Curcuma longa L. Water Extract Improves Dexamethasone-Induced Sarcopenia by Modulating the Muscle-Related Gene and Oxidative Stress in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Chemicals
2.2. Animal Experiments
2.3. Ladder Climbing Exercise
2.4. Grip Strength
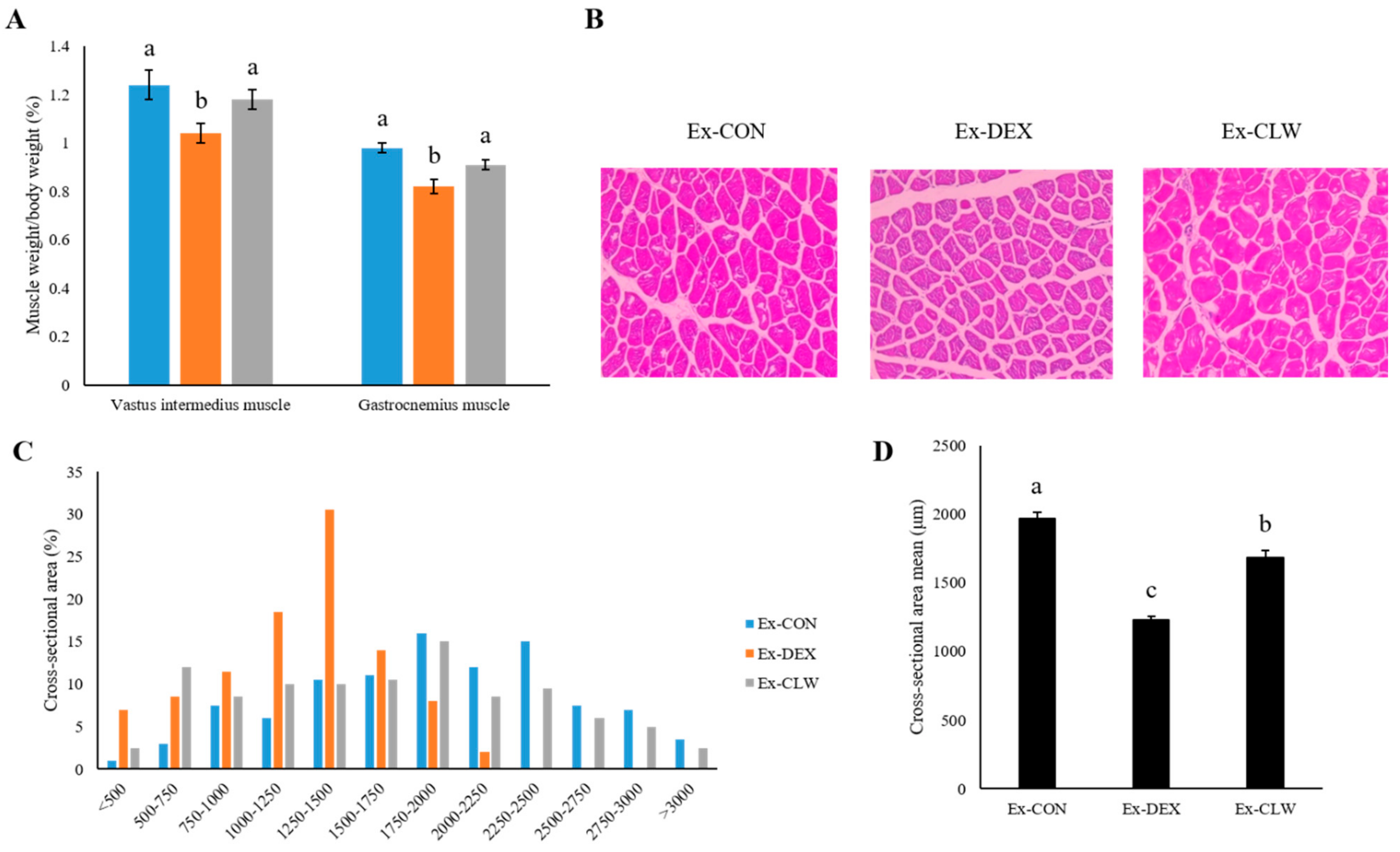
2.5. Evaluation of Muscle Histopathology
2.6. Real-Time Polymerase Chain Reaction
2.7. Western Blotting
2.8. Antioxidant Enzyme Activity
2.9. Statistical Analysis
3. Results
3.1. Effect of CLW on Grip Strength
3.2. Effect of CLW on Muscle Weight
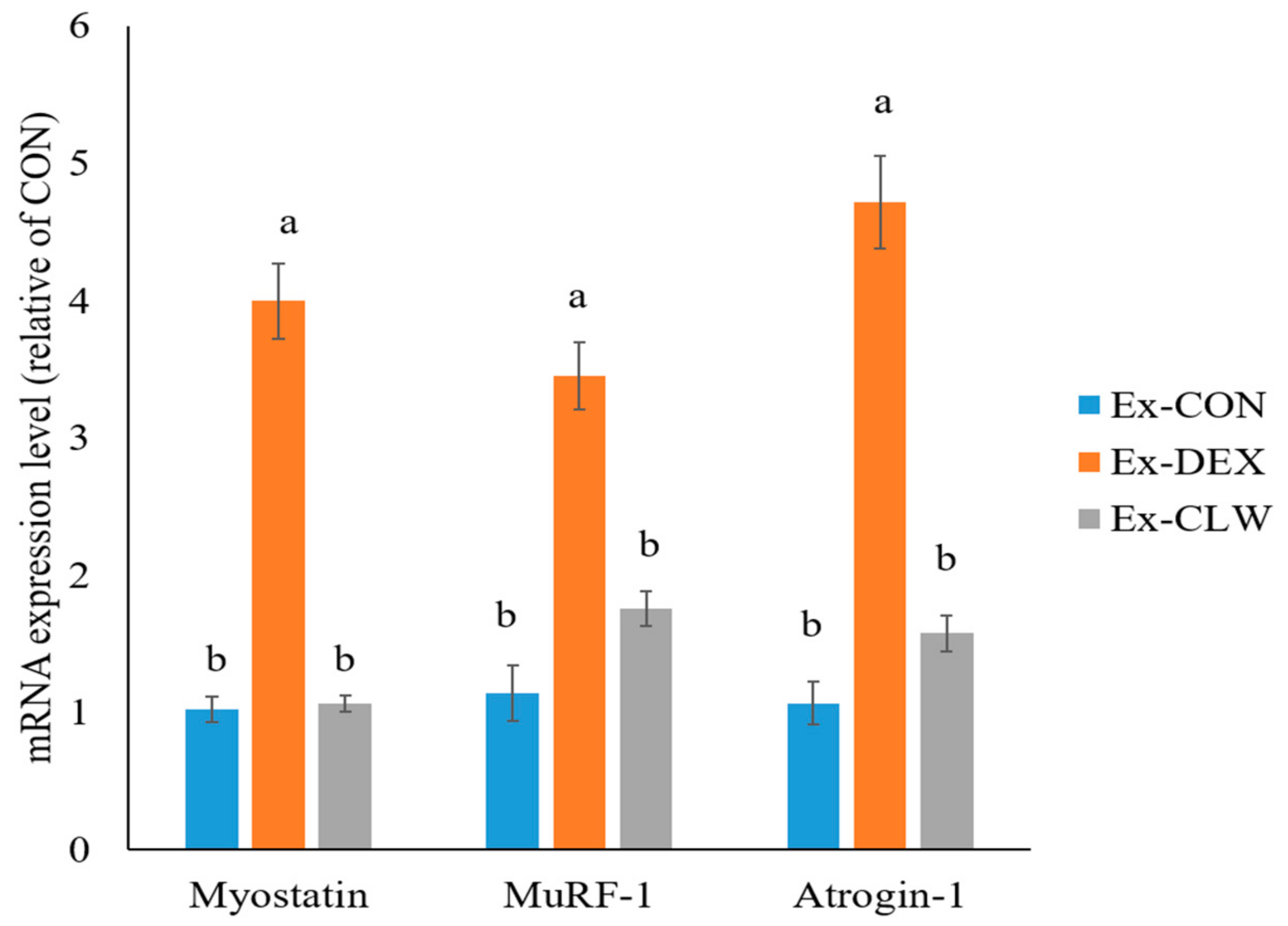
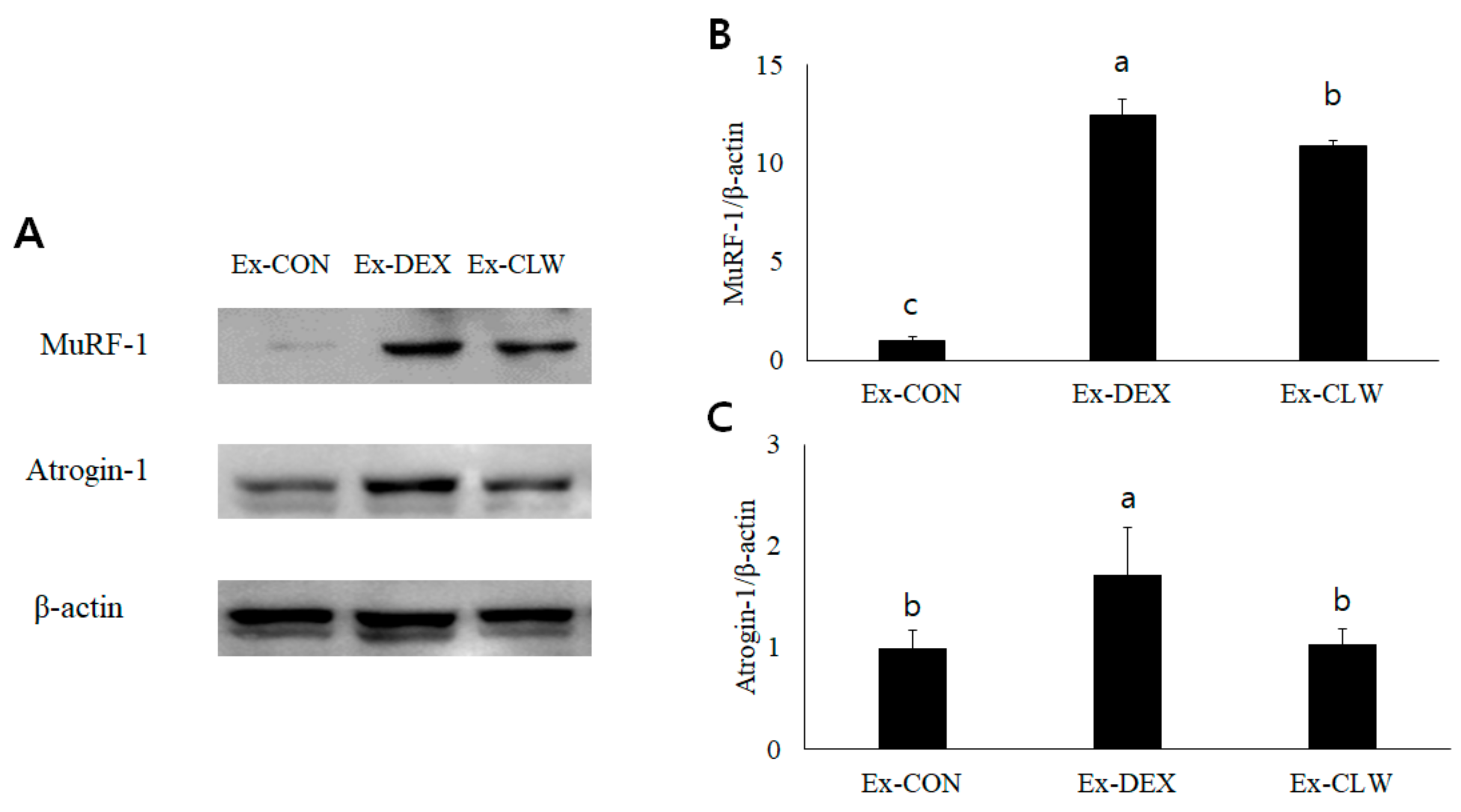
3.3. Effect of CLW on Muscle Atrophy-Related mRNA and Protein Expression
3.4. Effect of CLW on Oxidative Stress in Muscle
4. Discussion
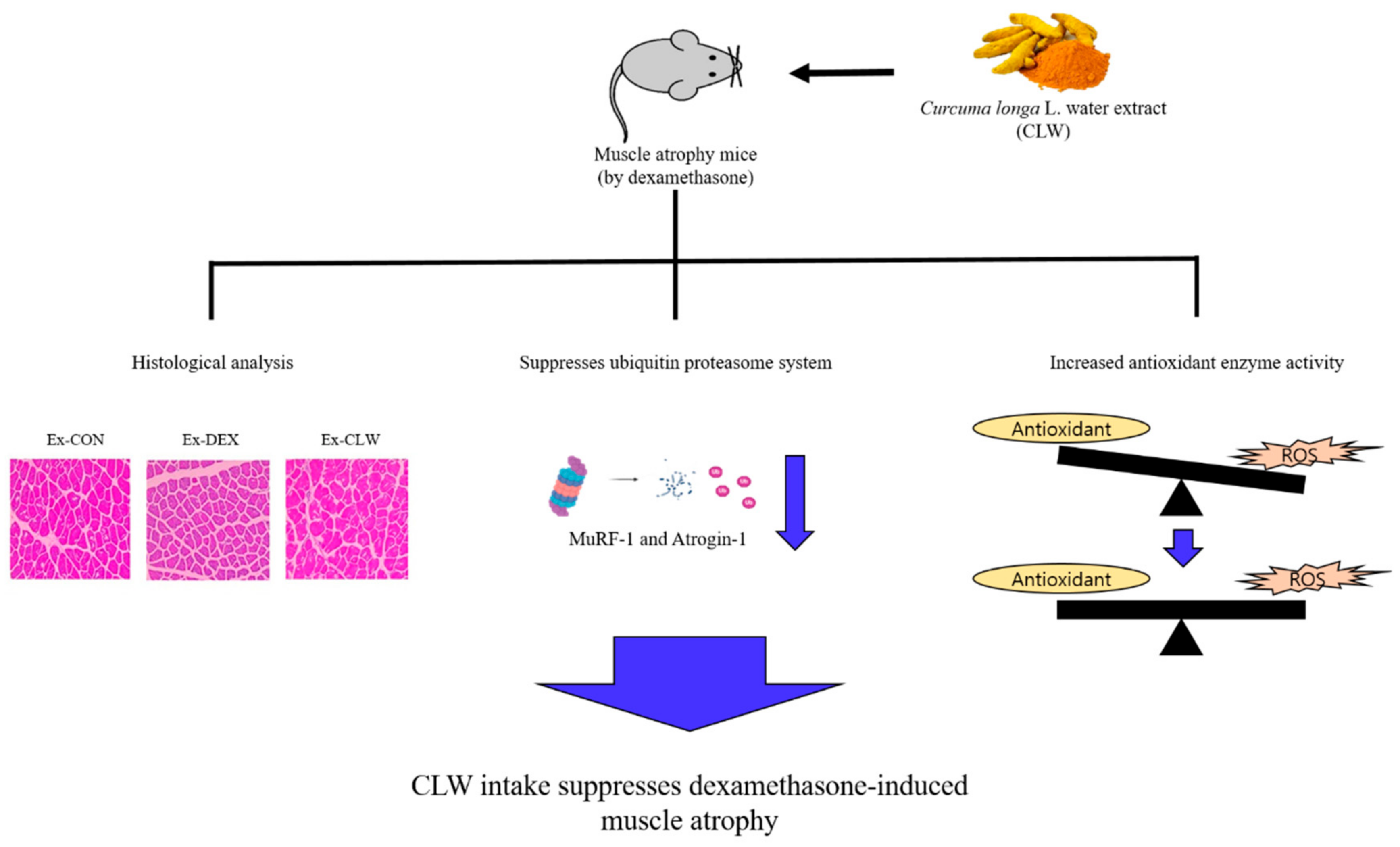
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leitner, L.M.; Wilson, R.J.; Yan, Z.; Godecke, A. Reactive oxygen species/nitric oxide mediated inter-organ communication in skeletal muscle wasting diseases. Antioxid. Redox Signal. 2017, 26, 700–717. [Google Scholar] [CrossRef]
- Yamamoto, D.; Maki, T.; Herningtyas, E.H.; Ikeshita, N.; Shibahara, H.; Sugiyzma, Y.; Nakanishi, S.; Iida, K.; Iguchi, G.; Takahashi, Y.; et al. Branched-Chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle Nerve 2010, 41, 819–827. [Google Scholar] [CrossRef]
- Rom, O.; Reznick, A.Z. The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Radic. Biol. Med. 2016, 98, 218–230. [Google Scholar] [CrossRef]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Morton, A.B.; Ahn, B.; Smuder, A.J. Free radical biology and medicine redox control of skeletal muscle atrophy. Free Radic. Biol. Med. 2016, 98, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Theilen, N.T.; Kunkel, G.H.; Tyagi, S.C. The role of exercise and TFAM in preventing skeletal muscle atrophy. J. Cell. Physiol. 2017, 232, 2348–2358. [Google Scholar] [CrossRef]
