Omega-Class Glutathione Transferases of Carcinogenic Liver Fluke, Clonorchis sinensis, Modulate Apoptosis and Differentiation of Host Cholangiocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rat Biliary Ductal Epithelium Experimentally Infected with C. sinensis
2.2. Expression of rCsGSTos
2.3. Antisera
2.4. HIBEC Culture and Uptake of rCsGSTos
2.5. Oxidative Stress
2.6. Quantitative RNA Sequencing (Quant-RNAseq)
2.7. Quantitative Real Time Reverse Transcription-PCR (qRT-PCR)
2.8. Data Analysis
2.9. Double Sandwich-ELISA
2.10. Cell Viability
2.11. Fluorescence-Activated Cell Sorting (FACS) Analysis
2.12. Western Blot Analysis
2.13. Immunohistochemical Staining
2.14. Statistical Analysis
3. Results
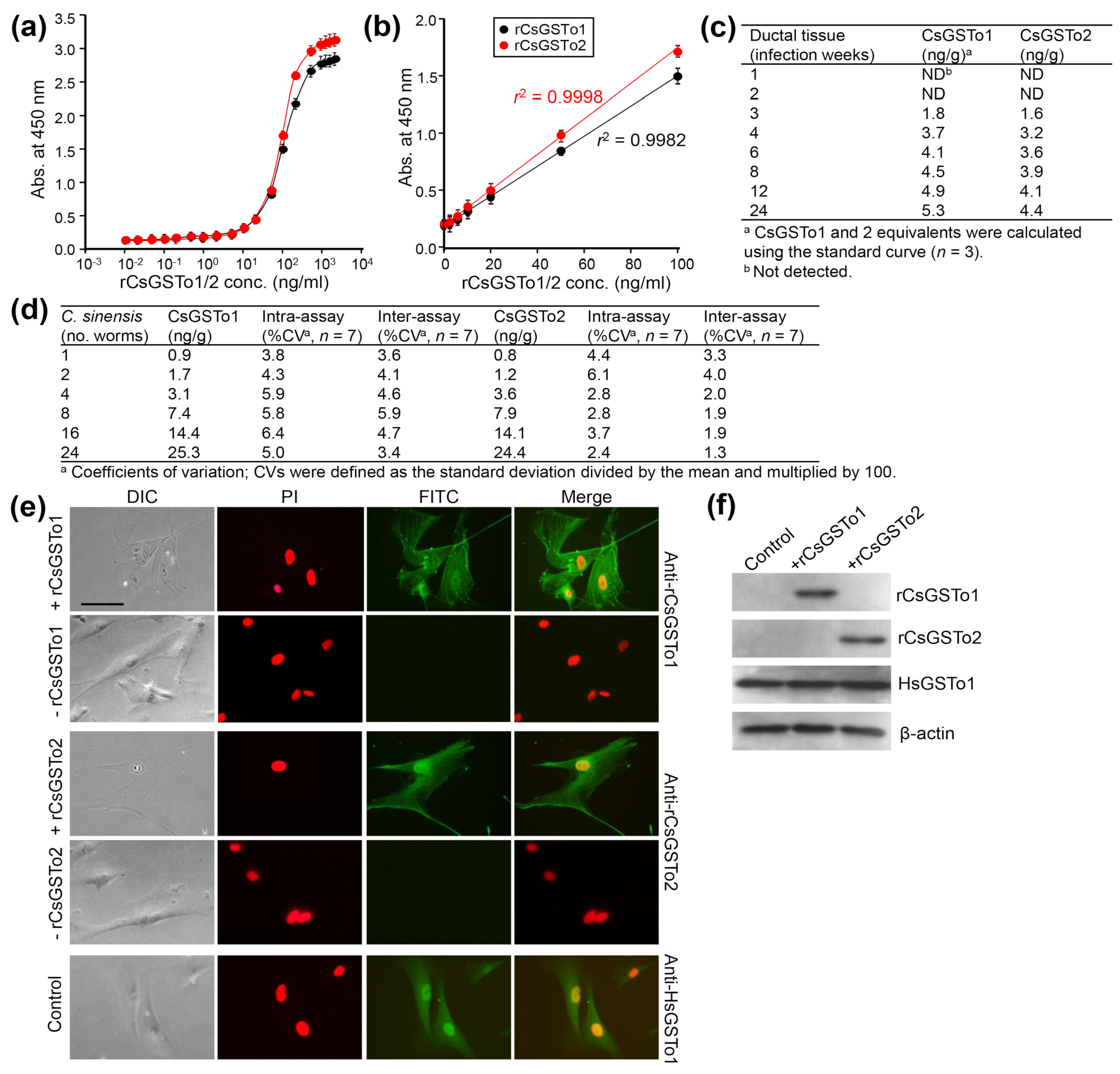
3.1. CsGSTo1 and CsGSTo2 Are Accumulated in Host Cholangiocytes In Vivo and In Vitro
3.2. rCsGSTos Inhibit Oxidative Stress-Induced Apoptosis
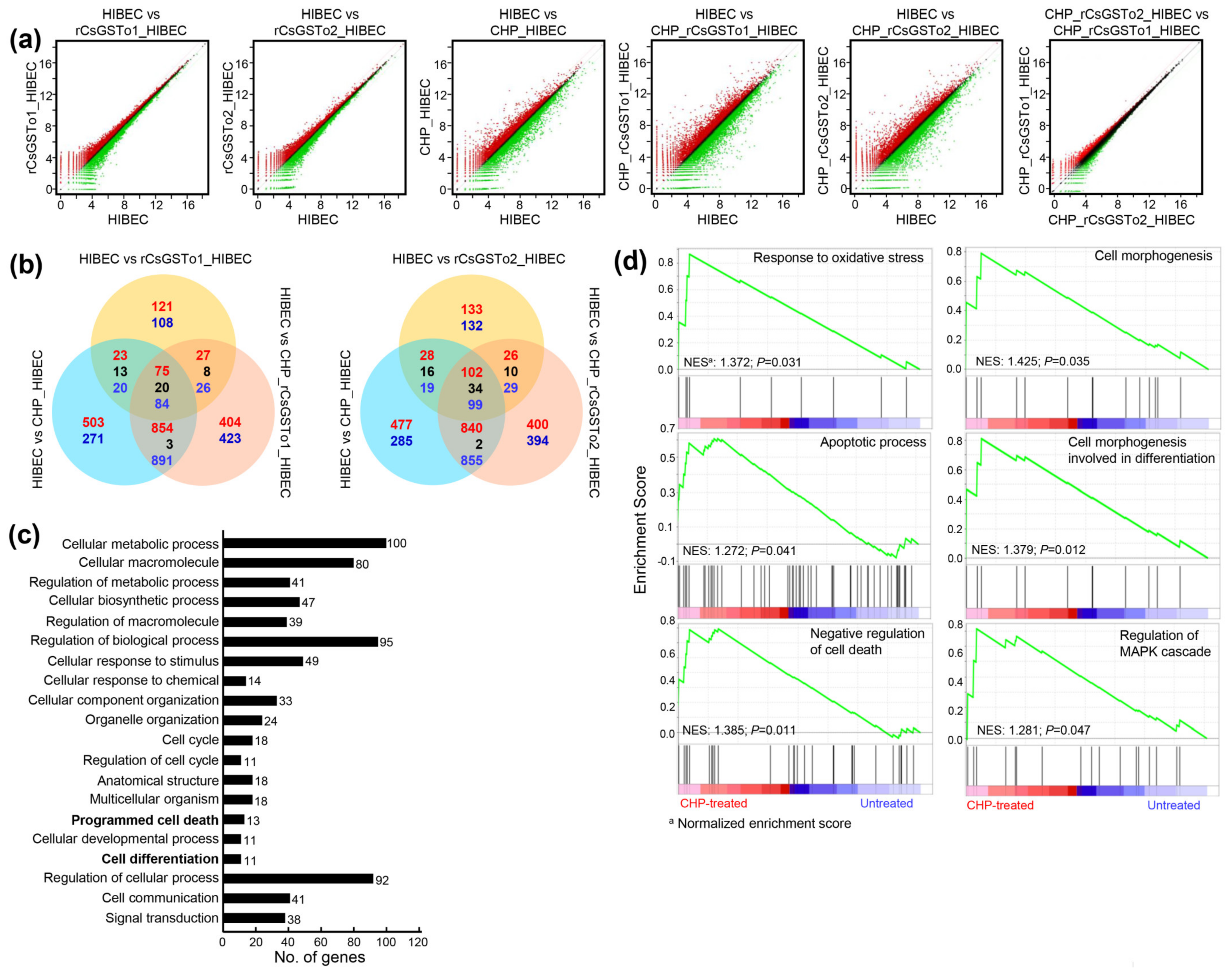
3.3. Functional Characterization of the Altered HIBEC Intracellular Environment in Response to rCsGSTos
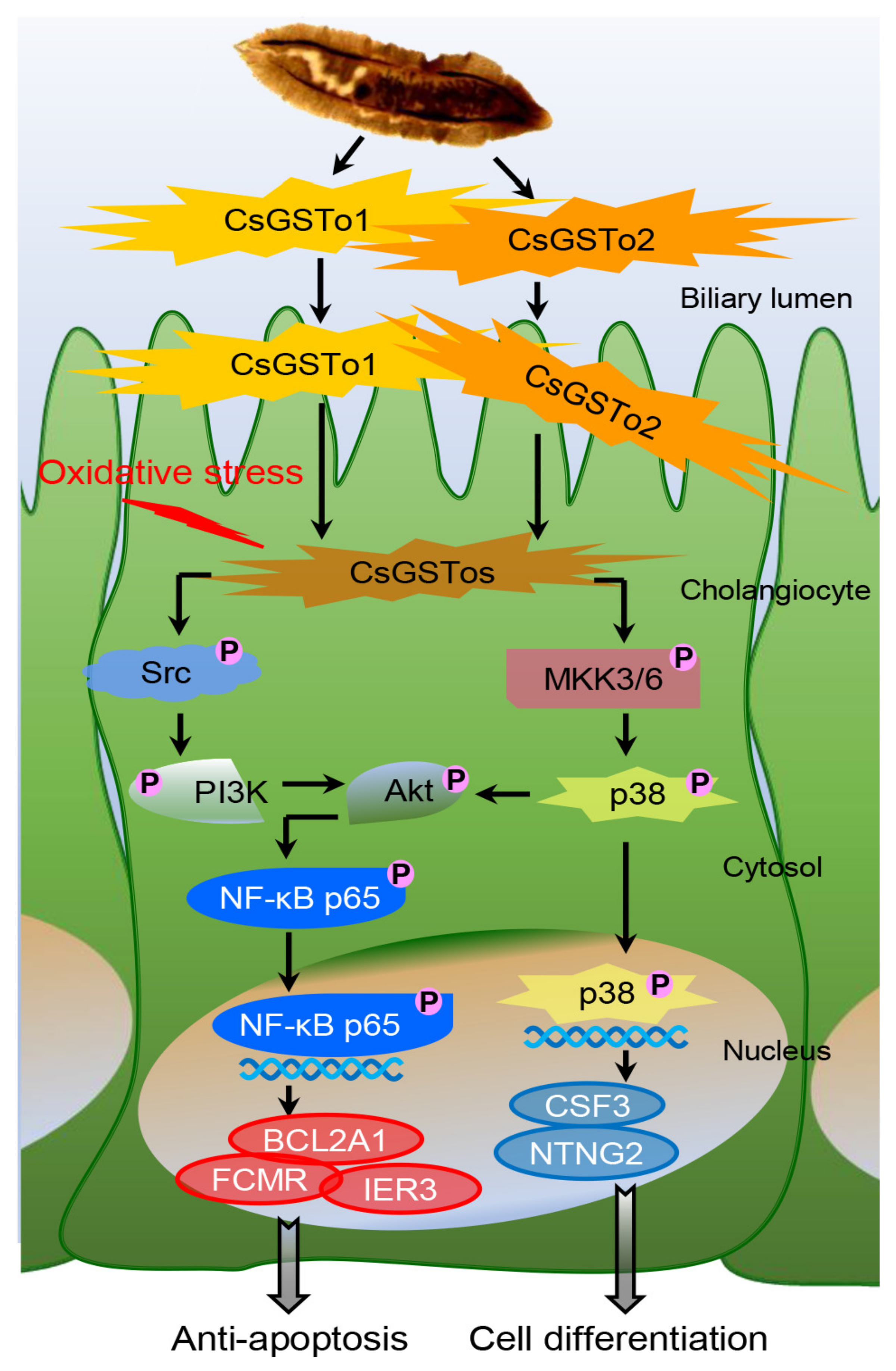
3.4. rCsGSTos Augment Cholangiocyte Survival through Activation of Src-PI3K/Akt-NF-κB p65 Signal Pathway Followed by Expressional Regulation of Genes Engaged in Apoptotic Process
3.5. rCsGSTos Induce Phosphorylation of MKK3/6 and p38 MAPK, thus Regulate the Expression of Genes Involved in Cell Differentiation and Are Located in the C. sinensis Infected Biliary Ductal Epithelium Where Cytokeratin 19 Is Expressed
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, M.B.; Utzinger, J.; Keiser, J.; Zhou, X.N. Clonorchiasis. Lancet 2016, 387, 800–810. [Google Scholar] [CrossRef]
- Jang, K.T.; Hong, S.M.; Lee, K.T.; Lee, J.G.; Choi, S.H.; Heo, J.S.; Choi, D.W.; Choi, D.; Lim, J.H. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch. 2008, 543, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Oh, J.K.; Lim, M.K.; Shin, A.; Kong, H.J.; Jung, K.W.; Won, Y.J.; Park, S.; Park, S.J.; Hong, S.T. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J. Korean Med. Sci. 2010, 25, 1011–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labib, P.L.; Goodchild, G.; Pereira, S.P. Molecular pathogenesis of cholangiocarcinoma. MMC Cancer 2019, 19, 185. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Pakharukova, M.Y.; Zaparina, O.G.; Kovner, A.V.; Mordvinov, V.A. Inhibition of Opisthorchis felineus glutathione-dependent prostaglandin synthase by resveratrol correlates with attenuation of cholangiocyte neoplasia in a hamster model of opisthorchiasis. Int. J. Parasitol. 2019, 49, 963–973. [Google Scholar] [CrossRef]
- Fedorova, O.S.; Kovshirina, Y.V.; Kovshirina, A.E.; Fedotova, M.M.; Deev, I.A.; Petrovskiy, F.I.; Filimonov, A.V.; Dmitrieva, A.I.; Kudyakov, L.A.; Saltykova, I.V.; et al. Opisthorchis felineus infection and cholangiocarcinoma in the Russian Federation: A review of medical statistics. Parasitol. Int. 2017, 66, 365–371. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Biological agents. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 341–371. [Google Scholar]
- Bae, Y.A.; Ahn, D.W.; Lee, E.G.; Kim, S.H.; Cai, G.B.; Kang, I.; Sohn, W.M.; Kong, Y. Differential activation of diverse glutathione transferases of Clonorchis sinensis in response to the host bile and oxidative stressors. PLoS Negl. Trop. Dis. 2013, 7, e2211. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.A.; Kim, J.G.; Kong, Y. Phylogenetic characterization of Clonorchis sinensis proteins homologous to the sigma-class glutathione transferase and their differential expression profiles. Mol. Biochem. Parasitol. 2016, 206, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Ahn, C.S.; Kim, S.H.; Bae, Y.A.; Kwon, N.Y.; Kang, I.; Yang, H.J.; Sohn, W.M.; Kong, Y. Clonorchis sinensis omega-class glutathione transferases play major roles in the protection of the reproductive system during maturation and the response to oxidative stress. Parasit. Vectors 2016, 9, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Board, P.G.; Coggan, M.; Chelvanayagam, G.; Easteal, S.; Jermiin, L.S.; Schulte, G.K.; Danley, D.E.; Hoth, L.R.; Griffor, M.C.; Kamath, A.V.; et al. Identification, characterization, and crystal structure of the omega class glutathione transferases. J. Biol. Chem. 2000, 275, 24798–24806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitbread, A.K.; Masoumi, M.; Tetlow, N.; Schmuck, E.; Coggan, M.; Board, P.G. Characterization of the omega class of glutathione transferases. Methods Enzymol. 2005, 401, 78–99. [Google Scholar]
