Optimized Supercritical CO2 Extraction Enhances the Recovery of Valuable Lipophilic Antioxidants and Other Constituents from Dual-Purpose Hop (Humulus lupulus L.) Variety Ella
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hop Pellets
2.2. Chemicals
2.3. Supercritical CO2 Extraction (SFE-CO2) of Hop Pellets
2.4. Determination of In Vitro Oxygen Radical Absorbance Capacity (ORAC)
2.5. Determination of α-and β-Acid Composition by UPLC-MSn Analysis
2.6. Determination of the Total Chlorophyll and Carotenoid Content
2.7. Determination of Volatile Compound Composition by SPME-GC×GC-TOF-MS Analysis
2.8. Experimental Design
2.9. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of SFE-CO2 of Hops at 10–15 MPa Pressure
3.2. Evaluation of SFE-CO2 of Hops at 24–45 MPa Pressure
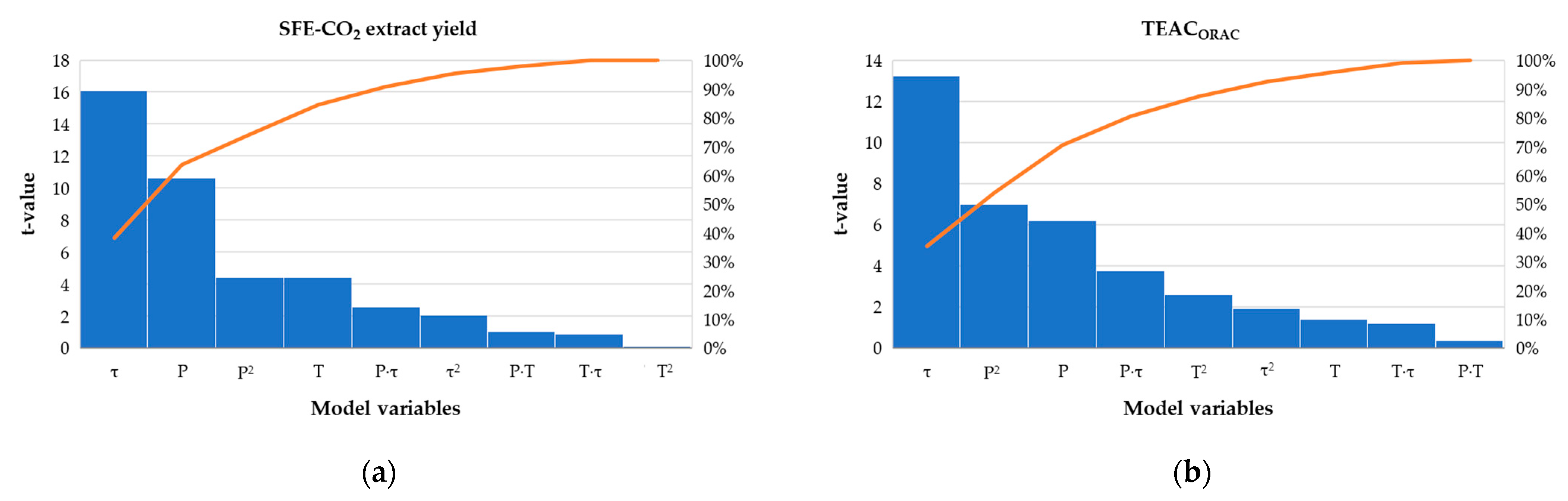
3.2.1. Central Composite Design and Model Analysis
3.2.2. Analysis of the Response Surface Plots
3.2.3. SFE-CO2 Optimization by the Desirability Function
3.3. Bitter Acid Profile of Hop Extracts Obtained under Different SFE-CO2 Conditions
3.4. Pigment Profile of Hop Extracts Obtained under Different SFE-CO2 Conditions
3.5. Volatile Compound Profile of Hop Extracts Obtained under Different SFE-CO2 Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically Active Compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrnčič, M.K.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Xiang, D.; Chen, X.; Huo, H. Role of characteristic components of Humulus lupulus in promoting human health. J. Agric. Food Chem. 2019, 67, 8291–8302. [Google Scholar] [CrossRef] [PubMed]
- Sanz, V.; Torres, M.D.; López Vilariño, J.M.; Domínguez, H. What is new on the hop extraction? Trends Food Sci. Technol. 2019, 93, 12–22. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Kupski, S.C.; Klein, E.J.; da Silva, E.A.; Palú, F.; Guirardello, R.; Vieira, M.G.A. Mathematical modeling of supercritical CO2 extraction of hops (Humulus lupulus L.). J. Supercrit. Fluids 2017, 130, 347–356. [Google Scholar] [CrossRef]
- Campalani, C.; Chioggia, F.; Amadio, E.; Gallo, M.; Rizzolio, F.; Selva, M.; Perosa, A. Supercritical CO2 extraction of natural antibacterials from low value weeds and agro-waste. J. CO2 Util. 2020, 40, 101198. [Google Scholar] [CrossRef]
- Formato, A.; Gallo, M.; Ianniello, D.; Montesano, D.; Naviglio, D. Supercritical fluid extraction of α- and β-acids from hops compared to cyclically pressurized solid-liquid extraction. J. Supercrit. Fluids 2013, 84, 113–120. [Google Scholar] [CrossRef]
- Van Opstaele, F.; Goiris, K.; De Rouck, G.; Aerts, G.; De Cooman, L. Production of novel varietal hop aromas by supercritical fluid extraction of hop pellets—Part 2: Preparation of single variety floral, citrus, and spicy hop oil essences by density programmed supercritical fluid extraction. J. Supercrit. Fluids 2012, 71, 147–161. [Google Scholar] [CrossRef]
- Van Opstaele, F.; Goiris, K.; De Rouck, G.; Aerts, G.; De Cooman, L. Production of novel varietal hop aromas by supercritical fluid extraction of hop pellets—Part 1: Preparation of single variety total hop essential oils and polar hop essences. J. Supercrit. Fluids 2012, 69, 45–56. [Google Scholar] [CrossRef]