- Mittal, A.; Bhatnagar, S.; Kumar, A.; Lach-Trifillieff, E.; Wauters, S.; Li, H.; Makonchuk, D.Y.; Glass, D.J.; Kumar, A. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J. Cell Biol. 2010, 188, 833–849. [Google Scholar] [CrossRef] [Green Version]
- Paul, P.K.; Bhatnagar, S.; Mishra, V.; Srivastava, S.; Darnay, B.G.; Choi, Y.; Kumar, A. The E3 ubiquitin ligase TRAF6 intercedes in starvation-induced skeletal muscle atrophy through multiple mechanisms. Mol. Cell. Biol. 2012, 32, 1248–1259. [Google Scholar] [CrossRef] [Green Version]
- Tryon, L.D.; Vainshtein, A.; Memme, J.M.; Crilly, M.J.; Hood, D.A. Recent advances in mitochondrial turnover during chronic muscle disuse. Integr. Med. Res. 2014, 3, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Krug, A.L.O.; Macedo, A.G.; Zago, A.S.; Rush, J.W.E.; Santos, C.F.; Amaral, S.L. High-Intensity resistance training attenuates dexamethasone-induced muscle atrophy. Muscle Nerve 2016, 53, 779–788. [Google Scholar] [CrossRef]
- Marcedo, A.G.; Krug, A.L.; Souza, L.M.; Martuscelli, A.M.; Constantino, P.B.; Zago, A.S.; Rush, J.W.E.; Santos, C.F.; Amaral, S.L. Time-Course changes of catabolic proteins following muscle atrophy induced by dexamethasone. Steroids 2016, 107, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Ku, S.K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of Fructus schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Noh, K.K.; Chung, K.W.; Choi, Y.J.; Park, M.H.; Jang, E.J.; Park, C.H.; Yoon, C.; Kim, N.D.; Kim, M.K.; Chung, H.Y. β-Hydroxy β-methylbutyrate improves dexamethasone-induced muscle atrophy by modulating the muscle degradation pathway in SD rat. PLoS ONE 2014, 9, e102947. [Google Scholar] [CrossRef]
