Sesamin Activates Nrf2/Cnc-Dependent Transcription in the Absence of Oxidative Stress in Drosophila Adult Brains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Stocks and Culture
2.2. Chemical Feeding
2.3. Observation of GFP and RFP Fluorescence
2.4. Quantitative Reverse Transcription Polymerase Chain Reaction
- RP49-Fw, 5′-TTCCTGGTGCACAACGTG-3′,
- RP49-Rv, 5′-TCTCCTTGCGCTTCTTGG-3′,
- GFP-Fw, 5′-AAGCTGACCCTGAAGTTCATCTGC-3′,
- GFP-Rv, 5′-CTTGTAGTTGCCGTCGTCCTTGAA-3′,
- Nrf2-Fw, 5′-TTACATCTACGAGTACGCCGC-3′,
- Nrf2-Rv, 5′-ACTGGAGCTCAAAACCGCTAA-3′,
- Keap1-Fw, 5′-CCACCGTGGAGCGTTATGATA-3′,
- Keap1-Rv, 5′-TTCCTGCATTCTGGACCAAGG-3′
2.5. Quantitation of GFP or RFP-Positive Area
2.6. Statistical Analysis
3. Results
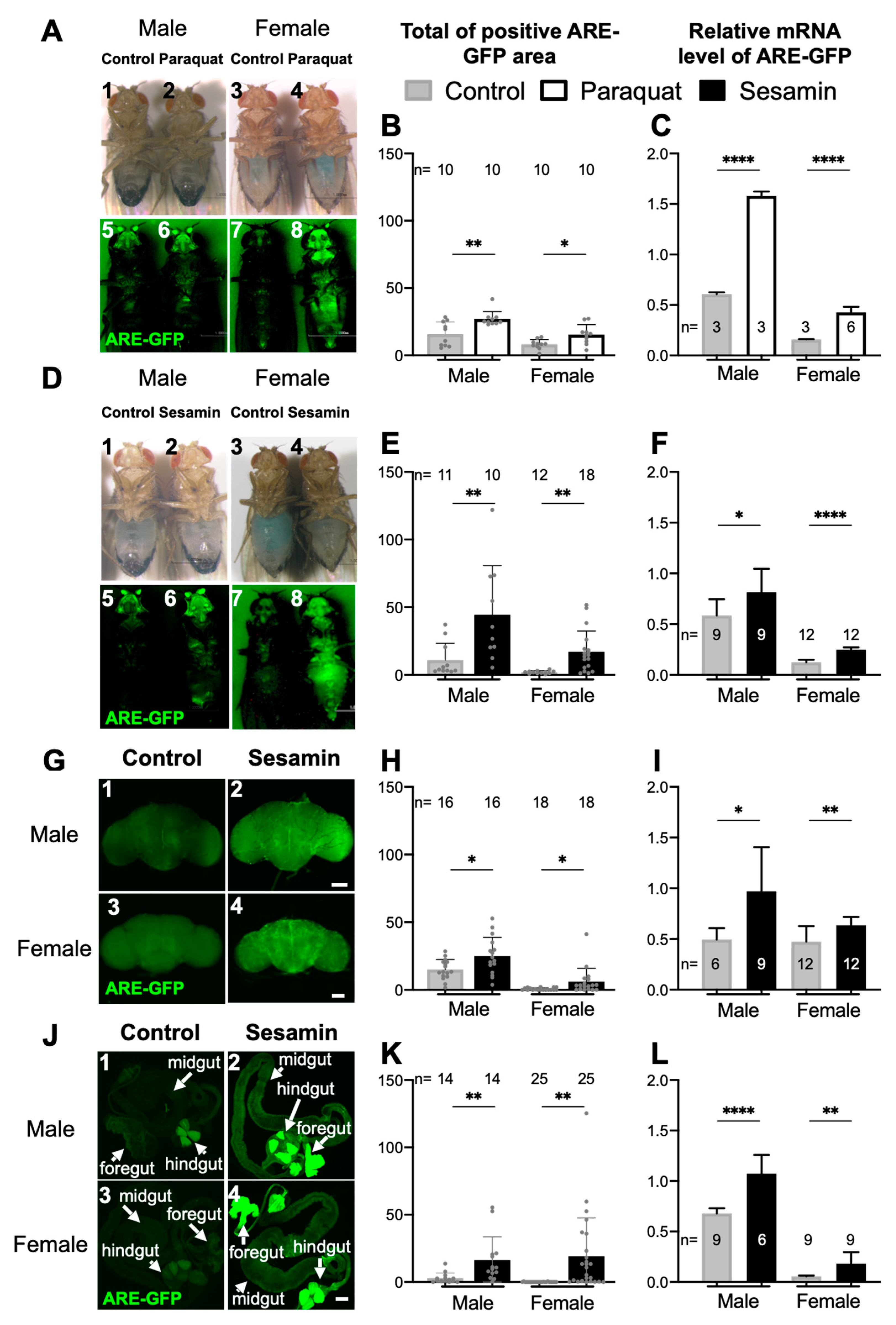
3.1. Sesamin Feeding Stimulated Expression of the ARE-GFP Reporter That Monitors Nrf2/Cnc-Dependent Transcription without External Oxidative Stress
3.2. Sesamin Feeding Stimulated the ARE-GFP Expression in Adult Brain and Gut
3.3. Sesamin Activated the Expression of the ARE-GFP Reporter in Adult Brains in a Nrf2/Cnc-Dependent Manner
3.4. Overexpression of Keap1 Abolished Induction of ARE-GFP Expression by Sesamin
3.5. Sesamin Feeding Stimulated Nrf2-Dependent Transcription in Several Types of Neurons Present in Adult Brains
3.6. Sesamin Suppressed Reduction of Glutamatergic, Cholinergic, and Dopaminergic Neurons Associated with Oxidative Stress Accumulation Induced by Sod1 or Sod2 Depletion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina-Vera, I.; Gómez-De-regil, L.; Gutiérrez-Solis, A.L.; Lugo, R.; Guevara-Cruz, M.; Pedraza-Chaverri, J.; Avila-Nava, A. Dietary strategies by foods with antioxidant effect on nutritional management of dyslipidemias: A systematic review. Antioxidants 2021, 10, 225. [Google Scholar] [CrossRef]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of dietary supplementation of astaxanthin and sesamin on daily fatigue: A randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, D.; Yasutake, Y.; Tomimori, N.; Ono, Y.; Shibata, H.; Hayashi, J. Sesame Lignans and Vitamin E Supplementation Improve Subjective Statuses and Anti-Oxidative Capacity in Healthy Humans with Feelings of Daily Fatigue. Glob. J. Health Sci. 2015, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ghafoorunissa, A.; Hemalatha, S.; Rao, M.V.V. Sesame lignans enhance antioxidant activity of vitamin E in lipid peroxidation systems. Mol. Cell. Biochem. 2004, 262, 195–202. [Google Scholar] [PubMed]
- Li, Z.L.; Gao, M.; Yang, M.S.; Xiao, X.F.; Liu, J.J.; Yang, B.C. Sesamin attenuates intestinal injury in sepsis via the HMGB1/TLR4/IL-33 signalling pathway. Pharm. Biol. 2020, 58, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.N.; Lin, C.S.; De Li, G.; Kuo, C.Y.; Kao, S.H. Sesamin inhibits cervical cancer cell proliferation by promoting p53/pten-mediated apoptosis. Int. J. Med. Sci. 2020, 17, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Kinugawa, S.; Matsushima, S.; Takemoto, D.; Furihata, T.; Mizushima, W.; Fukushima, A.; Yokota, T.; Ono, Y.; Shibata, H.; et al. Sesamin prevents decline in exercise capacity and impairment of skeletal muscle mitochondrial function in mice with high-fat diet-induced diabetes. Exp. Physiol. 