Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Isolation and Culture of Ovarian GCs and Oocytes
2.3. DON and Melatonin Treatment
2.4. Assessment of Cell Viability
2.5. Analysis of Apoptosis Using Flow Cytometry
2.6. Measurement of Reactive Oxygen Species (ROS) Using Flow Cytometry
2.7. Measurement of the Mitochondrial Membrane Potential (ΔΨm)
2.8. Determination of ATP Content
2.9. RNA Extraction and Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
2.10. Western Blotting Analysis
2.11. Data Analysis and Statistics
3. Results
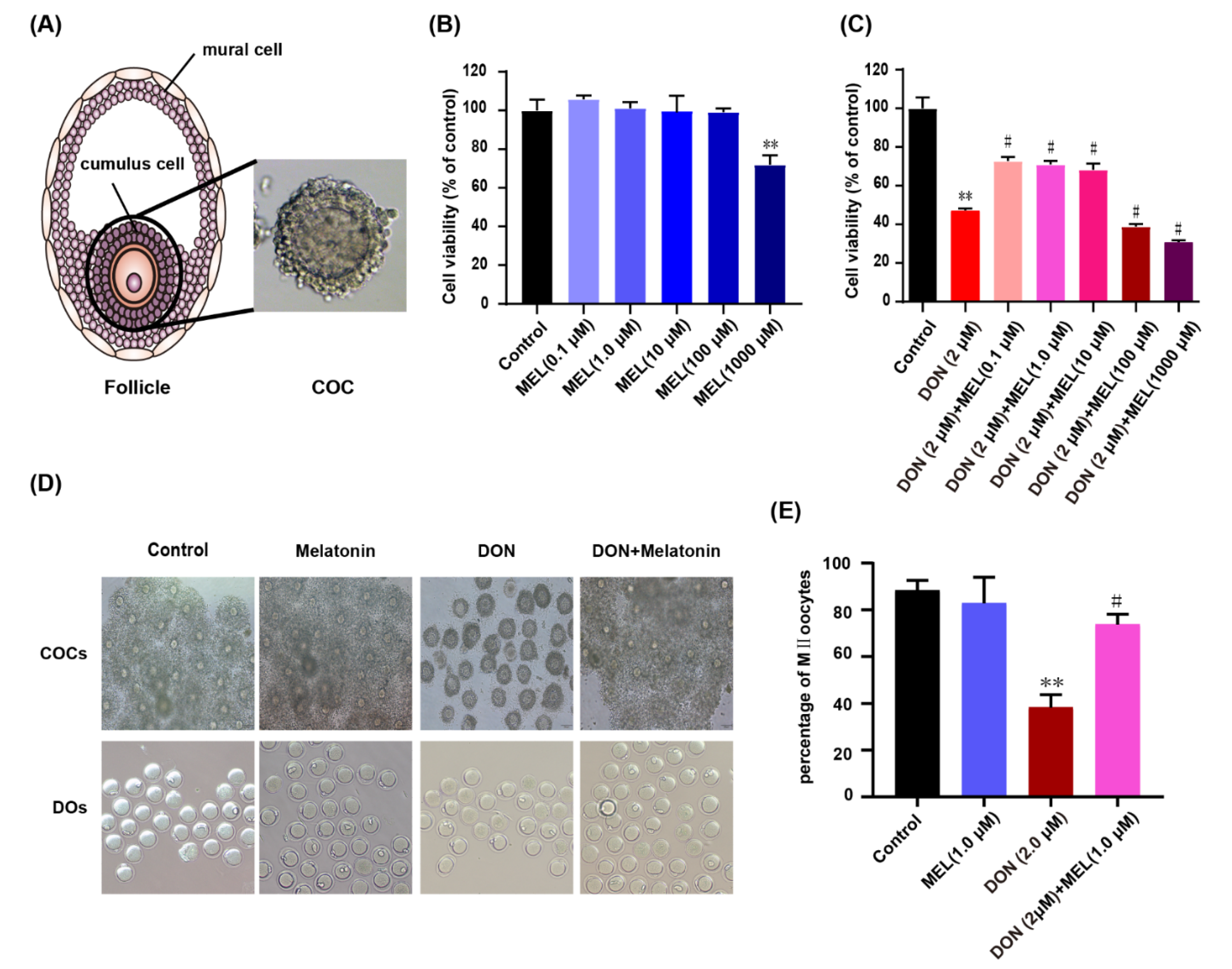
3.1. Effect of Melatonin on DON-Induced Cell Viability
3.2. Melatonin Protects against Meiotic Maturation Defects in DON Exposure Mouse Oocytes
3.3. Melatonin Decreases DON-Induced Murine GCs Apoptosis
3.4. Melatonin Decreases ROS Production in DON-Exposed Ovary GCs
3.5. Melatonin Ameliorates DON-Induced Mitochondrial Dysfunction
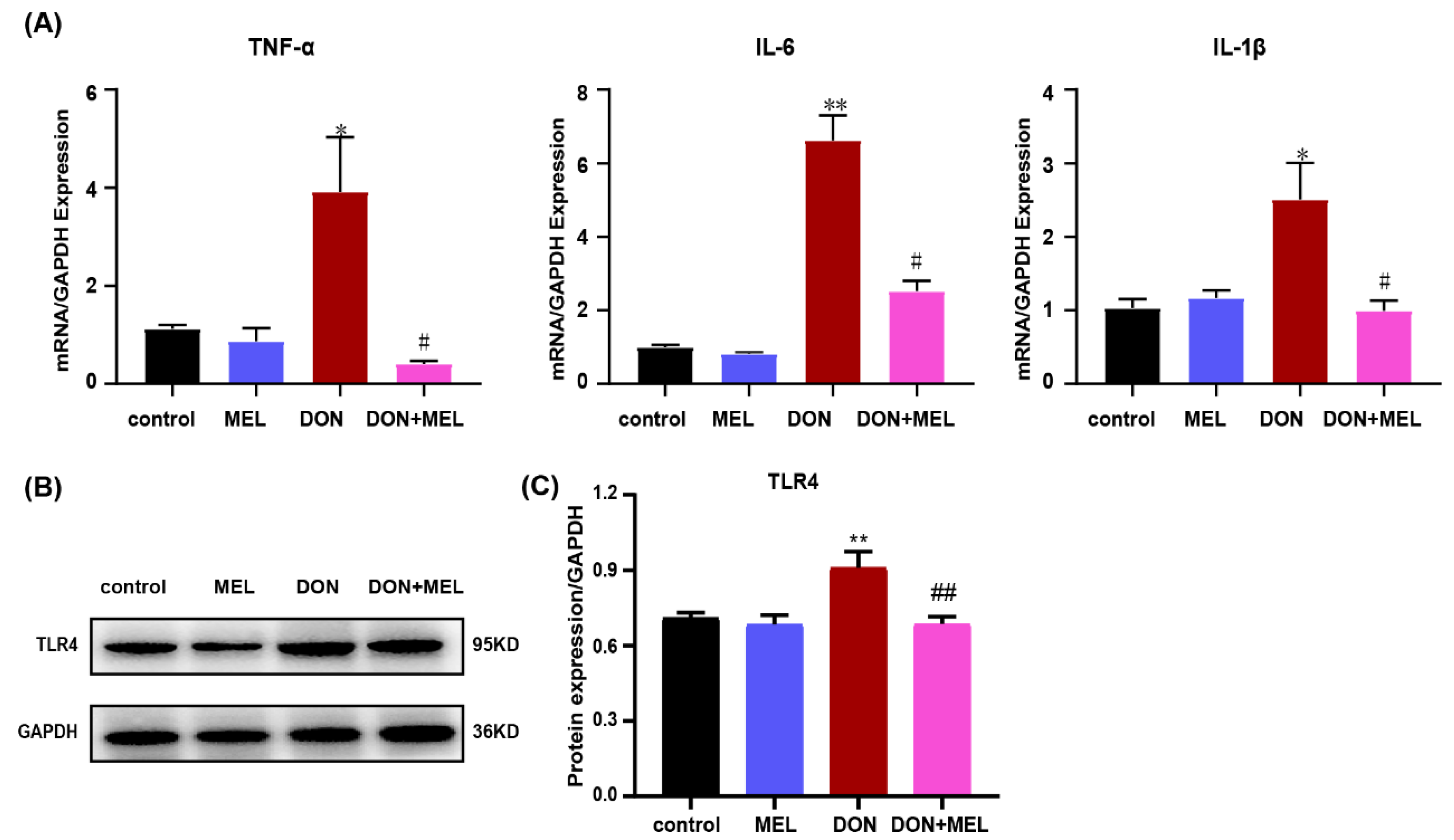
3.6. Melatonin Inhibits DON-Induced Inflammatory Cytokine Gene Expression
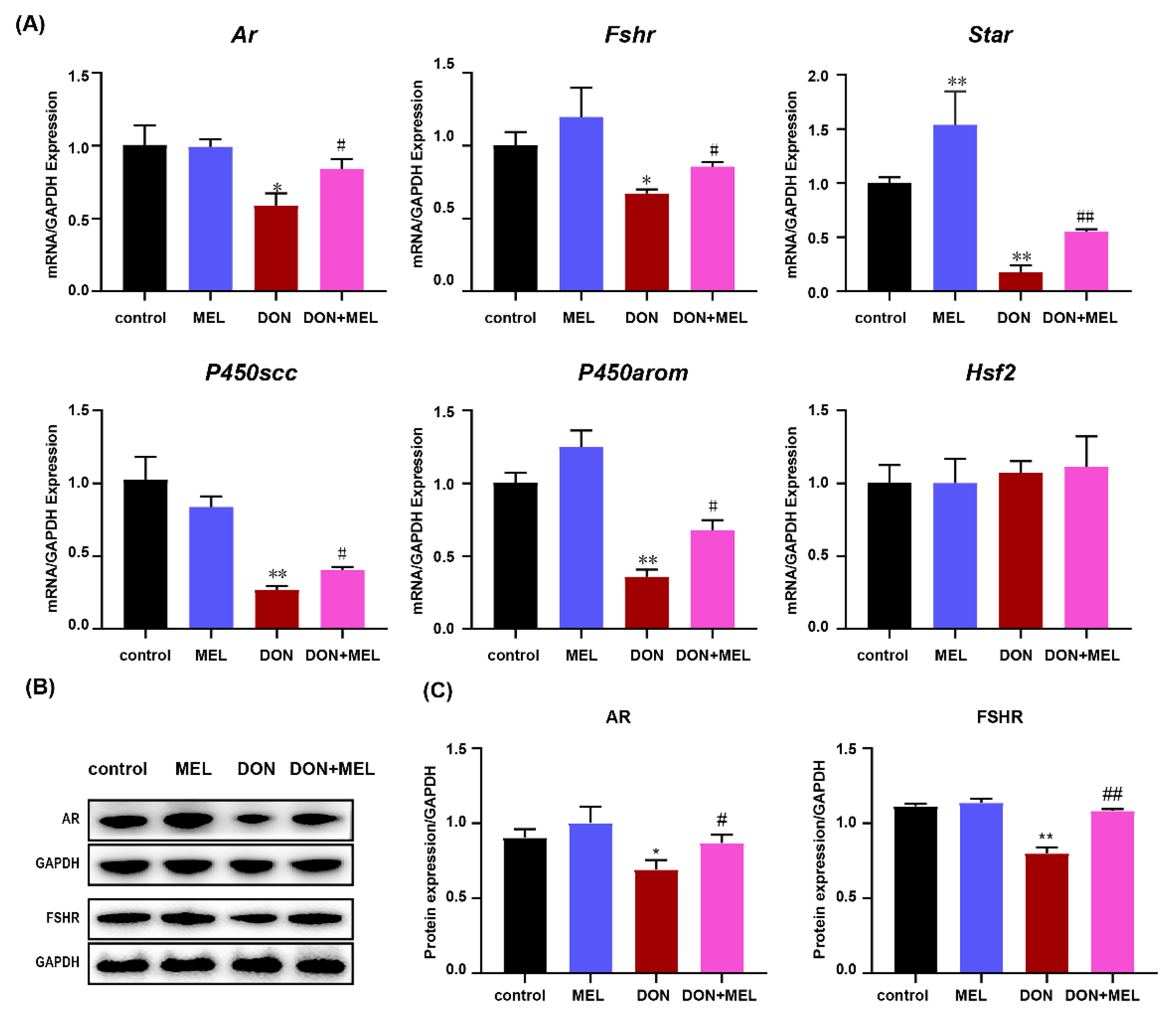
3.7. Melatonin Inhibits DON-Induced Downregulation of Reproduction Hormone Genes
3.8. Melatonin Reduces DON-Induced Inflammatory Response through NF-κB and MAPK Signaling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, R.; Albertini, D.F. The road to maturation: Somatic cell interaction and self-organization of the mammalian oocyte. Nat. Rev. Mol. Cell Biol. 2013, 14, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.W.; Zhang, M.; Shuhaibar, L.C.; Norris, R.P.; Geerts, A.; Wunder, F.; Eppig, J.J.; Potter, L.R.; Jaffe, L.A. Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev. Biol. 2012, 366, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manabe, N.; Goto, Y.; Matsuda-Minehata, F.; Inoue, N.; Maeda, A.; Sakamaki, K.; Miyano, T. Regulation Mechanism of Selective Atresia in Porcine Follicles: Regulation of Granulosa Cell Apoptosis during Atresia. J. Reprod. Dev. 2004, 50, 493–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Sun, A.; Li, H.; Tsinkgou, A.; Yu, J.; Ying, S.; Chen, Z.; Shi, Z. Molecular mechanisms of enhancing porcine granulosa cell proliferation and function by treatment in vitro with anti-inhibin alpha subunit antibody. Reprod Biol Endocrinol. 2015, 13, 26. [Google Scholar] [CrossRef] [Green Version]