- Paul, S.; Jakhar, R.; Bhardwaj, M.; Kang, C.S. Glutathione-S-transferase omega 1 (GSTO1-1) acts as mediator of signaling pathways involved in aflatoxin B1-induced apoptosis-autophagy crosstalk in macrophages. Free Radic. Biol. Med. 2015, 89, 1218–1230. [Google Scholar] [CrossRef]
- Yan, C.; Wang, Y.H.; Yu, Q.; Cheng, X.D.; Zhang, B.B.; Li, B.; Zhang, B.; Tang, R.X.; Zheng, K.Y. Clonorchis sinensis excretory/secretory products promote the secretion of TNF-alpha in the mouse intrahepatic biliary epithelial cells via Toll-like receptor 4. Parasit. Vectors 2015, 8, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Won, J.; Cho, Y.; Lee, D.; Jeon, B.Y.; Ju, J.W.; Chung, S.; Pak, J.H. Clonorchis sinensis excretory-secretory products increase malignant characteristics of cholangiocarcinoma cells in three-dimensional co-culture with biliary ductal plates. PLoS Pathog. 2019, 15, e1007818. [Google Scholar] [CrossRef]
- Pak, J.H.; Lee, J.Y.; Jeon, B.Y.; Dai, F.; Yoo, W.G.; Hong, S.J. Cytokine production in cholangiocarcinoma cells in response to Clonorchis sinensis excretory-secretory products and their putative protein components. Korean J. Parasitol. 2019, 57, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Ahn, C.S.; Sripa, B.; Eom, K.S.; Kang, I.; Sohn, W.M.; Nawa, Y.; Kong, Y. Clonorchis sinensis omega-class glutathione transferases are reliable biomarkers for serodiagnosis of clonorchiasis and opisthorchiasis. Clin. Microbiol. Infect. 2019, 25, 109.e1–109.e6. [Google Scholar] [CrossRef] [Green Version]
