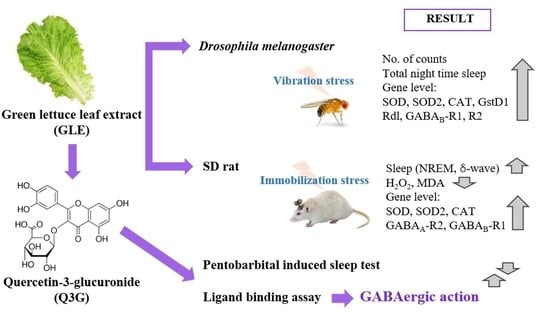
Effects of Green Lettuce Leaf Extract on Sleep Disturbance Control in Oxidative Stress-Induced Invertebrate and Vertebrate Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Extracts
2.2. Drosophila Melanogaster Stocks
2.3. Vibration Stress in Drosophila Melanogaster
2.4. Behavioral Tests in Drosophila Melanogaster
2.5. Animals
2.6. Immobilization Stress Procedure in Rats
2.7. EEG Recordings and Analysis in Rats
2.8. Measurement of Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA)
2.9. mRNA Expression of Oxidative-Related Factor and Neurotransmitter Receptors
2.10. Analysis of Polyphenols Contained in GLE
2.11. Pentobarbital-Induced Sleep Test
2.12. GABAA-BDZ Receptor-Binding Assay
2.13. Statistical Analysis
3. Results
3.1. Effects of Green Lettuce Leaf Extract (GLE) on Locomotor Activity in Vibration-Stressed Drosophila Melanogaster
3.2. Effects of Green Lettuce Leaf Extract (GLE) on Brain Receptor Expression in Vibration-Stressed Drosophila Melanogaster
3.3. Effect of Green Lettuce Leaf Extract (GLE) on Sleep Architecture Changed by Immobilization Stress in Rats
3.4. Effect of Green Lettuce Leaf Extract (GLE) on ROS Production by Immobilization Stress in Rats
3.5. Effects of Green Lettuce Leaf Extract (GLE) on Brain Receptor Expression by Immobilization Stress in Rats
3.6. Analysis of Green Lettuce Leaf Extract (GLE)
3.7. Effects of Green Lettuce Leaf Extract (GLE), Chlorogenic Acid, Scutellarin, and Q3G-Mediated Sleep Behavior in a Pentobarbital-Induced Sleep Mouse Model
3.8. GABAA-BDZ Receptor-Binding Activity of Green Lettuce Leaf Extract (GLE), Chlorogenic Acid, Scutellarin, and Q3G
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faludi, B.; Rozgonyi, R. Pharmacological and nonpharmacological treatment of insomnias with regard to sleep medicine. Ideggyogy. Szle. 2018, 71, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, F.; Salim, S. Sleep deprivation, oxidative stress and inflammation. Adv. Protein Chem. Struct. Biol. 2020, 119, 309–336. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, G.; Miguel-Puga, A.; Rodriguez, E.M.; Machado, S.; Manjarrez, E.; Arias-Carrion, O. Sleep deprivation and oxidative stress in animal models: A systematic review. Oxid. Med. Cell. Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, L.; Kalesan, B. Sleep duration and mortality: A systematic review and meta-analysis. J. Sleep Res. 2009, 18, 148–158. [Google Scholar] [CrossRef]
- Ramanathan, L.; Gulyani, S.; Nienhuis, R.; Siegel, J.M. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport 2002, 13, 1387–1390. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.H.; Abilio, V.C.; Takatsu, A.L.; Kameda, S.R.; Grassl, C.; Chehin, A.B.; Medrano, W.A.; Calzavara, M.B.; Registro, S.; Andersen, M.L.; et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology 2004, 46, 895–903. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Ji, L.L.; Cirelli, C. Sleep deprivation and cellular responses to oxidative stress. Sleep 2004, 27, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Schrire, Z.M.; Phillips, C.L.; Duffy, S.L.; Marshall, N.S.; Mowszowski, L.; La Monica, H.M.; Gordon, C.J.; Chapman, J.L.; Saini, B.; Lewis, S.J. Feasibility of 3-month melatonin supplementation for brain oxidative stress and sleep in mild cognitive impairment: Protocol for a randomised, placebo-controlled study. BMJ Open 2021, 11, e041500. [Google Scholar] [CrossRef]