- Gore-Langton, R.E. Follicle-stimulating hormone and estradiol regulate antrum-like reorganization of granulosa cells in rat preantral follicle cultures. Biol. Reprod. 1990, 43, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Xin, Z.; Jiang, S.; Jiang, P.; Yan, X.; Fan, C.; Di, S.; Wu, G.; Yang, Y.; Reiter, R.J.; Ji, G. Melatonin as a treatment for gastrointestinal cancer: A review. J. Pineal Res. 2015, 58, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A Review of the Multiple Actions of Melatonin on the Immune System. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Gonzalez-Arto, M.; Hamilton, T.R.D.S.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.; Pe, R.M.P.; Blanco, M.T.M.; Pérez, J.; et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tamura, H.; Tan, D.X.; Xu, X.Y. Melatonin and the circadian system: Contributions to successful female reproduction. Fertil. Steril. 2014, 102, 321–328. [Google Scholar] [CrossRef]
- Lan, M.; Han, J.; Pan, M.H.; Wan, X.; Pan, Z.N.; Sun, S.C. Melatonin protects against defects induced by deoxynivalenol during mouse oocyte maturation. J. Pineal Res. 2018, 65, e12477. [Google Scholar] [CrossRef] [PubMed]
- Suwannakot, K.; Sritawan, N.; Prajit, R.; Aranarochana, A.; Sirichoat, A.; Pannangrong, W.; Wigmore, P.; Welbat, J. Melatonin Protects against the Side-Effects of 5-Fluorouracil on Hippocampal Neurogenesis and Ameliorates Antioxidant Activity in an Adult Rat Hippocampus and Prefrontal Cortex. Antioxidants 2021, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Chen, B.; Ding, D.; Gao, M.; Chen, D.; Liu, Y.; Hao, Y.; Zou, W.; Ji, D.; Zhou, P.; et al. Melatonin promotes the development of immature oocytes from the COH cycle into healthy offspring by protecting mitochondrial function. J. Pineal Res. 2020, 68, e12621. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; He, C.; Zhu, K.; Xu, Z.; Ma, T.; Tao, J.; Liu, G. Melatonin protects porcine oocyte in vitro maturation from heat stress. J. Pineal Res. 2015, 59, 365–375. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Li, W.; Ao, H.; Zhang, Y.; Zhou, R.; Li, K. Effects of melatonin on the synthesis of estradiol and gene expression in pig granulosa cells. J. Pineal Res. 2019, 66, e12546. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, J.; Li, Y.; Zhu, K.; Xu, Z.; Song, Y.; Liu, G. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J. Pineal Res. 2015, 58, 300–309. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; He, C.; Zhang, L.; Tan, D.; Reiter, R.J.; Xu, J.; Ji, P.; Liu, G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Lan, M.; Zang, X.-W.; Li, Y.-L.; Cui, X.-S.; Kim, N.-H.; Sun, S.-C. Melatonin protects oocytes from MEHP exposure-induced meiosis defects in porcine. Biol. Reprod. 2018, 98, 286–298. [Google Scholar] [CrossRef]
- Urbanek, K.A.; Habrowska-Gorczynska, D.E.; Kowalska, K.; Stanczyk, A.; Dominska, K.; Piastowska-Ciesielska, A.W. Deoxynivalenol as potential modulator of human steroidogenesis. J. Appl. Toxicol. 2018, 38, 1450–1459. [Google Scholar] [CrossRef]
- Han, J.; Wang, Q.-C.; Zhu, C.-C.; Liu, J.; Zhang, Y.; Cui, X.-S.; Kim, N.-H.; Sun, S.-C. Deoxynivalenol exposure induces autophagy/apoptosis and epigenetic modification changes during porcine oocyte maturation. Toxicol. Appl. Pharmacol. 2016, 300, 70–76. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Kuca, K.; Dohnal, V.; Tian, Z. Deoxynivalenol: Signaling pathways and human exposure risk assessment--an update. Arch. Toxicol. 2014, 88, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lohrey, L.; Cramer, B.; Yuan, Z.; Humpf, H.U. Impact of physicochemical parameters on the decomposition of deoxynivalenol during extrusion cooking of wheat grits. J. Agric. Food Chem. 2011, 59, 12480–12485. [Google Scholar] [CrossRef] [PubMed]