- Demetris, A.J.; Markus, B.H.; Fung, J.J.; Makowka, L.; Graner, S.; Duquesnoy, R.; Starzl, T.E. Primary cultures of human intrahepatic (biliary) epithelial cells. Transplant. Proc. 1988, 20, 161–163. [Google Scholar] [PubMed]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Maphanao, P.; Thanan, R.; Loilome, W.; Chio-Srichan, S.; Wongwattanakul, M.; Sakonsinsiri, C. Synchrotron FTIR microspectroscopy revealed apoptosis-induced biomolecular changes of cholangiocarcinoma cells treated with ursolic acid. Biochim. Biophys. Acta 2020, 1864, 129708. [Google Scholar] [CrossRef]
- Kaczanowski, S. Apoptosis: Its origin, history, maintenance and the medical implications for cancer and aging. Phys. Biol. 2016, 13, 031001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Sripa, B.; Tangkawattana, S.; Brindley, P.J. Update on pathogenesis of opisthorchiasis and carcinoma. Adv. Parasitol. 2018, 102, 97–113. [Google Scholar]
- Kampkötter, A.; Volkmann, T.E.; de Castro, S.H.; Leiers, B.; Klotz, L.O.; Johnson, T.E.; Link, C.D.; Henkle-Dührsen, K. Functional analysis of the glutathione S-transferase 3 from Onchocerca volvulus (Ov-GST-3): A parasite GST confers increased resistance to oxidative stress in Caenorhabditis elegans. J. Mol. Biol. 2003, 325, 25–37. [Google Scholar] [CrossRef]
- Matchimakul, P.; Rinaldi, G.; Suttiprapa, S.; Mann, V.H.; Popratiloff, A.; Laha, T.; Pimenta, R.N.; Cochran, C.J.; Kaewkes, S.; Sripa, B.; et al. Apoptosis of cholangiocytes modulated by thioredoxin of carcinogenic liver fluke. Int. J. Biochem. Cell Biol. 2015, 65, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Vogler, M. BCL2A1: The underdog in the BCL2 family. Cell Death Differ. 2012, 19, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, S.L.; Careddu, G.; Stirparo, G.G.; Castagna, L.; Santoro, A.; Carlo-Stella, C. Dual PI3K/ERK inhibition induces necroptotic cell death of Hodgkin lymphoma cells through IER3 downregulation. Sci. Rep. 2016, 6, 35745. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.X.; Ao, Z.; Prasad, K.V.; Wu, R.; Schlossman, S.F. IEX-1L, an apoptosis inhibitor involved in NF-kappaB-mediated cell survival. Science 1988, 281, 998–1001. [Google Scholar] [CrossRef]
- Baptiste-Okoh, N.; Barsotti, A.M.; Prives, C. A role for caspase 2 and PIDD in the process of p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 1937–1942. [Google Scholar] [CrossRef] [Green Version]