2015, 100, 1319–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udomruk, S.; Kaewmool, C.; Phitak, T.; Pothacharoen, P.; Kongtawelert, P. Sesamin Promotes Neurite Outgrowth under Insufficient Nerve Growth Factor Condition in PC12 Cells through ERK1/2 Pathway and SIRT1 Modulation. Evid. Based Complement. Altern. Med. 2020, 2020, 9145458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, B.H.; Al Salam, S.; Al Suleimani, Y.; Al Za’abi, M.; Ashique, M.; Manoj, P.; Sudhadevi, M.; Al Tobi, M.; Nemmar, A. Ameliorative effect of sesamin in cisplatin-induced nephrotoxicity in rats by suppressing inflammation, oxidative/nitrosative stress, and cellular damage. Physiol. Res. 2020, 69, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Wu, P.Y.; Hou, C.W.; Chien, T.Y.; Chang, Q.X.; Wen, K.C.; Lin, C.Y.; Chiang, H.M. Protective effects of sesamin against UVB-induced skin inflammation and photodamage in vitro and in vivo. Biomolecules 2019, 9, 479. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhang, C.-L.; Shen, H.-Q.; Zhou, X.-F.; Li, J.-H.; Yu, J.-L.; An, Q.; Fu, B.-D.; Yi, P.-F. Sesamin protects against DSS-induced colitis in mice by inhibiting NF-κB and MAPK signaling pathways. Food Funct. 2021, 12, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Belur, P.D.; Iyyaswami, R. Use of antioxidants for enhancing oxidative stability of bulk edible oils: A review. Int. J. Food Sci. Technol. 2021, 56, 1–12. [Google Scholar] [CrossRef]
- Hsieh, P.F.; Hou, C.W.; Yao, P.W.; Wu, S.P.; Peng, Y.F.; Shen, M.L.; Lin, C.H.; Chao, Y.Y.; Chang, M.H.; Jeng, K.C. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J. Neuroinflammation 2011, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Le, T.D.; Nakahara, Y.; Ueda, M.; Okumura, K.; Hirai, J.; Sato, Y.; Takemoto, D.; Tomimori, N.; Ono, Y.; Nakai, M.; et al. Sesamin suppresses aging phenotypes in adult muscular and nervous systems and intestines in a Drosophila senescence-accelerated model. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1826–1839. [Google Scholar]
- Zuo, Y.; Peng, C.; Liang, Y.; Ma, K.Y.; Chan, H.Y.E.; Huang, Y.; Chen, Z.Y. Sesamin extends the mean lifespan of fruit flies. Biogerontology 2013, 14, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Yang, Z.; Liu, F.Y.; Jin, Y.G.; Zhang, N.; Ni, J.; Yuan, Y.; Liao, H.H.; Wu, Q.Q.; Xu, M.; et al. Sesamin Protects Against Cardiac Remodeling Via Sirt3/ROS Pathway. Cell. Physiol. Biochem. 2017, 44, 2212–2227. [Google Scholar] [CrossRef]
- Sayhan, M.B.; Oguz, S.; Salt, Ö.; Can, N.; Ozgurtas, T.; Yalta, T.D. Sesamin ameliorates mucosal tissue injury of mesenteric ischemia and reperfusion in an experimental rat model. Arch. Med. Sci. 2019, 15, 1582–1588. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Screening of phenolic compounds in Australian grown berries by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential. Antioxidants 2021, 10, 26. [Google Scholar] [CrossRef]