- Medvedova, M.; Kolesarova, A.; Capcarova, M.; Labuda, R.; Sirotkin, A.V.; Kovacik, J.; Bulla, J. The effect of deoxynivalenol on the secretion activity, proliferation and apoptosis of porcine ovarian granulosa cells in vitro. J. Environ. Sci. Health Part B. 2011, 46, 213–219. [Google Scholar] [CrossRef]
- Wang, H.; Zong, Q.; Wang, S.; Zhao, C.; Wu, S.; Bao, W. Genome-Wide DNA Methylome and Transcriptome Analysis of Porcine Intestinal Epithelial Cells upon Deoxynivalenol Exposure. J. Agric. Food Chem. 2019, 67, 6423–6431. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Risks to Human and Animal Health Related to the Presence of Deoxynivalenol and Its Acetylated and Modified Forms in Food and Feed, 2017/09/11 ed; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2017; p. e04718. [Google Scholar]
- Borutova, R.; Faix, S.; Placha, I.; Gresakova, L.; Cobanova, K.; Leng, L. Effects of deoxynivalenol and zearalenone on oxidative stress and blood phagocytic activity in broilers. Arch. Anim. Nutr. 2008, 62, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol Impairs Weight Gain and Affects Markers of Gut Health after Low-Dose, Short-Term Exposure of Growing Pigs. Toxins 2015, 7, 2071–2095. [Google Scholar] [CrossRef]
- Guerrero-Netro, H.M.; Chorfi, Y.; Price, C.A. Effects of the mycotoxin deoxynivalenol on steroidogenesis and apoptosis in granulosa cells. Reproduction 2015, 149, 555–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alm, H.; Greising, T.; Brussow, K.-P.; Torner, H.; Tiemann, U. The influence of the mycotoxins deoxynivalenol and zearalenol on in vitro maturation of pig oocytes and in vitro culture of pig zygotes. Toxicol. Vitro 2002, 16, 643–648. [Google Scholar] [CrossRef]
- Minervinia, F.; Dell’Aquilab, M.E.; Maritatob, F.; Minoiab, P.; Viscontia, A. Toxic effects of the mycotoxin zearalenone and its derivatives on in vitro maturation of bovine oocytes and 17β-estradiol levels in mural granulosa cell cultures. Toxicol. Vitro 2001, 15, 489–495. [Google Scholar] [CrossRef]