- John, K.; Alla, Y.; Meier, C.; Pützer, B.M. GRAMD4 mimics p53 and mediates the apoptotic function of p73 at mitochondria. Cell Death Differ. 2011, 18, 874–886. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, J.W.; Ko, K.P.; Ryu, B.K.; Lee, M.G.; Kim, H.J.; Chi, S.G. XAF1 forms a positive feedback loop with IRF-1 to drive apoptotic stress response and suppress tumorigenesis. Cell Death Dis. 2018, 9, 806. [Google Scholar] [CrossRef]
- Buccitelli, C.; Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 10, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Pannala, V.R.; Dash, R.K. Mechanistic characterization of the thioredoxin system in the removal of hydrogen peroxide. Free Radic. Biol. Med. 2015, 78, 42–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, R.N.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Williams, D.A. Interleukin-11: Review of molecular, cell biology, and clinical use. Blood 1997, 89, 3897–3908. [Google Scholar] [CrossRef]
- Schneider, A.; Kruger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.H.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef] [Green Version]
- Górka, B.; Skubis-Zegadło, J.; Mikula, M.; Bardadin, K.; Paliczka, E.; Czarnocka, B. NrCAM, a neuronal system cell-adhesion molecule, is induced in papillary thyroid carcinomas. Br. J. Cancer 2007, 97, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Dickens, M.; Raingeaud, J.; Davis, R.M.; Greenberg, M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995, 270, 1326–1331. [Google Scholar] [CrossRef]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signaling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [Green Version]
- Caraglia, M.; Giuberti, G.; Marra, M.; Addeo, R.; Montella, L.; Murolo, M.; Sperlongano, P.; Vincenzi, B.; Naviglio, S.; Del Prete, S.; et al. Oxidative stress and ERK1/2 phosphorylation as predictors of outcome in hepatocellular carcinoma patients treated with sorafenib plus octreotide LAR. Cell Death Dis. 2011, 2, e150. [Google Scholar] [CrossRef]