- Syrpas, M.; Bukauskaitė, J.; Paškauskas, R.; Bašinskienė, L.; Venskutonis, P.R. Recovery of lipophilic products from wild cyanobacteria (Aphanizomenon flos-aquae) isolated from the Curonian Lagoon by means of supercritical carbon dioxide extraction. Algal Res. 2018, 35, 10–21. [Google Scholar] [CrossRef]
- Kitrytė, V.; Laurinavičienė, A.; Syrpas, M.; Pukalskas, A.; Venskutonis, P.R. Modeling and optimization of supercritical carbon dioxide extraction for isolation of valuable lipophilic constituents from elderberry (Sambucus nigra L.) pomace. J. CO2 Util. 2020, 35, 225–235. [Google Scholar] [CrossRef]
- Kitrytė, V.; Narkevičiūtė, A.; Tamkutė, L.; Syrpas, M.; Pukalskienė, M.; Venskutonis, P.R. Consecutive high-pressure and enzyme assisted fractionation of blackberry (Rubus fruticosus L.) pomace into functional ingredients: Process optimization and product characterization. Food Chem. 2020, 312, 126072. [Google Scholar] [CrossRef] [PubMed]
- Syrpas, M.; Bukauskaitė, J.; Ramanauskienė, K.; Karosienė, J.R.; Majienė, D.; Bašinskienė, L.; Venskutonis, P.R. Ultrasound-assisted extraction and assessment of biological activity of phycobiliprotein-rich aqueous extracts from wild cyanobacteria (Aphanizomenon flos-aquae). J. Agric. Food Chem. 2019, 68, 1896–1909. [Google Scholar] [CrossRef] [PubMed]
- Syrpas, M.; Subbarayadu, K.; Kitrytė, V.; Venskutonis, P.R. High-pressure extraction of antioxidant-rich fractions from shrubby cinquefoil (Dasiphora fruticosa L. Rydb.) leaves: Process optimization and extract characterization. Antioxidants 2020, 9, 457. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Mouahid, A.; Seengeon, K.; Martino, M.; Crampon, C.; Kramer, A.; Badens, E. Selective extraction of neutral lipids and pigments from Nannochloropsis salina and Nannochloropsis maritima using supercritical CO2 extraction: Effects of process parameters and pre-treatment. J. Supercrit. Fluids 2020, 165, 104934. [Google Scholar] [CrossRef]
- Martins, Z.E.; Machado, J.C.; Cunha, S.C.; Barata, A.M.; Ferreira, I.M.P.L.V.O. A chemometric approach to compare Portuguese native hops with worldwide commercial varieties. J. Chemom. 2020, 34, e3285. [Google Scholar] [CrossRef]
- Rali, T.; Wossa, S.; Leach, D.; Waterman, P. Volatile chemical constituents of Piper aduncum L. and Piper gibbilimbum C. DC (Piperaceae) from Papua New Guinea. Molecules 2007, 12, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.D.; Wong, Y.F.; Shellie, R.A.; Marriott, P.J.; Whittock, S.P.; Koutoulis, A. Assessment of the phytochemical profiles of novel hop (Humulus lupulus L.) cultivars: A potential route to beer crafting. Food Chem. 2019, 275, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, C.D.; Serafini, L.A.; Dellacassa, E.; Lorenzo, D.; Moyna, P. Essential oil of Baccharis uncinella DC. From Southern Brazil. Flavour Fragr. J. 2001, 16, 286–288. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ. Corp.: Carol Stream, IL, USA, 2007; pp. 1–804. [Google Scholar]