- Magesh, S.; Chen, Y.; Hu, L. Small Molecule Modulators of Keap1-Nrf2-ARE Pathway as Potential Preventive and Therapeutic Agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.C.; Ji, J.A.; Jiang, Z.Y.; You, Q.D. The Keap1–Nrf2–ARE Pathway as a Potential Preventive and Therapeutic Target: An Update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef]
- Inoue, H.; Kobayashi, K.I.; Ndong, M.; Yamamoto, Y.; Katsumata, S.I.; Suzuki, K.; Uehara, M. Activation of NRF2/KEAP1 signaling and autophagy induction against oxidative stress in heart in iron deficiency. Biosci. Biotechnol. Biochem. 2015, 79, 1366–1368. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Jiang, T.; Liu, H.; Miao, Z.; Fang, D.; Zheng, L.; Zhao, J. Andrographolide protects chondrocytes from oxidative stress injury by activation of the Keap1–Nrf2–Are signaling pathway. J. Cell. Physiol. 2019, 234, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, N.; Bohmann, D. A versatile φC31 based reporter system for measuring AP-1 and NRF2 signaling in Drosophila and in tissue culture. PLoS ONE 2012, 7, e34063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Silva, M.F.; Pruccoli, L.; Morroni, F.; Sita, G.; Seghetti, F.; Viegas, C.; Tarozzi, A. The Keap1/Nrf2-ARE pathway as a pharmacological target for chalcones. Molecules 2018, 23, 1803. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.-H.; Zhang, B.; Fan, Y.; Lin, N.-M. Keap1–Nrf2 pathway: A promising target towards lung cancer prevention and therapeutics. Chronic Dis. Transl. Med. 2015, 1, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, S.; Sou, Y.S.; Uemura, T.; Kametaka, S.; Saito, T.; Ishimura, R.; Kouno, T.; Bedford, L.; Mayer, R.J.; Lee, M.S.; et al. Proteasome dysfunction activates autophagy and the Keap1-Nrf2 pathway. J. Biol. Chem. 2014, 289, 24944–24955. [Google Scholar] [CrossRef] [Green Version]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, L.F.; Schlosser, T.; Manning, S.A.; Parisot, J.P.; Street, I.P.; Richardson, H.E.; Humbert, P.O.; Brumby, A.M. An in vivo large-scale chemical screening platform using Drosophila for anti-cancer drug discovery. Dis. Model. Mech. 2013, 6, 521–529. [Google Scholar] [CrossRef] [Green Version]
- De Lazzari, F.; Sandrelli, F.; Whitworth, A.J.; Bisaglia, M. Antioxidant therapy in Parkinson’s disease: Insights from Drosophila melanogaster. Antioxidants 2020, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Oka, S.; Hirai, J.; Takashi, Y.N.Y.; Inoue, Y.H. A correlation of reactive oxygen species accumulation by depletion of superoxide dismutases with age-dependent impairment in the nervous system and muscles of Drosophila adults. Biogerontology 2015, 16, 485–501. [Google Scholar] [CrossRef]
- Kroeger, D.; Ferrari, L.L.; Petit, G.; Mahoney, C.E.; Fuller, P.M.; Arrigoni, E.; Scammell, T.E. Cholinergic, glutamatergic, and GABAergic neurons of the pedunculopontine tegmental nucleus have distinct effects on sleep/wake behavior in mice. J. Neurosci. 2017, 37, 1352–1366. [Google Scholar] [CrossRef]
- Crevier-Sorbo, G.; Rymar, V.V.; Crevier-Sorbo, R.; Sadikot, A.F. Thalamostriatal degeneration contributes to dystonia and cholinergic interneuron dysfunction in a mouse model of Huntington’s disease. Acta Neuropathol. Commun. 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. The cholinergic system, the adrenergic system and the neuropathology of alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 1273. [Google Scholar] [CrossRef] [PubMed]
