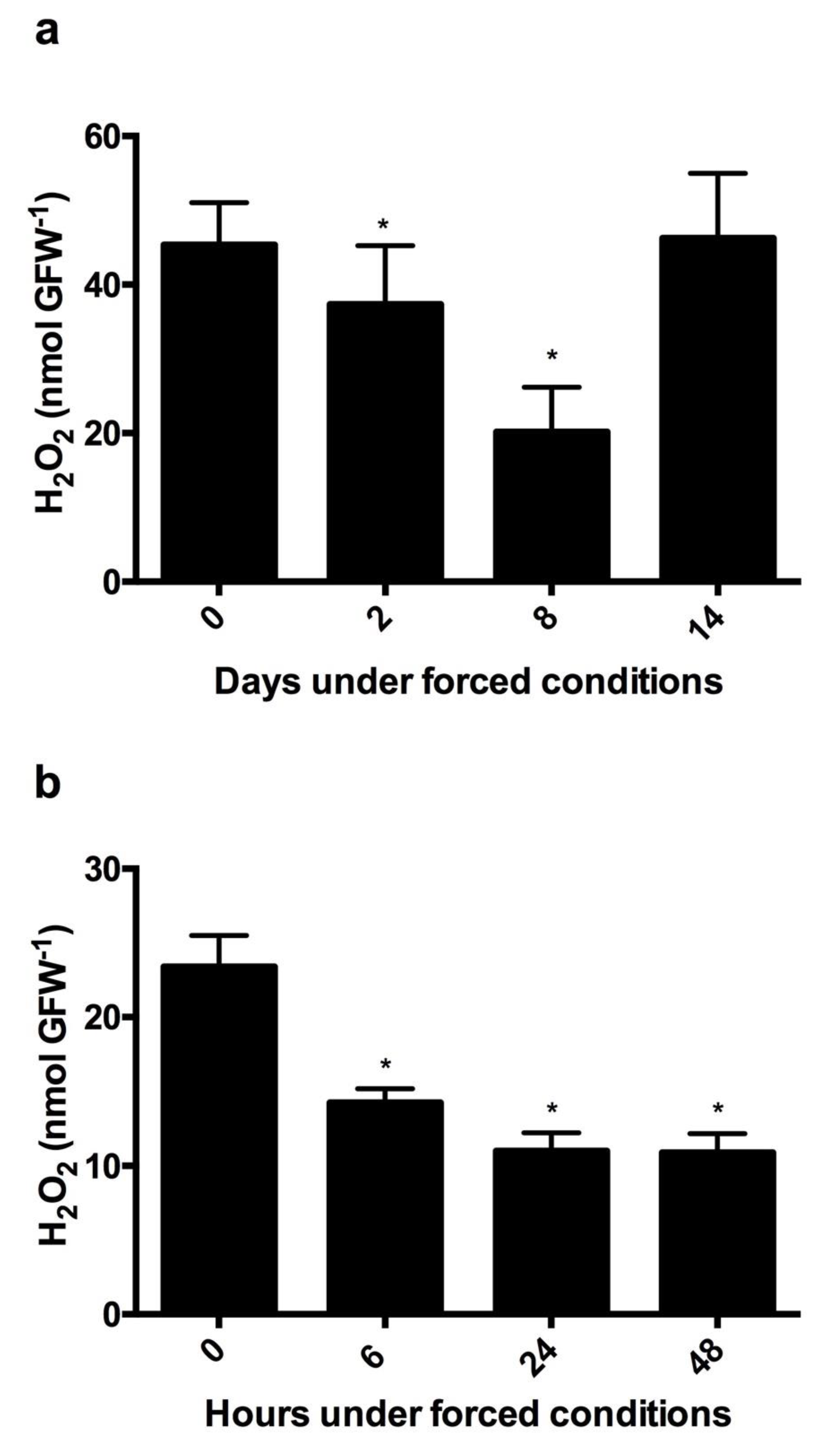
3.1. Depth of Endodormancy and H2O2 Content in Grapevine Buds
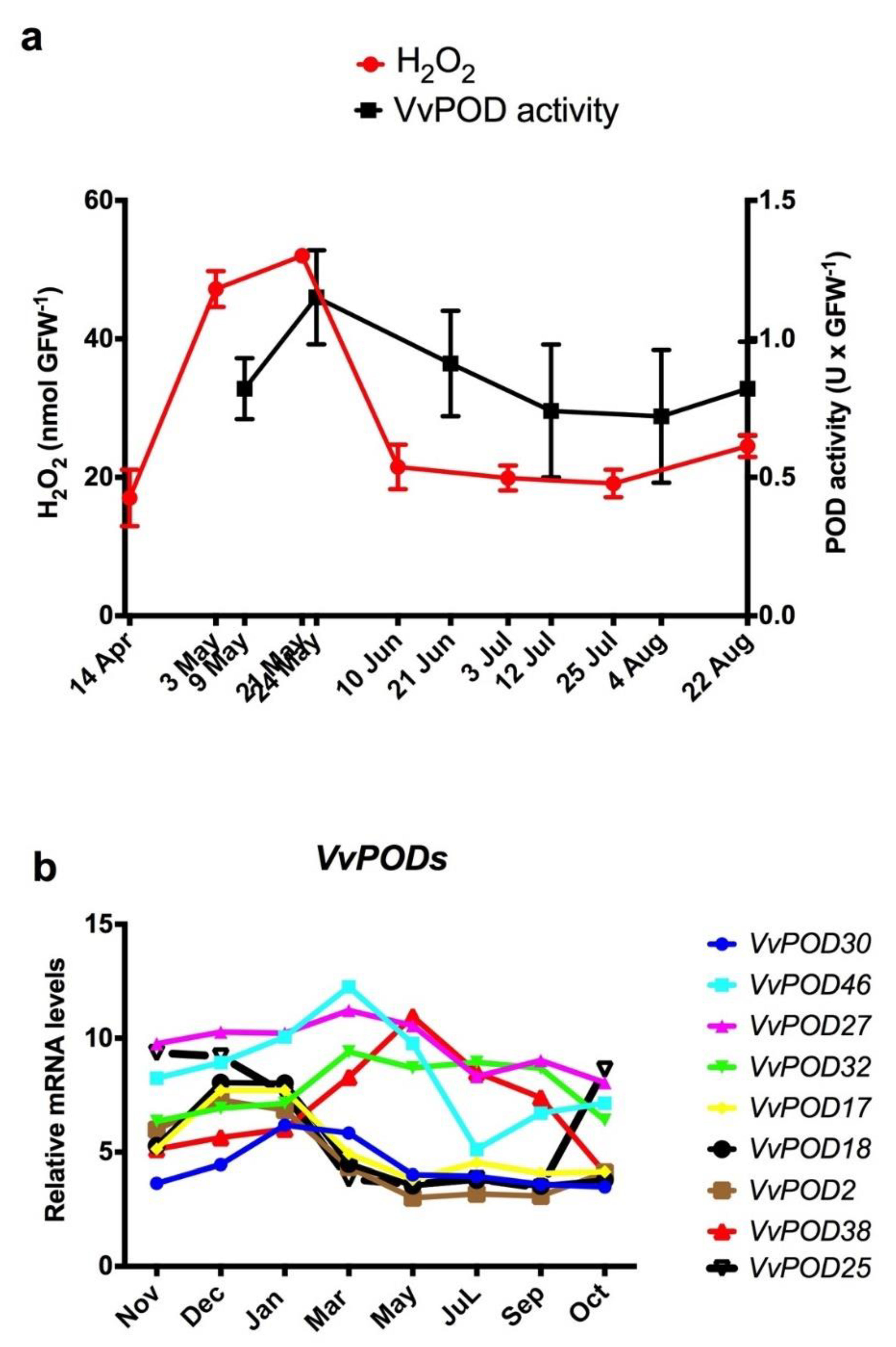
3.2. Increases in Peroxidase Activity and H2O2 Content Coincided through Endodormancy in the Grapevine Buds
3.3. Expression Profile of VvPOD Genes throughout the Endodormancy in Grapevine Buds
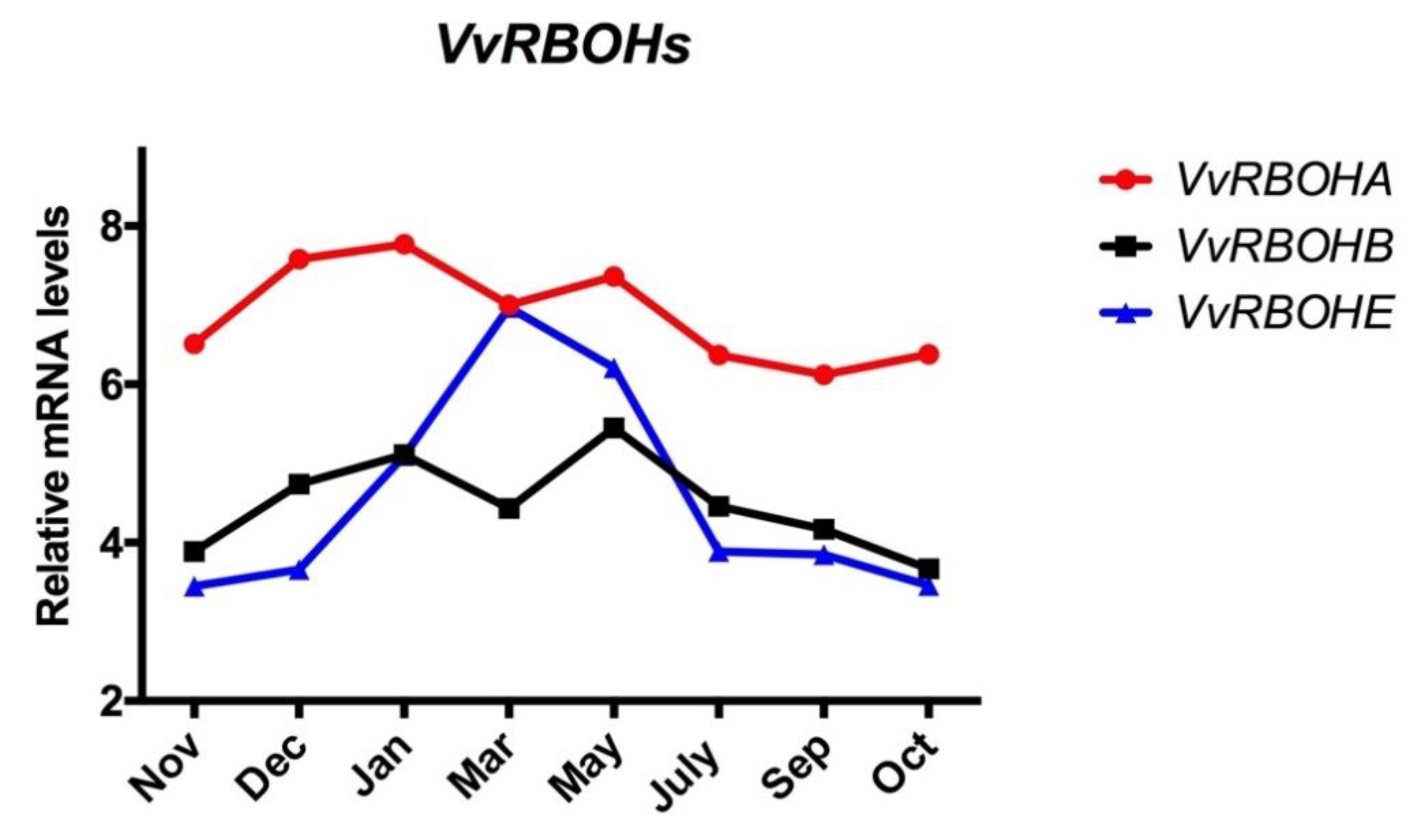
3.4. Expression Profile of RBOH throughout the Endodormancy Period in Grapevine Buds
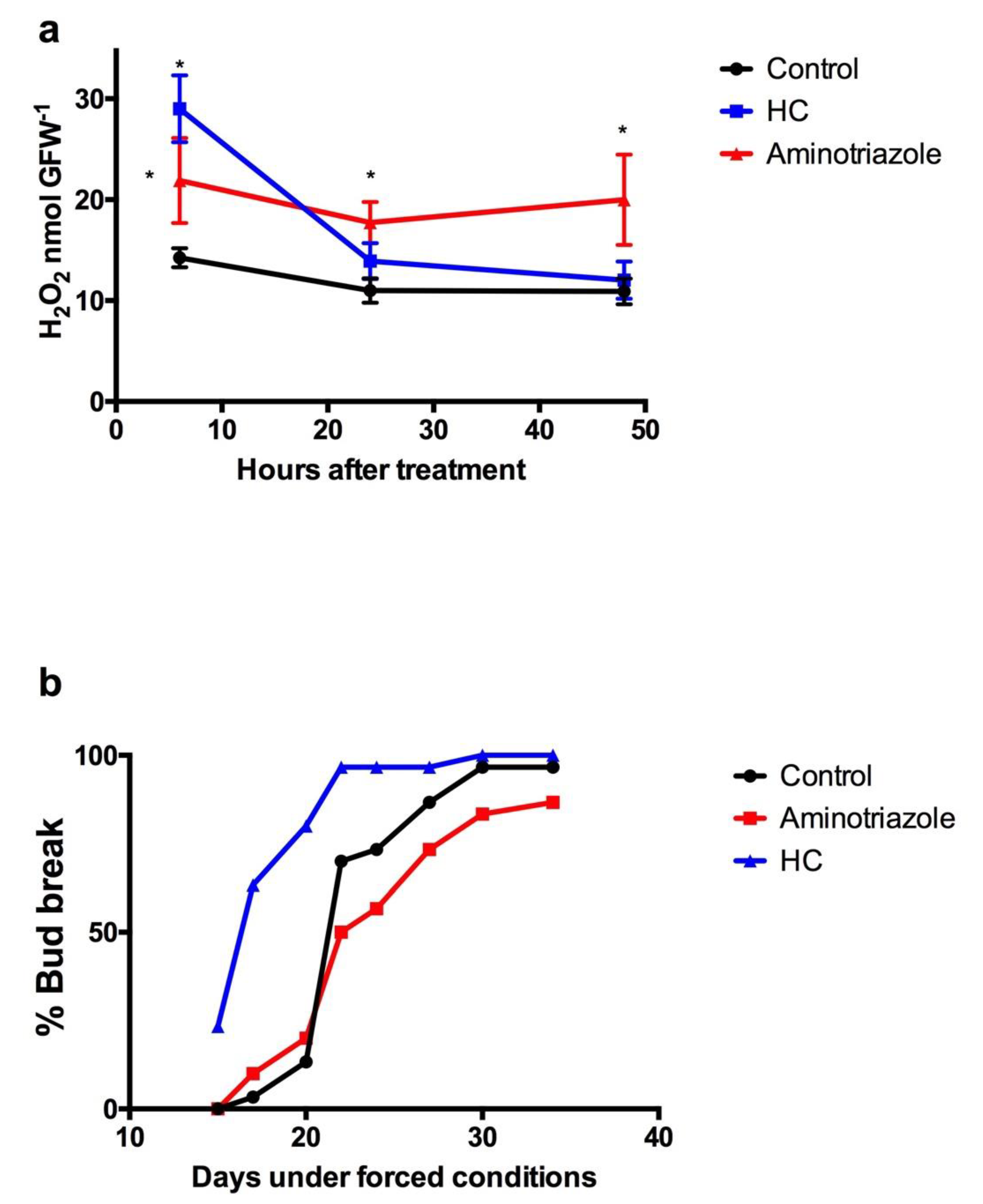
3.5. Scavenging Activity of H2O2 during Endodormancy in Grapevine Buds
3.6. Variations in the H2O2 Content during Budbreak in Grapevine Buds under Forced Conditions
3.7. Cytokinin-Inactivating Genes and Auxin Biosynthesis Genes Are Induced by H2O2 in Grapevine Buds, While Cytokinin Biosynthesis Genes Are Repressed
4. Discussion
4.1. ABA and H2O2 Accumulate in Grapevine Buds during ED
4.2. The Role of H2O2 during Bud ED Release and Budbreak
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kühn, N.; Ormeño-Nuñez, J.; Jaque-Zamora, G.; Pérez, F.J. Photoperiod modifies the diurnal expression profile of VvPHYA and VvPHYB transcripts in field-grown grapevine leaves. J. Plant Physiol. 2009, 66, 1172–1180. [Google Scholar] [CrossRef]
- Grant, T.N.L.; Gargrave, J.; Dami, I.E. Morphological physiological, and biochemical changes in Vitis genotype in responses to photoperiod regimes. Am. J. Enol. Vitic. 2013, 64, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Cragin, J.; Serpe, M.; Keller, M.; Shellie, K. Dormancy and cold hardiness transition in wine grape cultivars Chardonnay and Cabernet Sauvignon. Am. J. Enol. Vitic. 2017, 68, 195–202. [Google Scholar] [CrossRef]
- Lang, G.A. Dormancy: A new universal terminology. HortScience 1987, 22, 817–820. [Google Scholar]