- Brendel, S.; Hofmann, T.; Granvogl, M. Characterization of key aroma compounds in pellets of different hop varieties (Humulus lupulus L.) by means of the sensomics approach. J. Agric. Food Chem. 2019, 67, 12044–12053. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Comparison of the volatile composition in thyme honeys from several origins in Greece. J. Agric. Food Chem. 2007, 55, 8152–8157. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Essien, S.O.; Young, B.; Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Rivera, O.; Teuber, O.; Palma, M.T. Supercritical CO2 extraction of Chilean hop (Humulus lupulus) ecotypes. J. Sci. Food Agric. 2003, 83, 1349–1356. [Google Scholar] [CrossRef]
- Schuster, J.J.; Bahr, L.A.; Fehr, L.; Tippelt, M.; Schulmeyr, J.; Wuzik, A.; Braeuer, A.S. Online monitoring of the supercritical CO2 extraction of hop. J. Supercrit. Fluids 2018, 133, 139–145. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Rój, E.; Tadić, V.M.; Mišić, D.; Žižović, I.; Arsić, I.; Dobrzyńska-Inger, A.; Kostrzewa, D. Supercritical carbon dioxide hops extracts with antimicrobial properties. Open Chem. 2015, 13, 1157–1171. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine 2009, 16, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Gorjanović, S.; Pastor, F.T.; Vasić, R.; Novaković, M.; Simonović, M.; Milić, S.; Sužnjević, D. Electrochemical versus spectrophotometric assessment of antioxidant activity of hop (Humulus lupulus L.) products and individual compounds. J. Agric. Food Chem. 2013, 61, 9089–9096. [Google Scholar] [CrossRef] [PubMed]
- Ting, P.L.; Lusk, L.; Refling, J.; Kay, S.; Ryder, D. Identification of antiradical hop compounds. J. Am. Soc. Brew. Chem. 2008, 66, 116–126. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Composition and in vitro effects of cultivars of Humulus lupulus L. hops on cholinesterase activity and microbial growth. Nutrients 2019, 11, 1377. [Google Scholar] [CrossRef] [Green Version]
- Masek, A.; Chrzescijanska, E.; Kosmalska, A.; Zaborski, M. Characteristics of compounds in hops using cyclic voltammetry, UV-VIS, FTIR and GC-MS analysis. Food Chem. 2014, 156, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Cook, D.; Wilson, C.; Marriott, R.; Ford, R. Sensory properties of supercritical CO2 fractions extracted from Magnum hop essential oil. J. Inst. Brew. 2020, 126, 263–279. [Google Scholar] [CrossRef]
- Duarte, L.M.; Amorim, T.L.; Grazul, R.M.; de Oliveira, M.A.L. Differentiation of aromatic, bittering and dual-purpose commercial hops from their terpenic profiles: An approach involving batch extraction, GC–MS and multivariate analysis. Food Res. Int. 2020, 138, 109768. [Google Scholar] [CrossRef] [PubMed]
- Van Opstaele, F.; De Causmaecker, B.; Aerts, G.; De Cooman, L. Characterization of novel varietal floral hop aromas by headspace solid phase microextraction and gas chromatography-mass spectrometry/olfactometry. J. Agric. Food Chem. 2012, 60, 12270–12281. [Google Scholar] [CrossRef] [PubMed]
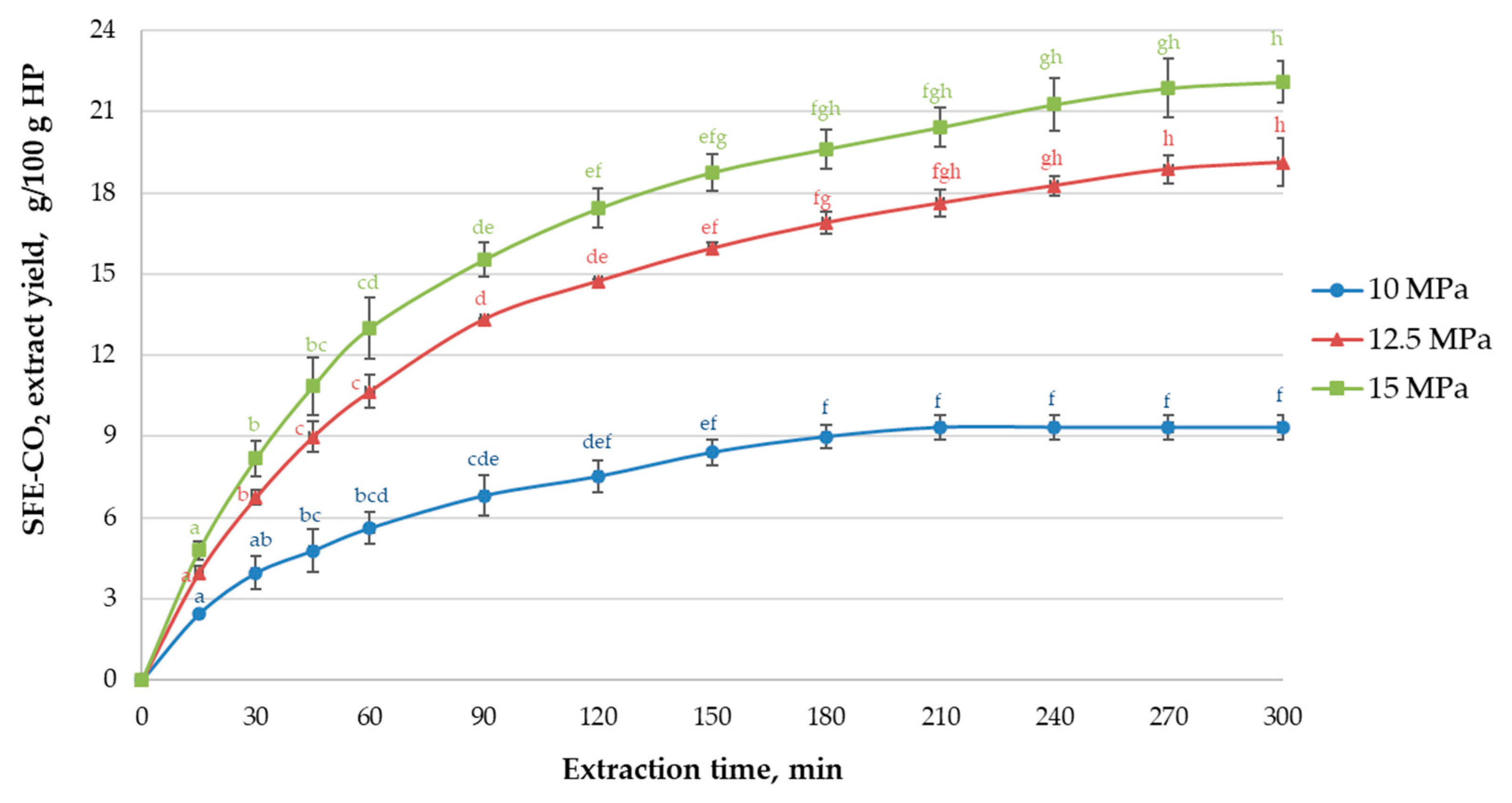
| Samples | SFE-CO2 Parameters | |||
|---|---|---|---|---|