- Qin, J.; Du, R.; Yang, Y.; Zhang, H.; Li, Q.; Liu, L.; Guan, H.; Hou, J.; An, X. Dexamethasone-Induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res. Vet. Sci. 2013, 94, 84–89. [Google Scholar] [CrossRef]
- Margolis, L.M.; Pasiakos, S.M. Optimizing intramuscular adaptations to aerobic exercise: Effects of carbohydrate restriction and protein supplementation on mitochondrial biogenesis. Adv. Nutr. 2013, 4, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Murton, A.J. Muscle protein turnover in the elderly and its potential contribution to the development of sarcopenia. Proc. Nutr. Soc. 2015, 74, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Porter, M.M.; Vandervoort, A.A.; Lexell, J. Aging of human muscle: Structure, function and adaptability. Scand. J. Med. Sci. Sports 1995, 5, 129–142. [Google Scholar] [CrossRef]
- Choi, E.J.; So, W.Y. Effects of resistance exercise intensity on cytokine and chemokine gene expression in atopic dermatitis mouse model. J. Mens Health 2018, 14, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Nicastro, H.; Zanchi, N.E.; Luz, C.R.; Moraes, W.M.A.M.; Ramona, P.; Filho, M.A.S.; Chaves, D.F.S.; Medeiros, A.; Brum, P.C.; Dardevet, D.; et al. Effects of leucine supplementation and resistance exercise on dexamethasone-induced muscle atrophy and insulin resistance in rats. Nutrition 2012, 28, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Labban, L. Medicinal and pharmacological properties of Turmeric (Curcuma longa): A review. Int. J. Pharm. Biomed. Res. 2014, 5, 17–23. [Google Scholar]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; EI-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [Green Version]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: A systematic review and meta-analysis of randomized clinical trials. J. Med. Food 2016, 19, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.M.D. Anticancer potential of curcumin (review). Anticancer Res. 2003, 398, 363–398. [Google Scholar]
- Araújo, C.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef]
- Kim, D.H.; Phillips, J.F.; Lockey, R.F. Oral curcumin supplementation in patients with atopic asthma. Allergy Rhinol. 2011, 2, e51–e53. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, P.; Khan, Z.; Chakraverty, N. Soluble curcumin: A promising oral supplement for health management. J. Appl. Pharm. Sci. 2011, 1, 1–7. [Google Scholar]
- Hewlings, S.; Kalman, D. Curcumin: A review of its effects on human health. Foods 2017, 6, 92–103. [Google Scholar] [CrossRef]
- Hamidie, R.D.R.; Yamada, T.; Ishizawa, R.; Saito, Y.; Masuda, K. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism 2015, 64, 1334–1347. [Google Scholar] [CrossRef]
- Huei-Chen, H.; Tong-Rong, J.; Sheau-Farn, Y. Inhibitory effect of curcumin, an anti-inflammatory agent, on vascular smooth muscle cell proliferation. Eur. J. Pharmacol. 1992, 221, 381–384. [Google Scholar] [CrossRef]
- Peterson, C.M.; Johannsen, D.L.; Ravussin, E. Skeletal muscle mitochondria and aging: A review. J. Aging Res. 2012, e194821. [Google Scholar] [CrossRef] [Green Version]
- Hood, D.A.; Uguccioni, G.; Vainshtein, A.; D’souza, D. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: Implications for health and disease. Compr. Physiol. 2011, 1, 1119–1134. [Google Scholar]
- Tidball, J.G. Mechanisms of muscle injury, repair, and regeneration. Compr. Physiol. 2011, 1, 2029–2062. [Google Scholar]