- Or, E.; Belausov, E.; Popilevski, I.; Tal, Y.B. Changes in endogenous ABA level in relation to the dormancy cycle in grapevines grown in a hot climate. J. Hortic. Sci. Biotechnol. 2000, 75, 190–194. [Google Scholar] [CrossRef]
- Rubio, S.; Noriega, X.; Pérez, F.J. ABA promotes starch synthesis and storage metabolism in dormant grapevine buds. J. Plant Physiol. 2019, 234, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.H.; Halaly, T.; Acheampong, A.K.; Takebayashi, Y.; Jikumaru, Y.; Kamiya, Y.; Or, E. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 2015, 66, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Vergara, R.; Noriega, X.; Pérez, F.J. ABA represses the expression of cell cycle genes an may modulate the development of endodormancy in grapevine buds. Front. Plant Sci. 2017, 8, 812. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Acheampog, A.K.; Shi, Z.; Mugzech, A.; Halaly-Basha, T.; Shaya, F.; Sun, Y.; Colova, V.; Mosquna, A.; Ophir, R.; et al. Abscisic acid catabolism enhances dormancy release of grapevine buds. Plant Cell Environ. 2018, 1, 14. [Google Scholar] [CrossRef]
- Neil, S.; Desikan, R.; Hancock, J. Hydrogen Peroxide signaling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Pérez, F.J.; Burgos, B. Alterations in the pattern of peroxidase isoenzymes and transient increases in its activity and in H2O2 levels take place during the dormancy cycle of grapevine buds: The effect of hydrogen cyanamide. Plant Growth Regul. 2004, 43, 213–220. [Google Scholar] [CrossRef]