| SFE-CO2 I 10 MPa, 40 °C, 300 min | SFE-CO2 II 12.5 MPa, 40 °C, 300 min | SFE-CO2 III 15 MPa, 40 °C, 300 min | SFE-CO2 IV 37 MPa, 43 °C, 80 min | |
| Extract yield, g/100 g HP | 9.33 ± 0.46 a | 19.11 ± 0.88 b | 22.09 ± 0.76 c | 26.32 ± 0.46 d |
| TEACORAC | ||||
| mg TE/g E | 1251.79 ± 6.30 a | 1281.94 ± 41.22 a | 1515.16 ± 26.30 b | 1481.17 ± 50.87 b |
| mg TE/g HP | 116.79 ± 0.59 a | 244.98 ± 7.88 b | 334.70 ± 5.81 c | 389.84 ± 13.39 d |
| Levels and Runs | SFE-CO2 Parameters | CO2 Density *, kg/m3 | RFI: Extract Yield, g/100 g HP | RFII: TEACORAC, mg TE/g HP | ||||
|---|---|---|---|---|---|---|---|---|
| P, MPa | T, °C | τ, min | Experimental | Predicted | Experimental | Predicted | ||
| Center | 35 | 50 | 60 | 899 | 23.24 ± 0.56 | 23.91 | 334.22 ± 14.15 | 344.65 |
| Axial | 25 | 50 | 60 | 834 | 20.15 ± 0.31 | 19.84 | 297.98 ± 19.92 | 295.34 |
| Axial | 35 | 40 | 60 | 935 | 25.12 ± 0.89 | 24.89 | 370.86 ± 12.90 | 360.63 |
| Factorial | 25 | 40 | 90 | 880 | 23.84 ± 1.32 | 23.60 | 351.05 ± 5.92 | 350.56 |
| Factorial | 25 | 60 | 30 | 787 | 13.85 ± 0.18 | 14.10 | 255.93 ± 7.42 | 253.41 |
| Axial | 35 | 50 | 90 | 899 | 25.74 ± 1.16 | 26.53 | 374.68 ± 5.43 | 368.99 |
| Center | 35 | 50 | 60 | 899 | 23.54 ± 0.56 | 23.91 | 338.53 ± 14.33 | 344.65 |
| Center | 35 | 50 | 60 | 899 | 24.22 ± 0.50 | 23.91 | 348.31 ± 14.74 | 344.65 |
| Factorial | 45 | 60 | 30 | 913 | 19.04 ± 0.62 | 19.38 | 304.38 ± 14.09 | 307.83 |
| Factorial | 45 | 40 | 90 | 975 | 27.57 ± 0.29 | 27.41 | 353.07 ± 19.91 | 358.56 |
| Factorial | 45 | 40 | 30 | 975 | 21.94 ± 0.47 | 22.15 | 304.98 ± 5.86 | 306.25 |
| Axial | 35 | 60 | 60 | 863 | 23.21 ± 0.54 | 23.01 | 355.29 ± 31.40 | 353.67 |
| Factorial | 25 | 60 | 90 | 787 | 22.72 ± 0.30 | 22.60 | 333.37 ± 9.10 | 335.06 |
| Factorial | 45 | 60 | 90 | 913 | 25.74 ± 0.66 | 25.44 | 347.98 ± 3.67 | 346.98 |
| Center | 35 | 50 | 60 | 899 | 23.14 ± 0.56 | 23.92 | 332.78 ± 14.09 | 344.65 |
| Center | 35 | 50 | 60 | 899 | 24.00 ± 0.55 | 23.91 | 345.15 ± 14.61 | 344.65 |
| Axial | 45 | 50 | 60 | 944 | 24.51 ± 0.23 | 24.39 | 335.77 ± 18.35 | 326.56 |
| Center | 35 | 50 | 60 | 899 | 24.52 ± 0.49 | 23.91 | 345.22 ± 2.21 | 344.65 |
| Factorial | 25 | 40 | 30 | 880 | 15.50 ± 0.27 | 15.90 | 251.79 ± 17.80 | 255.75 |
| Axial | 35 | 50 | 30 | 899 | 20.88 ± 1.28 | 19.65 | 308.17 ± 9.36 | 302.01 |
| Source | SS | df | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| RFI: Extract yield, g/100 g HP | |||||
| Model | 210.66 | 9 | 23.41 | 51.17 | <0.0001 * |
| P (pressure, MPa) | 51.71 | 1 | 51.71 | 113.06 | <0.0001 * |
| T (temperature, °C) | 8.85 | 1 | 8.85 | 19.36 | 0.0013 * |
| τ (time, min) | 118.34 | 1 | 118.34 | 258.72 | <0.0001 * |
| PT | 0.4802 | 1 | 0.4802 | 1.05 | 0.3297 ** |
| Pτ | 2.98 | 1 | 2.98 | 6.51 | 0.0288 * |
| Tτ | 0.3200 | 1 | 0.3200 | 0.6996 | 0.4224 ** |
| P² | 8.93 | 1 | 8.93 | 19.52 | 0.0013 * |
| T² | 0.0030 | 1 | 0.0030 | 0.0066 | 0.9368 ** |
| τ² | 1.86 | 1 | 1.86 | 4.06 | 0.0716 ** |
| Residual | 4.57 | 10 | 0.4574 | ||
| Lack of Fit | 3.03 | 5 | 0.6051 | 1.95 | 0.2399 ** |
| Pure Error | 1.55 | 5 | 0.3097 | ||
| Cor Total | 215.23 | 19 | |||