- Davoudian, P.A.; Nitabach, M.N. Dopaminergic mechanism underlying reward-encoding of punishment omission during reversal learning in Drosophila. Nat. Commun. 2021, 12, 1115. [Google Scholar]
- Yang, C.H.; Shih, M.F.M.; Chang, C.C.; Chiang, M.H.; Shih, H.W.; Tsai, Y.L.; Chiang, A.S.; Fu, T.F.; Wu, C.L. Additive Expression of Consolidated Memory through Drosophila Mushroom Body Subsets. PLoS Genet. 2016, 12, e1006061. [Google Scholar] [CrossRef]
- Plaçais, P.Y.; De Tredern, É.; Scheunemann, L.; Trannoy, S.; Goguel, V.; Han, K.A.; Isabel, G.; Preat, T. Upregulated energy metabolism in the Drosophila mushroom body is the trigger for long-term memory. Nat. Commun. 2017, 8, 15510. [Google Scholar] [CrossRef] [Green Version]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef]
- Dietzl, G.; Chen, D.; Schnorrer, F.; Su, K.C.; Barinova, Y.; Fellner, M.; Gasser, B.; Kinsey, K.; Oppel, S.; Scheiblauer, S.; et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007, 448, 151–156. [Google Scholar] [CrossRef]
- Fleige, S.; Walf, V.; Huch, S.; Prgomet, C.; Sehm, J.; Pfaffl, M.W. Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR. Biotechnol. Lett. 2006, 28, 1601–1613. [Google Scholar] [CrossRef]
- Kasprzak, D.; Krystkowiak, E.; Stępniak, I.; Galiński, M. Dissolution of cellulose in novel carboxylate-based ionic liquids and dimethyl sulfoxide mixed solvents. Eur. Polym. J. 2019, 113, 89–97. [Google Scholar] [CrossRef]
- Kong, P.; Chen, G.; Jiang, A.; Wang, Y.; Song, C.; Zhuang, J.; Xi, C.; Wang, G.; Ji, Y.; Yan, J. Sesamin inhibits IL-1β-stimulated inflammatory response in human osteoarthritis chondrocytes by activating Nrf2 signaling pathway. Oncotarget 2016, 7, 83720–83726. [Google Scholar] [CrossRef] [Green Version]
- Hamada, N.; Tanaka, A.; Fujita, Y.; Itoh, T.; Ono, Y.; Kitagawa, Y.; Tomimori, N.; Kiso, Y.; Akao, Y.; Nozawa, Y.; et al. Bioorganic & Medicinal Chemistry Involvement of heme oxygenase-1 induction via Nrf2/ARE activation in protection against H2O2-induced PC12 cell death by a metabolite of sesamin contained in sesame seeds. Bioorg. Med. Chem. 2011, 19, 1959–1965. [Google Scholar]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 Signaling Regulates Oxidative Stress Tolerance and Lifespan in Drosophila. Dev. Cell 2008, 14, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capo, F.; Wilson, A.; Di Cara, F. The intestine of Drosophila melanogaster: An emerging versatile model system to study intestinal epithelial homeostasis and host-microbial interactions in humans. Microorganisms 2019, 7, 336. [Google Scholar] [CrossRef] [Green Version]
- Micchelli, C.A.; Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 2006, 439, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Hindle, S.J.; Bainton, R.J. Barrier mechanisms in the Drosophila blood-brain barrier. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scopelliti, A.; Bauer, C.; Yu, Y.; Zhang, T.; Kruspig, B.; Murphy, D.J.; Vidal, M.; Maddocks, O.D.K.; Cordero, J.B. A Neuronal Relay Mediates a Nutrient Responsive Gut/Fat Body Axis Regulating Energy Homeostasis in Adult Drosophila. Cell Metab. 2019, 29, 269–284. [Google Scholar] [CrossRef] [Green Version]