- Pérez, F.J.; Lira, W. Possible role of catalase in post-dormancy bud break in grapevines. J. Plant Physiol. 2005, 162, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.J.; Vergara, R.; Rubio, S. H2O2 is involved in the dormancy-breaking effect of hydrogen cyanamide in grapevine buds. Plant Growth Regul. 2008, 55, 149–515. [Google Scholar] [CrossRef]
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud dormancy in perennial fruit tree species: A pivotal role for oxidative cues. Front. Plant Sci. 2018, 9, 657. [Google Scholar] [CrossRef]
- Vergara, R.; Parada, F.; Rubio, S.; Pérez, F.J. Hypoxia induces H2O2 production and activates antioxidant defense system in grapevine buds through mediation of H2O2 and ethylene. J. Exp. Bot. 2012, 63, 4123–4131. [Google Scholar] [CrossRef] [Green Version]
- Baxler-Burrel, A.; Yang, Z.; Springer, P.S.; Bailey-Seres, J. RopGAP4-dependent Rop-GTPase rheostat control of Arabidopsis oxygen de privation tolerance. Science 2002, 296, 2026–2028. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Xu, X.; Gao, M.; Li, J.; Guo, C.; Song, J.; Wang, X. Genome-Wide analysis of respiratory burst oxidase homologs in grape (Vitis vinifera L.). Int. J. Mol. Sci. 2013, 14, 24169–24186. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Riquelme, J.; Grimplet, J.; Martínez-Zapater, J.M.; Carmona, M.J. Transcriptome variation along bud development in grapevine. BMC Plant Biol. 2012, 12, 181. [Google Scholar] [CrossRef] [Green Version]
- Khalil-Ur-Rehman, M.; Wang, W.; Dong, Y.; Faheem, M.; Xu, Y.; Gao, Z.; Tao, J. Comparative transcriptomic and proteomic analysis to deeply investigate the role of hydrogen cyanamide in grape bud dormancy. Int. J. Mol. Sci. 2019, 20, 3528. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Wang, C.; Khan, N.; Chen, M.; Fu, W.; Guan, L.; Leng, X. Genome-wide identification of class III POD gene family and their expression profiling in grapevine (Vitis vinifera L.). BMC Genom. 2020, 21, 444. [Google Scholar] [CrossRef]