- Harsha, S.N.; Anilakumar, K.R. Anxiolytic property of Lactuca sativa, effect on anxiety behaviour induced by novel food and height. Asian Pac. J. Trop. Med. 2013, 6, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Ismail, H.; Dilshad, E.; Waheed, M.T.; Mirza, B. Transformation of lettuce with rol ABS genes: Extracts show enhanced antioxidant, analgesic, anti-inflammatory, antidepressant, and anticoagulant activities in rats. Appl. Biochem. Biotechnol. 2017, 181, 1179–1198. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce vatieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Kim, H.W.; Suh, H.J.; Choi, H.S.; Hong, K.B.; Jo, K. Effectiveness of the sleep enhancement by green romaine lettuce (Lactuca sativa) in a rodent model. Biol. Pharm. Bull. 2019, 42, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hong, K.B.; Kim, S.; Suh, H.J.; Jo, K. Creatine and taurine mixtures alleviate depressive-like behaviour in Drosophila melanogaster and mice via regulating Akt and ERK/BDNF pathways. Sci. Rep. 2020, 10, 11370. [Google Scholar] [CrossRef]
- Jo, K.; Choi, H.S.; Jeon, S.; Ahn, C.W.; Suh, H.J. Nelumbo nucifera seed extract promotes sleep in Drosophila melanogaster. Biol. Pharm. Bull. 2018, 41, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegde, P.; Singh, K.; Chaplot, S.; Shankaranarayana Rao, B.S.; Chattarji, S.; Kutty, B.M.; Laxmi, T.R. Stress-induced changes in sleep and associated neuronal activity in rat hippocampus and amygdala. Neuroscience 2008, 153, 20–30. [Google Scholar] [CrossRef]
- Jo, K.; Suh, H.J.; Choi, H.S. Polygonatum sibiricum rhizome promotes sleep by regulating non-rapid eye movement and GABAergic/serotonergic receptors in rodent models. Biomed. Pharmacother. 2018, 105, 167–175. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, Y.C.; Han, K.S.; Singh, H.; Yoon, M.; Park, J.H.; Cho, C.W.; Cho, S. Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 2013, 136, 160–163. [Google Scholar] [CrossRef]
- Roth, T.; Seiden, D.; Sainati, S.; Wang-Weigand, S.; Zhang, J.; Zee, P. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006, 7, 312–318. [Google Scholar] [CrossRef]
- Fang, X.; Hao, J.F.; Zhou, H.Y.; Zhu, L.X.; Wang, J.H.; Song, F.Q. Pharmacological studies on the sedative-hypnotic effect of Semen Ziziphi spinosae (Suanzaoren) and Radix et Rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine 2010, 17, 75–80. [Google Scholar] [CrossRef]
- Choi, H.S.; Hong, K.B.; Han, S.H.; Suh, H.J. Valerian/Cascade mixture promotes sleep by increasing non-rapid eye movement (NREM) in rodent model. Biomed. Pharmacother. 2018, 99, 913–920. [Google Scholar] [CrossRef]
- Jo, K.; Kim, S.; Hong, K.B.; Suh, H.J. Nelumbo nucifera promotes non-rapid eye movement sleep by regulating GABAergic receptors in rat model. J. Ethnopharmacol. 2021, 267, 113511. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, J.W.; Ahn, Y.-J.; Kwon, H.W. Neuroprotective properties of phytochemicals against paraquat-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Pestic. Biochem. Phys. 2012, 104, 118–125. [Google Scholar] [CrossRef]