- Ukeda, H.; Kawana, D.; Maeda, S.; Sawamura, M. Spectrophotometric assay for superoxide dismutase based on the reduction of highly water-soluble tetrazolium salts by xanthine-xanthine oxidase. Biosci. Biotechnol. Biochem. 1999, 63, 485–488. [Google Scholar] [CrossRef]
- Aebi, H. Oxygen radicals in biological systems. Methods Enzymol. 1947, 105, 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef]
- Antonio, S.R.D.; Heloisa, M.B.; Roney, L.M.; Paulo, L.; Manoel, B.B.; Norma, T.F.; Ayrton, C.M.; Margaret, C. Effects of dexamethasone on lymphocyte proliferation and cytokine production in rheumatoid arthritis. J. Rheumatol. 2002, 29, 46–51. [Google Scholar]
- Qureshi, F.; Zaritsky, A.; Poirier, M.P. Comparative efficacy of oral dexamethasone versus oral prednisone in acute pediatric asthma. J. Pediatr. 2001, 139, 20–26. [Google Scholar] [CrossRef]
- Boumpas, D.T.; Chrousos, G.P.; Wilder, R.L.; Cupps, T.R.; Balow, J.E. Glucocorticoid therapy for immune-mediated diseases: Basic and clinical correlates. Ann. Intern. Med. 1993, 119, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Longui, C.A. Glucocorticoid therapy: Minimizing side effects. J. Pediatr. 2007, 83, 163–177. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Yang, X.; Wang, R.X.; Jiao, H.O.; Zhao, J.P.; Song, Z.G.; Lin, H. Leucine alleviates dexamethasone-induced suppression of muscle protein synthesis via synergy involvement of mTOR and AMPK pathways. Biosci. Rep. 2016, 36, e00346. [Google Scholar] [CrossRef] [Green Version]
- Pikir, B.S.; Subagjo, A.; Wardhani, D.E.; Oktaviono, Y.H.; Nugraha, R.A. Comparison of carotid intima media thickness between women with history of preeclampsia and normal pregnancy: A meta-analysis of systematic review. medRxiv 2021, e228825. [Google Scholar] [CrossRef]