- Koussa, T.; Broquedis, M.; Bouard, J. Changes of abscisic acid during the development of latent buds particularly in the phase of dormancy break. Vitis 1994, 33, 63–67. [Google Scholar]
- Dennis, G. Problems in standardizing methods for evaluating the chilling requirements for the breaking of dormancy in buds of woody plants. HortScience 2003, 38, 347–350. [Google Scholar] [CrossRef] [Green Version]
- Camargo Alvarez, H.; Salazar-Gutierrez, M.; Zapata, D.; Keller, M.; Hoogenboom, G. Time to event analysis to evaluate dormancy status of single-bud cuttings: An example for grapevines. Plant Methods 2018, 14, 94. [Google Scholar] [CrossRef]
- Vergara, R.; Pérez, F.J. Similarities between natural and chemically induced bud-endodormancy release in grapevine Vitis vinifera L. Sci. Hortic. 2010, 125, 648–653. [Google Scholar] [CrossRef]
- Rubio, S.; Dantas, D.; Bressan-Smith, R.; Pérez, F.J. Relationship between endodormancy and cold hardiness in grapevine buds. J. Plant Growth Regul. 2016, 35, 266–275. [Google Scholar] [CrossRef]
- Pérez, F.J.; Rubio, S. An improved chemiluminescence method for hydrogen peroxide determinations in plant tissues. Plant Growth Regul. 2006, 48, 89–95. [Google Scholar] [CrossRef]
- Pérez, F.J.; Gómez, M. Gibberelic acid stimulation of isoperoxidase from pedicel of grape. Phytochemistry 1998, 48, 411–414. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Noriega, X.; Burgos, B.; Pérez, F.J. Short-day photoperiod triggers and low temperatures increase expression of peroxidase RNA transcripts and basic peroxidase isoenzyme activity in grapevine-buds. Phytochemitry 2007, 68, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the www for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Time to event survival data. BMJ 1998, 317, 468–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, P. The reaction between aminotriazole and catalase. Biochem. Biophys. Acta 1962, 59, 414–420. [Google Scholar] [CrossRef]
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin–auxin crosstalk. Trends Plant Sci. 2009, 14, 557–562. [Google Scholar] [CrossRef]
- Noriega, X.; Pérez, F.J. ABA biosynthesis genes are down-regulated while auxin and cytokinin biosynthesis gene are up-regulated during the release of grapevine from endodormancy. J. Plant Growth Regul. 2017, 36, 814–823. [Google Scholar] [CrossRef]
- Assmann, S.M.; Shimazaki, K.I. The multisensory guard cell, stomatal responses to blue light and abscisic acid. Plant Physiol. 1999, 119, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Potikha, T.S.; Collins, C.C.; Johnson, D.I.; Delmer, D.P.; Levine, A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999, 119, 849–858. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, L.; Dong, F.; Gao, J.; Galbraith, W.; Song, C. Hydrogen Peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001, 126, 1428–1448. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Kao, C.H. Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci. 2001, 160, 323–329. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Li, Y.; Zhang, J. ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol. 2011, 54, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Shu, S.; Gao, P.; Li, L.; Yuan, Y.; Sun, J.; Guo, S. Abscicic acid-induced H2O2 accumulation enhances antioxidant capacity in pumpkin-grafted cucumber leaves under Ca(NO3)2 stress. Front. Plant Sci. 2016, 7, 1489. [Google Scholar] [CrossRef]