| RFII TEACORAC, mg TE/g HP | |||||
| Model | 20,284.94 | 9 | 2253.88 | 35.20 | <0.0001 * |
| P (pressure, MPa) | 2435.47 | 1 | 2435.47 | 38.03 | 0.0001 * |
| T (temperature, °C) | 121.10 | 1 | 121.10 | 1.89 | 0.1991 ** |
| τ (time, min) | 11,215.80 | 1 | 11,215.80 | 175.15 | <0.0001 * |
| PT | 7.70 | 1 | 7.70 | 0.1203 | 0.7359 ** |
| Pτ | 903.34 | 1 | 903.34 | 14.11 | 0.0037 * |
| Tτ | 86.53 | 1 | 86.53 | 1.35 | 0.2721 ** |
| P² | 3123.23 | 1 | 3123.23 | 48.77 | <0.0001 * |
| T² | 429.66 | 1 | 429.66 | 6.71 | 0.0269 * |
| τ² | 230.26 | 1 | 230.26 | 3.60 | 0.0872 ** |
| Residual | 640.35 | 10 | 64.03 | ||
| Lack of Fit | 432.78 | 5 | 86.56 | 2.08 | 0.2196 ** |
| Pure Error | 207.57 | 5 | 41.51 | ||
| Cor Total | 20,925.29 | 19 | |||
| Samples | SFE-CO2 Parameters | |||
|---|---|---|---|---|
| SFE-CO2 I 10 MPa, 40 °C, 300 min | SFE-CO2 II 12.5 MPa, 40 °C, 300 min | SFE-CO2 III 15 MPa, 40 °C, 300 min | SFE-CO2 IV 37 MPa, 43 °C, 80 min | |
| Bitter Acid Content | ||||
| α-Bitter acids | ||||
| Cohumulone | ||||
| mg/g E | 144.59 ± 2.89 a | 182.60 ± 8.47 b | 234.54 ± 0.24 c | 186.95 ± 8.03 b |
| mg/g HP | 13.49 ± 0.27 a | 34.90 ± 1.62 b | 51.81 ± 0.05 c | 49.21 ± 2.11 c |
| Adhumulone + humulone | ||||
| mg/g E | 246.37 ± 4.29 a | 349.19 ± 17.43 b | 360.15 ± 6.17 b | 335.92 ± 2.27 b |
| mg/g HP | 22.99 ± 0.40 a | 66.73 ± 3.33 b | 79.56 ± 1.36 c | 88.41 ± 0.60 d |
| Total α-bitter acids | ||||
| mg/g E | 390.96 ± 7.18 a | 531.79 ± 25.90 b | 594.69 ± 6.41 c | 522.87 ± 10.30 b |
| mg/g HP | 36.48 ± 0.67 a | 101.63 ± 4.95 b | 131.37 ± 1.42 c | 137.62 ± 2.71 c |
| β-Bitter acids | ||||
| Colupulone | ||||
| mg/g E | 217.38 ± 6.33 b | 186.47 ± 7.83 a | 212.82 ± 6.08 b | 225.94 ± 13.05 b |
| mg/g HP | 20.28 ± 0.59 a | 35.63 ± 1.50 b | 47.01 ± 1.34 c | 59.47 ± 3.43 d |
| Adlupulone + lupulone | ||||
| mg/g E | 109.69 ± 3.06 a | 116.45 ± 6.38 ab | 125.11 ± 1.68 b | 119.09 ± 6.39 ab |
| mg/g HP | 10.23 ± 0.29 a | 22.25 ± 1.22 b | 27.64 ± 0.37 c | 31.34 ± 1.68 d |
| Total β-bitter acids | ||||
| mg/g E | 327.07 ± 9.39 ab | 302.92 ± 14.20 a | 337.93 ± 4.40 b | 345.03 ± 6.66 b |
| mg/g HP | 30.51 ± 0.88 a | 57.88 ± 2.71 b | 74.65 ± 0.97 c | 90.81 ± 1.75 d |
| Total hop bitter acids | ||||
| mg/g E | 718.03 ± 16.57 a | 834.71 ± 40.10 b | 932.62 ± 2.01 c | 867.90 ± 16.96 b |
| mg/g HP | 66.99 ± 1.55 a | 159.51 ± 7.66 b | 206.02 ± 0.44 c | 228.43 ± 4.46 d |
| α-acid/β-acid ratio | 1.19 | 1.76 | 1.76 | 1.52 |
| Pigment Content | ||||
| Chlorophylls | ||||
| Chlorophyll A | ||||
| μg/g E | -ND | 10.60 ± 0.12 a | 41.19 ± 0.14 b | 146.13 ± 1.45 c |
| μg/g HP | -ND | 2.02 ± 0.02 a | 9.10 ± 0.03 b | 38.46 ± 0.38 c |
| Chlorophyll B | ||||
| μg/g E | -ND | 12.84 ±1.16 a | 20.48 ± 1.42 b | 20.10 ± 0.83 b |
| μg/g HP | -ND | 2.45 ± 0.22 a | 4.52 ± 0.31 b | 5.29 ± 0.22 c |
| Total chlorophylls | ||||
| μg/g E | -ND | 23.43 ± 1.04 a | 61.67 ± 1.28 b | 166.23 ± 2.28 c |
| μg/g HP | -ND | 4.48 ± 0.20 a | 13.62 ± 0.28 b | 43.75 ± 0.60 c |
| Carotenoids | ||||
| Total carotenoids | ||||
| μg/g E | 20.72 ± 1.18 a | 76.80 ± 3.39 b | 124.26 ± 0.59 c | 235.12 ± 1.33 d |