- Sim, S.; Yong, T.S.; Park, S.J.; Im, K.I.; Kong, Y.; Ryu, J.S.; Min, D.Y.; Shin, M.H. NADPH oxidase-derived reactive oxygen species-mediated activation of ERK1/2 is required for apoptosis of human neutrophils induced by Entamoeba histolytica. J. Immunol. 2005, 174, 4279–4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.S.N.; Kwek, J.; Wong, C.K.E.; Saner, N.J.; Yap, C.; Felquer, F.; Morris, M.B.; Gardner, D.K.; Rathjen, P.D.; Rathjen, J. Src family kinases and p38 mitogen-activated protein kinases regulate pluripotent cell differentiation in culture. PLoS ONE 2016, 11, e0163244. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; Abdelrasoul, H.; Aboelmagd, M.; Tawila, A.M. The role of the MAPK signaling, topoisomerase and dietary bioactives in controlling cancer incidence. Diseases 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.D.; Gerber, M.A. Development of intrahepatic bile ducts in humans. Immunohistochemical study using monoclonal cytokeratin antibodies. Arch. Pathol. Lab. Med. 1989, 113, 1135–1138. [Google Scholar] [PubMed]
- Zong, Y.; Stanger, B.Z. Molecular mechanisms of bile duct development. Int. J. Biochem. Cell Biol. 2011, 43, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickson, G.R.; O’Farrell, P.H. Anillin: A pivotal organizer of the cytokinetic machinery. Biochem. Soc. Trans. 2008, 36, 439–441. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, L.; He, W.; Liu, T.; Zhao, Y.; Chen, H.; Li, Y. ESCO2 knockdown inhibits cell proliferation and induces apoptosis in human gastric cancer cells. Biochem. Biophys. Res. Commun. 2018, 496, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.M.; Hanson, A.; Kavanagh, J.A.; Waddell, D.S. Fam83d modulates MAP kinase and AKT signaling and is induced during neurogenic skeletal muscle atrophy. Cell. Signal. 2020, 70, 109576. [Google Scholar] [CrossRef] [PubMed]
- Pajaud, J.; Kumar, S.; Rauch, C.; Morel, F.; Aninat, C. Regulation of signal transduction by glutathione transferases. Int. J. Hepatol. 2012, 2012, 137676. [Google Scholar] [CrossRef] [PubMed]



| KEGG ID | Signaling Pathways a | No. of Gene | Related Genes |
|---|---|---|---|
| hsa04621 | NOD-like receptor | 10 | NEK7, CXCL1, CXCL3, IRF7, OAS1, OAS2, OAS3, CCL5, STAT1, CASP8b |