- Todorovic, M.; Wood, S.A.; Mellick, G.D. Nrf2: A modulator of Parkinson’s disease? J. Neural Transm. 2016, 123, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.C.; Sykiotis, G.P.; Bohmann, D. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson’s disease. Dis. Model. Mech. 2011, 4, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.H.; Basil, A.H.; Hang, L.; Tan, R.; Goh, K.L.; O’Neill, S.; Zhang, X.; Yu, F.; Lim, K.L. Genetic or pharmacological activation of the Drosophila PGC-1α ortholog spargel rescues the disease phenotypes of genetic models of Parkinson’s disease. Neurobiol. Aging 2017, 55, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Baghdoyan, H.A.; Lydic, R. Neuropharmacology of sleep and wakefulness. Sleep Med. Clin. 2010, 5, 513–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palavicino-Maggio, C.B.; Chan, Y.B.; McKellar, C.; Kravitz, E.A. A small number of cholinergic neurons mediate hyperaggression in female Drosophila. Proc. Natl. Acad. Sci. USA 2019, 116, 17029–17038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuno, M.; Horiuchi, J.; Ofusa, K.; Masuda, T.; Saitoe, M. Inhibiting Glutamate Activity during Consolidation Suppresses Age-Related Long-Term Memory Impairment in Drosophila. iScience 2019, 15, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Masuda-Nakagawa, L.M.; Ito, K.; Awasaki, T.; O’Kane, C.J. A single GABAergic neuron mediates feedback of odor-evoked signals in the mushroom body of larval Drosophila. Front. Neural Circuits 2014, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Cheung, S.K.; Scott, K. GABAA receptor-expressing neurons promote consumption in Drosophila melanogaster. PLoS ONE 2017, 12, e0175177. [Google Scholar] [CrossRef] [Green Version]
- Aso, Y.; Hattori, D.; Yu, Y.; Johnston, R.M.; Iyer, N.A.; Ngo, T.T.B.; Dionne, H.; Abbott, L.F.; Axel, R.; Tanimoto, H.; et al. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 2014, 3, e04577. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Breda, C.; Robinson, S.; Giorgini, F.; Steinert, J.R. Drosophila Nrf2/Keap1 mediated redox signaling supports synaptic function and longevity and impacts on circadian activity. Front. Mol. Neurosci. 2019, 12, 86. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.D.; Inoue, Y.H. Sesamin Activates Nrf2/Cnc-Dependent Transcription in the Absence of Oxidative Stress in Drosophila Adult Brains. Antioxidants 2021, 10, 924. https://doi.org/10.3390/antiox10060924
Le TD, Inoue YH. Sesamin Activates Nrf2/Cnc-Dependent Transcription in the Absence of Oxidative Stress in Drosophila Adult Brains. Antioxidants. 2021; 10(6):924. https://doi.org/10.3390/antiox10060924
Chicago/Turabian StyleLe, Tuan Dat, and Yoshihiro H. Inoue. 2021. "Sesamin Activates Nrf2/Cnc-Dependent Transcription in the Absence of Oxidative Stress in Drosophila Adult Brains" Antioxidants 10, no. 6: 924. https://doi.org/10.3390/antiox10060924