| μg/g HP | 1.93 ± 0.11 a | 14.68 ± 0.65 b | 27.45 ± 0.13 c | 61.88 ± 0.35 d |
| Compound | Exact Mass | RIexp | RIlit A | Odor Type: Description B,C | SFE-CO2 Conditions | |||
|---|---|---|---|---|---|---|---|---|
| SFE-CO2 I 10 MPa, 40 °C, 300 min | SFE-CO2 II 12.5 MPa, 40 °C, 300 min | SFE-CO2 III 15 MPa, 40 °C, 300 min | SFE-CO2 IV 37 MPa, 43 °C, 80 min | |||||
| Monoterpenes, % of the total GC peak area | ||||||||
| α-Pinene | 136.1252 | 950 | 946 [24] | Herbal: herbal, fresh, terpenic, fruity, sweet, green, pine, earthy, woody | 0.09 ± 0.00 a | 0.05 ± 0.00 a | 0.08 ± 0.02 a | 0.39 ± 0.03 b |
| Camphene | 136.1252 | 971 | 972 [25] | Woody: camphoreous, cooling minty, citrus, green, spicy | 0.02 ± 0.01 a | 0.04 ± 0.00 a | 0.06 ± 0.03 a | 0.13 ± 0.05 a |
| β-Pinene | 136.1252 | 1000 | 989 [26] | Herbal: cooling, dry, woody, piney, spicy, eucalyptus | 3.21 ± 0.07 a | 4.21 ± 0.05 b | 3.74 ± 0.21 b | 7.02 ± 0.08 c |
| β-Myrcene | 136.1252 | 1000 | 995 [26] | Spicy: peppery, terpenic, balsamic, metallic, musty, fruity, ethereal, herbaceous, woody | 3.02 ± 0.01 a | 3.82 ± 0.04 b | 3.81 ± 0.04 b | 6.23 ± 0.17 c |
| p-Cymene | 136.1252 | 1015 | 1015 [27] | Terpenic: woody, fresh, terpenic, citrus, lemon, spicy | 0.41 ± 0.01 c | 0.20 ± 0.00 b | 0.14 ± 0.00 a | 0.39 ± 0.00 c |
| (E)-β-Ocimene | 136.1252 | 1059 | 1052 [26] | Floral: herbal, mild, citrus, sweet, orange, lemon, tropical, green, woody | 1.53 ± 0.02 d | 1.34 ± 0.01 c | 0.61 ± 0.00 a | 1.18 ± 0.00 b |
| γ-Terpinene | 136.1252 | 1074 | 1068 [25] | Terpenic: citrus, terpenic, herbal, oily, tropical, fruity, sweet | 0.36 ± 0.04 a | 0.36 ± 0.00 a | 0.32 ± 0.00 a | -ND |
| Terpinolene | 136.1252 | 1104 | 1105 [25] | Herbal: fresh, woody, sweet, piney, citrus, anise | 0.08 ± 0.01 a | 0.09 ± 0.00 a | 0.11 ± 0.02 a | 0.11 ± 0.00 a |
| β-Linalool | 154.1358 | 1119 | 1109 [26] | Floral: citrus, orange, floral, sweet, rose, woody, green | 1.78 ± 0.08 a | 1.97 ± 0.04 ab | 1.97 ± 0.02 ab | 2.15 ± 0.14 b |
| Total monoterpenes | 10.50 | 12.08 | 10.84 | 17.60 | ||||
| Sesquiterpenes, % of the total GC peak area | ||||||||
| α-Copaene | 204.1878 | 1375 | 1374 [28] | Woody: woody, spicy, earthy | 0.18 ± 0.01 a | 0.24 ± 0.00 c | 0.21 ± 0.00 b | -ND |
| α-Ylangene | 204.1878 | 1401 | 1390 [24] | Fruity | 3.14 ± 0.01 a | 3.53 ± 0.04 b | 3.47 ± 0.01 b | 4.01 ± 0.00 c |
| β-Caryophyllene | 204.1878 | 1438 | 1428 [24] | Spicy: musty, green, woody, clove, dry | 0.64 ± 0.00 b | 0.67 ± 0.00 c | 0.61 ± 0.00 a | 0.76 ± 0.00 d |
| Aromadendrene | 204.1878 | 1439 | 1439 [28] | Sweet, dry | 1.09 ± 0.01 a | 1.00 ± 0.07 a | 1.00 ± 0.00 a | 1.08 ± 0.00 a |
| β-Humulene | 204.1878 | 1457 | 1457 [27] | -NR | 6.30 ± 0.48 a | 6.67 ± 0.30 a | 6.98 ± 0.02 b | 6.31 ± 0.22 a |
| α-Humulene | 204.1878 | 1504 | 1505 [25] | Woody: woody, spicy, clove | 9.88 ± 0.02 b | 9.71 ± 0.08 b | 7.23 ± 0.00 a | 7.96 ± 0.78 a |
| β-Selinene | 204.1878 | 1514 | 1524 [24] | Herbal | -ND | 4.30 ± 0.00 b | 4.61 ± 0.00 c | 3.88 ± 0.00 a |
| α-Selinene | 204.1878 | 1534 | 1533 [24] | Pepper, orange | 14.86 ± 0.00 c | 10.55 ± 0.00 b | 5.90 ± 0.00 a | 5.49 ± 0.01 a |