| hsa04064 | NF-kappa B | 9 | GADD45G, EDARADD, CXCL1, CXCL3, ICAM1, PIDD1, PTGS2, BCL2A1, TNFSF11 |
| hsa04010 | MAPK | 8 | GADD45G, DUSP8, EPHA2, MECOM, HSPA1L, HSPA6, MAPK7, MAPK8IP1 |
| hsa04657 | IL-17 | 7 | CSF3c, CXCL1, CXCL3, IL17D, MAPK7, PTGS2, CASP8 |
| hsa04115 | p53 | 6 | GADD45G, CD82, PIDD1, CASP8, CCNB2, CDK1 |
| hsa04668 | TNF | 6 | CXCL1, CXCL3, ICAM1, PTGS2, CCL5, CASP8 |
| hsa04630 | JAK/STAT | 5 | IL24, CSF3, IL11, IL17D, STAT1 |
| hsa04071 | Sphingolipid | 5 | S1PR1, S1PR3, CERS2, ABCC1, BDKRB2 |
| hsa04020 | Calcium | 4 | AGTR1, EDNRB, PHKG2, BDKRB2 |
| hsa04620 | Toll-like receptor | 4 | IRF7, CCL5, STAT1, CASP8 |
| hsa04922 | Glucagon | 4 | SIK1, CRTC2, PHKG2, PPP4C |
| hsa04915 | Estrogen | 4 | HBEGF, HSPA1L, HSPA6, KRT15 |
| hsa04062 | Chemokine | 4 | CXCL1, CXCL3, CCL5, STAT1 |
| hsa04625 | C-type lectin receptor | 4 | IL17D, PTGS2, STAT1, CASP8 |
| hsa04068 | FoxO | 4 | PLK4, GADD45G, S1PR1, CCNB2 |
| hsa04622 | RIG-I-like receptor | 3 | IRF7, CASP8, ISG15 |
| hsa04151 | PI3K/Akt | 3 | CSF3, EPHA2, CRTC2 |
| hsa03320 | PPAR | 3 | ANGPTL4, ACSL5, PPARG |
| hsa04022 | cGMP/PKG | 3 | AGTR1, EDNRB, BDKRB2 |
| hsa04921 | Oxytocin | 3 | KCNJ2, MAPK7, PTGS2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, C.-S.; Kim, J.-G.; Kang, I.; Kong, Y. Omega-Class Glutathione Transferases of Carcinogenic Liver Fluke, Clonorchis sinensis, Modulate Apoptosis and Differentiation of Host Cholangiocytes. Antioxidants 2021, 10, 1017. https://doi.org/10.3390/antiox10071017
Ahn C-S, Kim J-G, Kang I, Kong Y. Omega-Class Glutathione Transferases of Carcinogenic Liver Fluke, Clonorchis sinensis, Modulate Apoptosis and Differentiation of Host Cholangiocytes. Antioxidants. 2021; 10(7):1017. https://doi.org/10.3390/antiox10071017
Chicago/Turabian StyleAhn, Chun-Seob, Jeong-Geun Kim, Insug Kang, and Yoon Kong. 2021. "Omega-Class Glutathione Transferases of Carcinogenic Liver Fluke, Clonorchis sinensis, Modulate Apoptosis and Differentiation of Host Cholangiocytes" Antioxidants 10, no. 7: 1017. https://doi.org/10.3390/antiox10071017