- Pilati, S.; Bagagli, G.; Sonego, P.; Moretto, M.; Brazzale, D.; Castorina, G.; Simoni, L.; Tonelli, C.; Guella, G.; Engelen, K.; et al. Abscisic acid is a major regulator of grape berry ripening onset. New insights into ABA signaling network. Front. Plant Sci. 2017, 8, 1093. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, P.; Lecourieux, D.; Kappel, C.; Cluzet, S.; Cramer, G.; Delrot, S.; Lecorieux, F. The basic Leucine-Zipper transcription factor ABSICISIC ACID RESPONSE ELEMENT-BINDING FACTOR2 is an important transcriptional regulator of absicisic-dependent grape berry ripening processes. Plant Physiol. 2014, 164, 365–383. [Google Scholar] [CrossRef] [Green Version]
- Sudawan, B.; Chang, C.; Chao, H.; Ku, M.S.; Yen, Y. Hydrogen cyanamide breaks grapevine buds dormancy in the summer through transient activation of gene expression and accumulatio of reactive oxygen and nitrogen species. BMC Plant Biol. 2016, 16, 202. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, H.; Zrig, A.; Geuns, J.M.C.; Khemira, H. Near-lethal heat treatment induced metabolic changes associated with endodormancy release of Superior Seedless grapevine cv. (Vitis vinifera L.) buds. Aust. J. Crop. Sci. 2014, 8, 486–494. [Google Scholar]
- Porcher, A.; Guérin, V.; Montrichard, F.; Lebrec, A.; Lothier, J.; Vian, A. Ascorbate glutathione-dependent H2O2 scavenging is an important process in axillary bud outgrowth in rosebush. Ann. Bot. 2020, 126, 1049–1062. [Google Scholar] [CrossRef]
- Azizi, P.; Rafi, M.Y.; Maziah, M.; Abdullah, S.N.A.; Hanafi, M.M.; Latif, M.A.; Rashid, A.A.; Sahebi, M. Understanding the shoot apical meristem regulation: Astudy of the phytohormones, auxin and cytokinin, in rice. Mech. Dev. 2015, 135, 1–15. [Google Scholar] [CrossRef]