| δ-Cadinene | 204.1878 | 1554 | 1556 [24] | Herbal: thyme, herbal, woody, dry | 1.51 ± 0.00 a | 1.58 ± 0.03a | 2.41 ± 0.01 a | 4.65 ± 0.08 b |
| Calamenene | 202.1722 | 1564 | 1562 [24] | Herbal, spicy | 0.27 ± 0.00 a | 0.31 ± 0.00 b | 0.77 ± 0.00 c | 0.84 ± 0.00 d |
| α-Calacorene | 200.1565 | 1583 | 1590 [24] | Woody: dry, woody | 0.21 ± 0.01 c | 0.17 ± 0.00 b | 0.20 ± 0.00 c | 0.13 ± 0.00 a |
| Caryophyllene oxide | 220.1827 | 1635 | 1617 [26] | Woody: sweet, fresh, dry, woody, spicy, fruity, sawdust, herbal | 0.21 ± 0.00 | -ND | 0.41 ± 0.00 | -ND |
| Total sesquiterpenes | 38.29 | 38.73 | 33.80 | 35.11 | ||||
| Alcohols, % of the total GC peak area | ||||||||
| 3-Methyl-2-buten-1-ol | 86.0732 | 799 | 785 [24] | Fruity: sweet, fruity, alcoholic, green | 0.20 ± 0.00 a | 0.44 ± 0.00 b | 0.50 ± 0.00 c | -ND |
| 2-Undecanol | 170.1671 | 1314 | 1302 [24] | Waxy: fresh, waxy, cloth, sarsaparilla | 1.74 ± 0.08 b | 1.89 ± 0.13 b | 1.80 ± 0.07 b | 1.48 ± 0.13 a |
| Total alcohols | 1.94 | 2.33 | 2.3 | 1.48 | ||||
| Aldehydes, % of the total GC peak area | ||||||||
| 3-Methyl-2-butenal | 84.0575 | 814 | 794 [24] | Fruity: sweet, fruity, pungent, nutty, almond, cherry | 0.24 ± 0.01 b | 0.24 ± 0.05 b | 0.27 ± 0.06 b | 0.04 ± 0.01 a |
| Total aldehydes | 0.24 | 0.24 | 0.27 | 0.04 | ||||
| Ketones, % of the total GC peak area | ||||||||
| 2-Undecanone | 170.1671 | 1271 | 1294 [28] | Fruity: waxy, fruity, creamy, fatty, pineapple, orris, floral | 0.63 ± 0.00 a | 0.60 ± 0.02 a | 0.64 ± 0.00 a | 0.58 ± 0.00 a |
| 2-Tridecanone | 198.1984 | 1514 | 1504 [26] | Waxy: fatty, waxy, dairy, milky, coconut, nutty, herbal, earthy | 0.67±0.00 a | 0.64±0.00 a | -ND | -ND |
| Total ketones | 1.30 | 1.24 | 0.64 | 0.58 | ||||
| Esters, % of the total GC peak area | ||||||||
| 2-methylpropyl 2-methylpropanoate | 144.1150 | 921 | 918 [26] | Fruity: ethereal, fruity, tropical, fruity, pineapple | 0.08 ± 0.03 a | 0.09 ± 0.04 a | 0.16 ± 0.00 ab | 0.27 ± 0.01 b |
| 3-methylbutyl propanoate | 144.1150 | 979 | 977 [26] | Fruity: sweet, fruity, apple, apple, raspberry, banana | 0.56 ± 0.06 a | 0.69 ± 0.07 a | 0.59 ± 0.00 a | 1.05 ± 0.10 b |
| Methyl hexanoate | 130.0994 | 936 | 927 [24] | Fruity: fruity, pineapple, thinner, acetone | 0.09 ± 0.00 | 0.10 ± 0.01 | 0.16 ± 0.00 | 0.11 ± 0.02 |
| Pentyl 2-methylpropanoate | 158.1307 | 1022 | 1020 [26] | Fruity: fruity, apple, banana, apricot, buttery | 1.37 ± 0.02 a | 1.52 ± 0.18 a | 1.68 ± 0.09 a | 2.76 ± 0.05 b |
| Methyl heptanoate | 144.1150 | 1037 | 1030 [26] | Fruity: sweet, fruity, waxy, floral, berry, apple | 0.56 ± 0.00 a | 0.65 ± 0.05 ab | 0.60 ± 0.04 a | 0.77 ± 0.06 b |
| Methyl 6-methylheptanoate | 158.1307 | 1096 | 1092 [24] | -NR | 0.72 ± 0.02 a | 1.02 ± 0.00 b | 1.14 ± 0.00 c | 0.96 ± 0.05 b |
| 2-Methylbutyl 3-methylbutanoate | 172.1463 | 1111 | 1113 [24] | Fruity: herbal, earthy, apple, green | -ND | -ND | 0.52 ± 0.06 | 0.63 ± 0.09 |
| Methyl octanoate | 158.1307 | 1135 | 1130 [26] | Waxy: waxy, green, sweet, orange, aldehydic, vegetable, herbal | 1.16 ± 0.01a | 1.33 ± 0.08 a | 1.28 ± 0.06 a | 1.20 ± 0.05 a |