- Sharifian, F.E.; Bahrami, F.; Yeganegi, H.; Afra, M.G. Alteration in REM sleep and sleep spindles’ characteristics by a model of immobilization stress in rat. Sleep Biol. Rhythms 2020, 18, 233–241. [Google Scholar] [CrossRef]
- Dewasmes, G.; Loos, N.; Delanaud, S.; Dewasmes, D.; Ramadan, W. Pattern of rapid-eye movement sleep episode occurrence after an immobilization stress in the rat. Neurosci. Lett. 2004, 355, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Hoang Do, J.P.; Chung, S.; Beier, K.T.; Bikov, M.; Saffari Doost, M.; Dan, Y. Regulation of REM and non-REM sleep by periaqueductal GABAergic neurons. Nat. Commun. 2018, 9, 354. [Google Scholar] [CrossRef] [Green Version]
- Busek, P.; Vankova, J.; Opavsky, J.; Salinger, J.; Nevsimalova, S. Spectral analysis of the heart rate variability in sleep. Physiol. Res. 2005, 54, 369–376. [Google Scholar]
- Imeri, L.; Opp, M.R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009, 10, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef]
- Ismail, A.; Marjan, Z.M.; Foong, C.W. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Agbor, G.A.; Oben, J.E.; Ngogang, J.Y.; Xinxing, C.; Vinson, J.A. Antioxidant capacity of some herbs/spices from Cameroon: A comparative study of two methods. J. Agric. Food Chem. 2005, 53, 6819–6824. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.A.; Bennett, M.H.; Lewis, M.J.; Mansfield, J.W.; Beale, M.H. Metabolite profiling of sesquiterpene lactones from Lactuca species: Major latex components are novel oxalate and sulfate conjugates of lactucin and its derivatives. J. Biol. Chem. 2000, 275, 26877–26884. [Google Scholar] [CrossRef]
- Chu, Y.F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef]
- Mulabagal, V.; Ngouajio, M.; Nair, A.; Zhang, Y.; Gottumukkala, A.L.; Nair, M.G. In vitro evaluation of red and green lettuce (Lactuca sativa) for functional food properties. Food Chem. 2010, 118, 300–306. [Google Scholar] [CrossRef]
- Gromek, D.; Kisiel, W.; Klodzińska, A.; Chojnacka-Wójcik, E. Biologically active preparations from Lactuca virosa L. Phytother. Res. 1992, 6, 285–287. [Google Scholar] [CrossRef]
- Park, I.; Ochiai, R.; Ogata, H.; Kayaba, M.; Hari, S.; Hibi, M.; Katsuragi, Y.; Satoh, M.; Tokuyama, K. Effects of subacute ingestion of chlorogenic acids on sleep architecture and energy metabolism through activity of the autonomic nervous system: A randomised, placebo-controlled, double-blinded cross-over trial. Br. J. Nutr. 2017, 117, 979–984. [Google Scholar] [CrossRef] [Green Version]
- Jager, A.K.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471–1485. [Google Scholar] [CrossRef] [Green Version]
- You, Y.X.; Shahar, S.; Haron, H.; Yahya, H.M.; Din, N.C. Relationship between traditional Malaysian vegetables (Ulam) intake and cognitive status among middle-aged adults from low cost residential areas. JSKM 2020, 17. [Google Scholar] [CrossRef]
- Tang, H.; Tang, Y.; Li, N.G.; Lin, H.; Li, W.; Shi, Q.; Zhang, W.; Zhang, P.; Dong, Z.; Shen, M.; et al. Comparative metabolomic analysis of the neuroprotective effects of scutellarin and scutellarein against ischemic insult. PLoS ONE 2015, 10, e0131569. [Google Scholar] [CrossRef]