- Neto, W.K.; Silva, W.D.A.; Ciena, A.P.; Anaruma, C.A.; Gama, E.F. Vertical climbing for rodent resistance training : A discussion about training parameters. Int. J. Sports Sci. 2016, 6, 36–49. [Google Scholar]
- Zimmerman, M.; Pfohl, B.M.; Coryell, W.H. Appetite and weight change and the dexamethasone suppression test. Biol. Psychiatry 1984, 19, 923–928. [Google Scholar]
- Edelstein, C.K.; Roy Byrne, B.; Fawzy, F.I.; Dornfeld, L. Effects of weight loss on the dexamethasone suppression test. Am. J. Psychiatry 1983, 140, 338–341. [Google Scholar]
- Ono, T.; Takada, S.; Kinugawa, S.; Tsutsui, H. Curcumin ameliorates skeletal muscle atrophy in type 1 diabetic mice by inhibiting protein ubiquitination. Exp. Physiol. 2015, 100, 1052–1063. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, Y.; Egawa, K.; Kanzaki, N.; Izumo, T.; Rogi, T.; Shibata, H. Quercetin glycosides prevent dexamethasone-induced muscle atrophy in mice. Biochem. Biophys. Rep. 2019, 18, 100618. [Google Scholar] [CrossRef]
- Sun, L.J.; Sun, Y.N.; Chen, S.J.; Liu, S.; Jiang, G.R. Resveratrol attenuates skeletal muscle atrophy induced by chronic kidney disease via MuRF1 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 487, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.Y.; Ku, S.K.; Lee, K.W.; Song, H.; An, W.G. Muscle-Protective effects of Schisandrae fructus extracts in old mice after chronic forced exercise. J. Ethnopharmacol. 2018, 212, 175–187. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef]
- Hemdan, D.I.I.; Hirasak, K.; Nakao, R.; Kohno, S.; Kagawa, S.; Abe, T.; Harada-Sukeno, A.; Okumura, Y.; Nakaya, Y.; Terao, J.; et al. Polyphenols prevent clinorotation-induced expression of atrogenes in mouse C2C12 skeletal myotubes. J. Med. Investig. 2009, 56, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Busch, C.J.; Binder, C.J. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 398–406. [Google Scholar] [CrossRef]
