Blanching Pre-Treatment Promotes High Yields, Bioactive Compounds, Antioxidants, Enzyme Inactivation and Antibacterial Activity of ‘Wonderful’ Pomegranate Peel Extracts at Three Different Harvest Maturities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Sample Preparation and Blanching Procedure
2.4. Phytochemical Extraction
2.5. Determination of Phytochemicals and Antioxidant Activity
2.5.1. Extract Yield
2.5.2. Total Phenolic Content (TPC)
2.5.3. Total Tannin Content (TTC)
2.5.4. Total Flavonoid Content (TFC)
2.5.5. Total Anthocyanin Content (TAC)
2.5.6. Ascorbic Acid Content (Vitamin C)
2.5.7. 2,2-Diphenyl-1-picryl Hydrazyl (DPPH) Antioxidant Assay
2.5.8. Ferric Ion Reducing Antioxidant Power (FRAP) Antioxidant Assay
2.5.9. 2,2-Azino-Bis (3-Ethylbenzothiazoline-6-sulphonic Acid) (ABTS) Antioxidant Assay
2.6. Enzyme Activity Evaluation
2.6.1. Sample Preparation
2.6.2. Polyphenol Oxidase (PPO) Assay
2.6.3. Peroxidase (POD) Assay
2.7. Ultra-Liquid Chromatography Mass Spectrometry
2.7.1. Determination of Individual Phenolic Acids and Flavonoid Concentration
2.7.2. Data Acquisition
2.8. Microdilution Antimicrobial Assay
2.9. Statistical Analyses
3. Results
3.1. Polyphenol Analysis (Extract Yield, TPC, TTC, TFC, TAC, and Vitamin C)
3.2. Antioxidant Activity (DPPH, FRAP, and ABTS)
3.3. Enzyme Activity (PPO and POD)
3.4. Antibacterial Activity
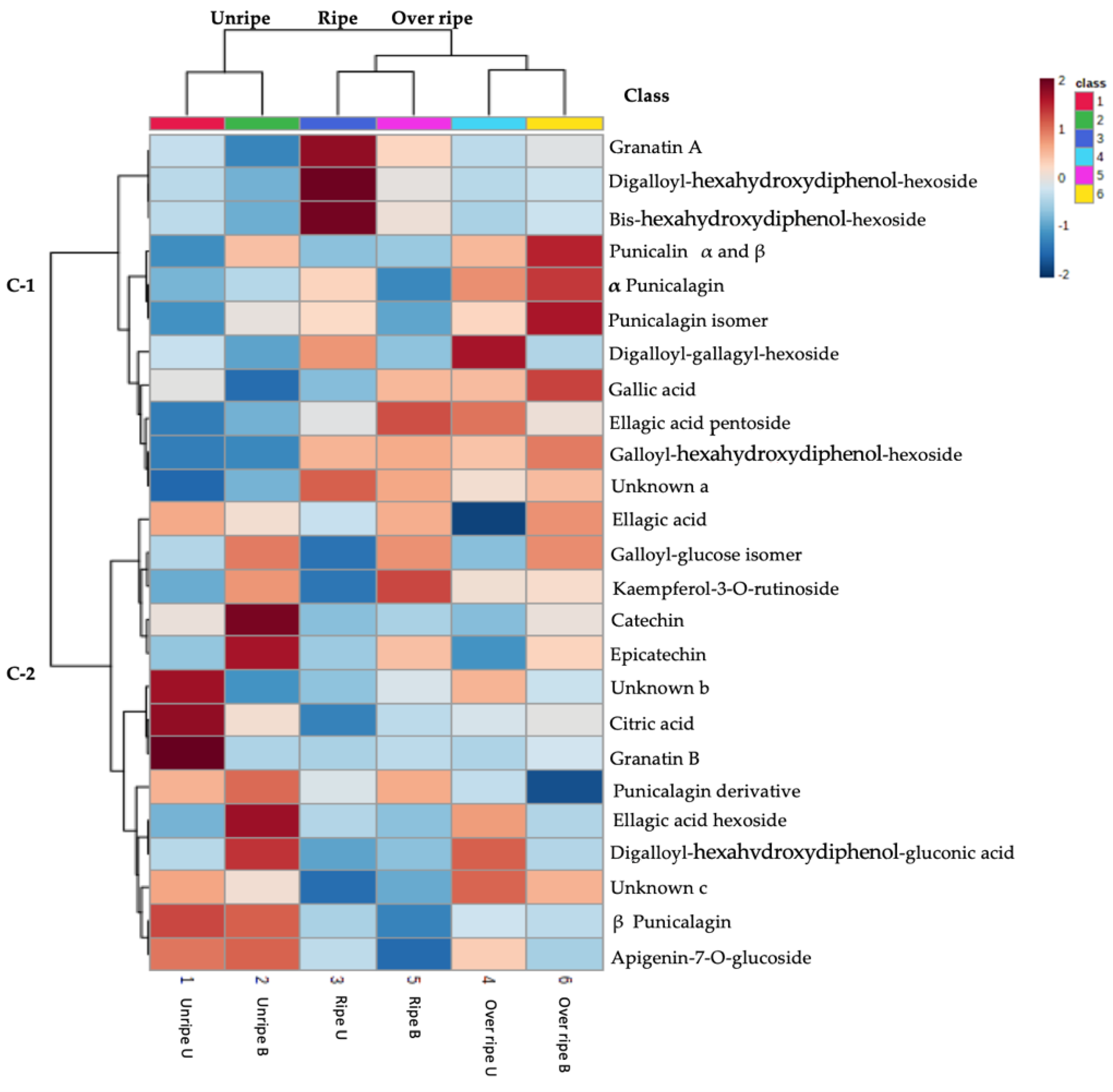
3.5. Correlation Matrix and Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Said, F.; Opara, U.L.; Al-Yahyai, R. Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman. J. Food Eng. 2009, 90, 129–134. [Google Scholar] [CrossRef]
- Fawole, O.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci. Hortic. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef]
- Opara, I.; Fawole, O.; Kelly, C.; Opara, U. Quantification of On-Farm Pomegranate Fruit Postharvest Losses and Waste, and Implications on Sustainability Indicators: South African Case Study. Sustainability 2021, 13, 5168. [Google Scholar] [CrossRef]
- Opara, I.; Fawole, O.; Opara, U. Postharvest Losses of Pomegranate Fruit at the Packhouse and Implications for Sustainability Indicators. Sustainability 2021, 13, 5187. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.; Opara, U.L. Influence of packaging system and long term storage on physiological attributes, biochemical quality, volatile composition and antioxidant properties of pomegranate fruit. Sci. Hortic. 2016, 211, 140–151. [Google Scholar] [CrossRef]
- Pomegranate Association of South Africa (POMASA). Pomegranate Industry Overview. Available online: http://www.hortgro.co.za/portfolio/pomegranates (accessed on 20 March 2020).