- Noriega, X.; Pérez, F.J. Cell cycle genes are activated earlier than respiratory genes during release of grapevine buds from endodormancy. Plant Signal. Behav. 2017, 12, e1321189. [Google Scholar] [CrossRef]
- Pérez, F.J.; Noriega, X. Sprouting of paradormant and end dormant grapevine buds under conditions of forced growth: Similarities and differences. Planta 2018, 248, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Xia, X.J.; Guo, X.; Zhou, Y.H.; Shi, K.; Zhou, J.; Yu, J.Q. Apoplastic H2O2 plays a critical role in axillary bud outgrowth by altering auxin and cytokinin homeostasis in tomato plants. New Phytol. 2016, 211, 1266–1278. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, F.J.; Noriega, X.; Rubio, S. Hydrogen Peroxide Increases during Endodormancy and Decreases during Budbreak in Grapevine (Vitis vinifera L.) Buds. Antioxidants 2021, 10, 873. https://doi.org/10.3390/antiox10060873
Pérez FJ, Noriega X, Rubio S. Hydrogen Peroxide Increases during Endodormancy and Decreases during Budbreak in Grapevine (Vitis vinifera L.) Buds. Antioxidants. 2021; 10(6):873. https://doi.org/10.3390/antiox10060873
Chicago/Turabian StylePérez, Francisco Javier, Ximena Noriega, and Sebastián Rubio. 2021. "Hydrogen Peroxide Increases during Endodormancy and Decreases during Budbreak in Grapevine (Vitis vinifera L.) Buds" Antioxidants 10, no. 6: 873. https://doi.org/10.3390/antiox10060873
APA StylePérez, F. J., Noriega, X., & Rubio, S. (2021). Hydrogen Peroxide Increases during Endodormancy and Decreases during Budbreak in Grapevine (Vitis vinifera L.) Buds. Antioxidants, 10(6), 873. https://doi.org/10.3390/antiox10060873