- Cheng, S.H.; Ismail, A.; Anthony, J.; Ng, O.C.; Hamid, A.A.; Barakatun-Nisak, M.Y. Eight weeks of cosmos caudatus (Ulam raja) supplementation improves glycemic status in patients with type 2 diabetes: A randomized controlled trial. Evid-Based Complement. 2015, 2015, 405615. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Ambrogini, P.; Del Grande, P.; Ninfali, P. Enhancement of flavonoid ability to cross the blood-brain barrier of rats by co-administration with α-tocopherol. Food Funct. 2015, 6, 394–400. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.M.; Ferrari, P.F.; Parmigiani, S.; Miczek, K.A. Escalated aggressive behavior: Dopamine, serotonin and GABA. Eur. J. Pharmacol. 2005, 526, 51–64. [Google Scholar] [CrossRef]
- Thompson, J.M.; Pappu, A.; Pandhare, A.; Jansen, M. Complex modulation of the GABAA α1β2γ2 receptor function by bupropion. Biophys. J. 2015, 108, 433a. [Google Scholar] [CrossRef] [Green Version]
- Kopp, C.; Rudolph, U.; Löw, K.; Tobler, I. Modulation of rhythmic brain activity by diazepam: GABAA receptor subtype and state specificity. Proc. Natl. Acad. Sci. USA 2004, 101, 3674–3679. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Yang, H.; Jeon, Y.J.; Lee, C.J.; Jin, Y.H.; Baek, N.I.; Kim, D.; Kang, S.M.; Yoon, M.; Yong, H.; et al. Phlorotannins of the edible brown seaweed Ecklonia cava Kjellman induce sleep via positive allosteric modulation of gamma-aminobutyric acid type A-benzodiazepine receptor: A novel neurological activity of seaweed polyphenols. Food Chem. 2012, 132, 1133–1142. [Google Scholar] [CrossRef]
- Schramm, A.; Ebrahimi, S.N.; Raith, M.; Zaugg, J.; Rueda, D.C.; Hering, S.; Hamburger, M. Phytochemical profiling of Curcuma kwangsiensis rhizome extract, and identification of labdane diterpenoids as positive GABAA receptor modulators. Phytochemistry 2013, 96, 318–329. [Google Scholar] [CrossRef]
- Komaki, A.; Khaledi Nasab, Z.; Shahidi, S.; Sarihi, A.; Salehi, I.; Ghaderi, A. Anxiolytic effects of acute injection of hydro-alcoholic extract of lettuce in the elevated plus-maze task in rats. Avicenna J. Neuro Psycho Physiol. 2014, 1, 14–19. [Google Scholar] [CrossRef]







| Sample (Final Concentration, mg/mL) | 0.1 | 1 | 10 |
|---|---|---|---|
| Green lettuce leaf extract | 1.36 ± 0.14 | 11.90 ± 1.35 | 16.17 ± 2.13 |
| Chlorogenic acid | n.d. | n.d. | n.d. |
| Scutellarin | n.d. | n.d. | n.d. |
| Q3G | 16.58 ± 0.39 | 51.11 ± 0.34 | 88.13 ± 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, K.; Kim, S.; Ahn, Y.; Suh, H.J. Effects of Green Lettuce Leaf Extract on Sleep Disturbance Control in Oxidative Stress-Induced Invertebrate and Vertebrate Models. Antioxidants 2021, 10, 970. https://doi.org/10.3390/antiox10060970
Jo K, Kim S, Ahn Y, Suh HJ. Effects of Green Lettuce Leaf Extract on Sleep Disturbance Control in Oxidative Stress-Induced Invertebrate and Vertebrate Models. Antioxidants. 2021; 10(6):970. https://doi.org/10.3390/antiox10060970
Chicago/Turabian StyleJo, Kyungae, Singeun Kim, Yejin Ahn, and Hyung Joo Suh. 2021. "Effects of Green Lettuce Leaf Extract on Sleep Disturbance Control in Oxidative Stress-Induced Invertebrate and Vertebrate Models" Antioxidants 10, no. 6: 970. https://doi.org/10.3390/antiox10060970
APA StyleJo, K., Kim, S., Ahn, Y., & Suh, H. J. (2021). Effects of Green Lettuce Leaf Extract on Sleep Disturbance Control in Oxidative Stress-Induced Invertebrate and Vertebrate Models. Antioxidants, 10(6), 970. https://doi.org/10.3390/antiox10060970