- Alm, H.; Brüssow, K.-P.; Torner, H.; Vanselow, J.; Tomek, W.; Dänicke, S.; Tiemann, U. Influence of Fusarium-toxin contaminated feed on initial quality and meiotic competence of gilt oocytes. Reprod. Toxicol. 2006, 22, 44–50. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Waskiewicz, A.; Beszterda, M.; Kostecki, M.; Zielonka, L.; Golinski, P.; Gajecki, M. Deoxynivalenol in the gastrointestinal tract of immature gilts under per os toxin application. Toxins 2014, 6, 973–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Dejie, L.; Xiaojing, S.; Tiancheng, W.; Yongguo, C.; Zhengtao, Y.; Naisheng, Z. Magnolol inhibits the inflammatory response in mouse mammary epithelial cells and a mouse mastitis model. Inflammation 2015, 38, 16–26. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21944. [Google Scholar] [CrossRef]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in Deoxynivalenol induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Fan, M.; Meng, T.; Chen, X.; Jiang, Y.; Zhu, D.; Hu, W.; Gong, J.; Feng, S.; et al. Deoxynivalenol induces apoptosis in PC12 cells via the mitochondrial pathway. Environ. Toxicol. Pharmacol. 2016, 43, 193–202. [Google Scholar] [CrossRef]
- Andersson, S.G.; Karlberg, O.; Canback, B.; Kurland, C.G. On the origin of mitochondria: A genomics perspective. Phil. Trans. R Soc. Lond. B 2003, 358, 165–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018, 69, 62–72. [Google Scholar] [CrossRef]
- Pivovarova, N.B.; Andrews, S.B. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 2010, 277, 3622–3636. [Google Scholar] [CrossRef] [Green Version]
- Palmeira, C.M.; Teodoro, J.S.; Amorim, J.A.; Steegborn, C.; Sinclair, D.A.; Rolo, A.P. Mitohormesis and metabolic health: The interplay between ROS, cAMP and sirtuins. Free. Radic. Biol. Med. 2019, 141, 483–491. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Bin-Umer, M.A.; McLaughlin, J.E.; Basu, D.; McCormick, S.; Tumer, N.E. Trichothecene mycotoxins inhibit mitochondrial translation--implication for the mechanism of toxicity. Toxins 2011, 3, 1484–1501. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Wang, Y.Y.; Fan, H.R.; Wu, C.J.; Kumar, A.; Yang, L.G. Preventive effects of beta-cryptoxanthin against cadmium-induced oxidative stress in the rat testis. Asian J. Androl. 2016, 18, 920–924. [Google Scholar] [PubMed]