| Hexyl 2-methylpropanoate | 172.1463 | 1158 | 1151 [26] | Green: sweet, green, fruity, apple, pear, grape, ripe, berry | 0.15 ± 0.06 a | 0.28 ± 0.00 abc | 0.61 ± 0.04 c | 0.36 ± 0.01 b |
| Heptyl propanoate | 172.1463 | 1206 | 1207 [24] | Floral: rose, apricot | 0.61 ± 0.03 ab | 0.62 ± 0.01 ab | 0.67 ± 0.00 b | 0.56 ± 0.00 a |
| Methyl 8-nonenoate | 170.1307 | 1222 | 1218 [26] | -NR | -ND | 0.55 ± 0.03 a | 0.50 ± 0.00 a | 0.49 ± 0.00 a |
| Methyl nonanoate | 172.1463 | 1238 | 1229 [26] | Fruity: sweet, fruity, pear, waxy, tropical, winey | 0.91 ± 0.01 | 2.18 ± 0.12 bc | 2.83 ± 0.04 c | 2.13 ± 0.00 b |
| Heptyl 2-methylpropanoate | 186.1620 | 1255 | 1249 [26] | Fruity: fruity, sweet, green, warm, floral, tropical, chamomile, tea, green | 0.39 ± 0.00 | 0.49 ± 0.00 | 0.53 ± 0.07 a | 0.42 ± 0.02 a |
| 2-Methylbutyl hexanoate | 186.1620 | 1263 | 1246 [24] | Fruity: fruity, ethereal | 0.06 ± 0.00 | 0.12 ± 0.00 | -ND | -ND |
| Methyl 4-decenoate | 184.1463 | 1322 | 1316 [26] | Fruity: fruity, pear, mango, fishy, peach, green | 5.51 ± 0.04 a | 10.89 ± 0.22 c | 11.08 ± 0.27 c | 8.38 ± 0.06 b |
| Total esters | 12.17 | 20.53 | 22.35 | 20.09 | ||||
| Fatty acids, % of the total GC peak area | ||||||||
| 2-Methylpropanoic acid | 88.05240 | 778 | 762 [29] | Acidic: sour, cheesy, dairy, buttery, rancid, phenolic, fatty, sweaty | 0.17 ± 0.00 a | 0.17 ± 0.02 a | 0.20 ± 0.02 a | 0.26 ± 0.05 a |
| 3-Methylbutanoic acid | 102.0681 | 850 | 865 [29] | Cheesy: dairy, acidic, sour, pungent, fruity, fatty, sweaty, rancid | 0.11 ± 0.01 a | 0.13 ± 0.00 a | 0.14 ± 0.00 a | 0.11 ± 0.01 a |
| Heptanoic acid | 130.0994 | 1089 | 1072 [24] | Cheesy: rancid, sour, cheesy, waxy, sweaty, fermented, pineapple, fruity | 0.24 ± 0.00 a | 0.41 ± 0.01 c | 0.31 ± 0.02 b | 0.28 ± 0.02 ab |
| Octanoic acid | 144.1150 | 1189 | 1191 [30] | Fatty: fatty, waxy, rancid, oily, vegetable, cheesy | 0.20 ± 0.00 a | 0.25 ± 0.00 c | 0.22 ± 0.00 b | 0.28 ± 0.00 d |
| Total fatty acids | 0.72 | 0.96 | 0.87 | 0.93 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagybákay, N.E.; Syrpas, M.; Vilimaitė, V.; Tamkutė, L.; Pukalskas, A.; Venskutonis, P.R.; Kitrytė, V. Optimized Supercritical CO2 Extraction Enhances the Recovery of Valuable Lipophilic Antioxidants and Other Constituents from Dual-Purpose Hop (Humulus lupulus L.) Variety Ella. Antioxidants 2021, 10, 918. https://doi.org/10.3390/antiox10060918
Nagybákay NE, Syrpas M, Vilimaitė V, Tamkutė L, Pukalskas A, Venskutonis PR, Kitrytė V. Optimized Supercritical CO2 Extraction Enhances the Recovery of Valuable Lipophilic Antioxidants and Other Constituents from Dual-Purpose Hop (Humulus lupulus L.) Variety Ella. Antioxidants. 2021; 10(6):918. https://doi.org/10.3390/antiox10060918
Chicago/Turabian StyleNagybákay, Nóra Emilia, Michail Syrpas, Vaiva Vilimaitė, Laura Tamkutė, Audrius Pukalskas, Petras Rimantas Venskutonis, and Vaida Kitrytė. 2021. "Optimized Supercritical CO2 Extraction Enhances the Recovery of Valuable Lipophilic Antioxidants and Other Constituents from Dual-Purpose Hop (Humulus lupulus L.) Variety Ella" Antioxidants 10, no. 6: 918. https://doi.org/10.3390/antiox10060918