- Jeong, H.J.; Kim, S.; Park, J.; Kim, K.M.; Kim, K.; Jun, W. Antioxidant activities and protective effects of hot water extract from Curcuma longa L. on oxidative stress-induced C2C12 myoblasts. J. Korean Soc. Food Sci. Nutr. 2017, 46, 1408–1413. [Google Scholar]

| Group | Superoxide Dismutase (U/mg Protein) | Catalase (U/mg Protein) | Glutathione Peroxidase (U/mg Protein) | Malondialdehyde (μg/mg Protein) |
|---|---|---|---|---|
| Ex-CON | 27.76 ± 0.94 | 1.41 ± 0.08 | 0.30 ± 0.01 | 0.73 ± 0.11 |
| Ex-DEX | 23.33 ± 0.35 * | 1.14 ± 0.16 * | 0.27 ± 0.01 * | 1.02 ± 0.06 * |
| Ex-CLW | 26.85 ± 1.02 | 1.56 ± 0.22 | 0.29 ± 0.01 | 0.65 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, K.; Park, J.; Jun, W. Curcuma longa L. Water Extract Improves Dexamethasone-Induced Sarcopenia by Modulating the Muscle-Related Gene and Oxidative Stress in Mice. Antioxidants 2021, 10, 1000. https://doi.org/10.3390/antiox10071000
Kim S, Kim K, Park J, Jun W. Curcuma longa L. Water Extract Improves Dexamethasone-Induced Sarcopenia by Modulating the Muscle-Related Gene and Oxidative Stress in Mice. Antioxidants. 2021; 10(7):1000. https://doi.org/10.3390/antiox10071000
Chicago/Turabian StyleKim, Shintae, Kyungmi Kim, Jeongjin Park, and Woojin Jun. 2021. "Curcuma longa L. Water Extract Improves Dexamethasone-Induced Sarcopenia by Modulating the Muscle-Related Gene and Oxidative Stress in Mice" Antioxidants 10, no. 7: 1000. https://doi.org/10.3390/antiox10071000
APA StyleKim, S., Kim, K., Park, J., & Jun, W. (2021). Curcuma longa L. Water Extract Improves Dexamethasone-Induced Sarcopenia by Modulating the Muscle-Related Gene and Oxidative Stress in Mice. Antioxidants, 10(7), 1000. https://doi.org/10.3390/antiox10071000