- Li, D.; Fu, Y.; Zhang, W.; Su, G.; Liu, B.; Guo, M.; Li, F.; Liang, D.; Liu, Z.; Zhang, X.; et al. Salidroside attenuates inflammatory responses by suppressing nuclear factor-kappaB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflamm. Res. 2013, 62, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.R.; Wang, Y.Y.; Dan, X.G.; Kumar, A.; Ye, T.Z.; Yu, Y.Y.; Yang, L.G. Anti-inflammatory potential of beta-cryptoxanthin against LPS-induced inflammation in mouse Sertoli cells. Reprod. Toxicol. 2016, 60, 148–155. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, B.; Zhang, N.; Liu, Z.; Liang, D.; Li, F.; Cao, Y.; Feng, X.; Zhang, X.; Yang, Z. Magnolol inhibits lipopolysaccharide-induced inflammatory response by interfering with TLR4 mediated NF-κB and MAPKs signaling pathways. J. Ethnopharmacol. 2013, 145, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.-C.; Cho, W.-K.; Jeong, Y.H.; Im, G.Y.; Kim, A.; Hwang, Y.-H.; Kim, T.; Song, K.H.; Ma, J.Y. A Novel Herbal Medicine KIOM-MA Exerts an Anti-Inflammatory Effect in LPS-Stimulated RAW 264.7 Macrophage Cells. Evid. Based Complement. Altern. Med. 2012, 2012, 462383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, M.S.; Ghosh, S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Wang, S.; Wang, H.; Sun, M.; Wu, S.; Bao, W. Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects. Antioxidants 2021, 10, 1045. https://doi.org/10.3390/antiox10071045
Fan H, Wang S, Wang H, Sun M, Wu S, Bao W. Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects. Antioxidants. 2021; 10(7):1045. https://doi.org/10.3390/antiox10071045
Chicago/Turabian StyleFan, Hairui, Shiqin Wang, Haifei Wang, Mingan Sun, Shenglong Wu, and Wenbin Bao. 2021. "Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects" Antioxidants 10, no. 7: 1045. https://doi.org/10.3390/antiox10071045
APA StyleFan, H., Wang, S., Wang, H., Sun, M., Wu, S., & Bao, W. (2021). Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects. Antioxidants, 10(7), 1045. https://doi.org/10.3390/antiox10071045