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200. [Google Scholar] [CrossRef] [Green Version]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Alvarez, J.A. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, R.; Fawole, O.; Stander, M.; Opara, U.L. Preharvest and postharvest factors influencing bioactive compounds in pomegranate (Punica granatum L.)—A review. Sci. Hortic. 2014, 178, 114–123. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hortic. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Attanayake, R.; Rajapaksha, R.; Weerakkody, P.; Bandaranayake, P.C.G. The Effect of Maturity Status on Biochemical Composition, Antioxidant Activity, and Anthocyanin Biosynthesis Gene Expression in a Pomegranate (Punica granatum L.) Cultivar with Red Flowers, Yellow Peel, and Pinkish Arils. J. Plant Growth Regul. 2019, 38, 992–1006. [Google Scholar] [CrossRef]
- Li, R.; Chen, X.G.; Jia, K.; Liu, Z.P.; Peng, H.Y. A systematic determination of polyphenols constituents and cytotoxic ability in fruit parts of pomegranates derived from five Chinese cultivars. SpringerPlus 2016, 5, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurhuda, H.H.; Maskat, M.Y.; Mamot, S.; Afiq, J.; Aminah, A. Effect of blanching on enzyme and antioxidant activities of rambutan (Nephelium lappaceum) peel. Int. Food Res. J. 2013, 20, 1725–1730. [Google Scholar]
- Xiao, H.-W.; Pan, Z.; Deng, L.-Z.; El Mashad, H.; Yang, X.-H.; Mujumdar, A.S.; Gao, Z.-J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Ben Zid, M.; Dhuique-Mayer, C.; Bellagha, S.; Sanier, C.; Collignan, A.; Servent, A.; Dornier, M. Effects of Blanching on Flavanones and Microstructure of Citrus aurantium Peels. Food Bioprocess Technol. 2015, 8, 2246–2255. [Google Scholar] [CrossRef]
- Duarte, Y.; Chaux, A.; Lopez, N.; Largo, E.; Ramírez, C.; Nuñez, H.; Simpson, R.; Vega, O. Effects of Blanching and Hot Air Drying Conditions on the Physicochemical and Technological Properties of Yellow Passion Fruit (Passiflora edulis Var. Flavicarpa) by-Products. J. Food Process. Eng. 2017, 40, e12425. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Chanshotikul, N.; Assawajitpukdee, C.; Khamjae, T. Effects of blanching and drying on fiber rich powder from pitaya (Hylocereus undatus) peel. Int. Food Res. J. 2013, 20, 1595–1600. [Google Scholar]
- Chung, Y.-C.; Chiang, B.-H.; Wei, J.-H.; Wang, C.-K.; Chen, P.-C.; Hsu, C.-K. Effects of blanching, drying and extraction processes on the antioxidant activity of yam (Dioscorea alata). Int. J. Food Sci. Technol. 2008, 43, 859–864. [Google Scholar] [CrossRef]
- Kaseke, T.; Fawole, O.A.; Mokwena, L.; Opara, U.L. Effect of cultivar and blanching of pomegranate seeds on physicochemical properties, nutritional qualities and antioxidant capacity of extracted oil. J. Food Meas. Charact. 2021, 15, 93–106. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Tsige, A.A.; Opara, U.L.; Fawole, O.A. Mathematical Modelling of Blanch-Assisted Drying of Pomegranate (Punica granatum) Arils in a Hot-Air Drier. Processes 2020, 8, 611. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Effect of solvent extraction and blanching pre-treatment on phytochemical, antioxidant properties, enzyme inactivation and antibacterial activities of ‘Wonderful’ pomegranate peel extracts. Processes 2021, 9, 1012. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Z.; Ma, H.; Atungulu, G.G. Extract of Phenolics from Pomegranate Peels. Open Food Sci. J. 2011, 5, 17–25. [Google Scholar] [CrossRef]
- Foujdar, R.; Bera, M.B.; Chopra, H.K. Optimization of process variables of probe ultrasonic-assisted extraction of phenolic compounds from the peel of Punica granatum Var. Bhagwa and its chemical and bioactivity characterization. J. Food Process. Preserv. 2019, 44, 1–16. [Google Scholar] [CrossRef]
- Makkar, P.S.M. Quantification of Tannins in Tree Foliage. Laboratory Manual. Joint FAO/IAEA Division of Nuclear Tecnhiques in Food and Agriculture. 2000. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:33048138 (accessed on 21 April 2021).
- Yang, J.; Martinson, T.; Liu, R.H. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009, 116, 332–339. [Google Scholar] [CrossRef]
- Wrolstad, R.E. Color and Pigment Analyses in Fruit Products; Agriculiural Experiment Station, Oregon State University: Corvallis, OR, USA, 1993. [Google Scholar]
- Karioti, A.; Hadjipavlou-Litina, D.; Mensah, M.L.K.; Fleischer, T.C.; Skaltsa, H. Composition and Antioxidant Activity of the Essential Oils ofXylopia aethiopica(Dun) A. Rich. (Annonaceae) Leaves, Stem Bark, Root Bark, and Fresh and Dried Fruits, Growing in Ghana. J. Agric. Food Chem. 2004, 52, 8094–8098. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Determination of optimal extraction conditions for phenolic compounds from: Pistacia atlantica leaves using the response surface methodology. Anal. Methods 2016, 8, 6107–6114. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind. Crop. Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- González, E.M.; de Ancos, B.; Cano, M.P. Partial Characterization of Polyphenol Oxidase Activity in Raspberry Fruits. J. Agric. Food Chem. 1999, 47, 4068–4072. [Google Scholar] [CrossRef] [Green Version]
- Arendse, E.; Fawole, O.; Magwaza, L.; Nieuwoudt, H.; Opara, U.L. Evaluation of biochemical markers associated with the development of husk scald and the use of diffuse reflectance NIR spectroscopy to predict husk scald in pomegranate fruit. Sci. Hortic. 2018, 232, 240–249. [Google Scholar] [CrossRef]
- Meighani, H.; Ghasemnezhad, M.; Bakshi, D. Evaluation of biochemical composition and enzyme activities in browned arils of pomegranate fruits. Int. J. Hortic. Sci. Technol. 2014, 1, 53–65. [Google Scholar] [CrossRef]
- Eloff, J.N. A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deylami, M.Z.; Rahman, R.A.; Tan, C.P.; Bakar, J.; Olusegun, L. Effect of blanching on enzyme activity, color changes, anthocyanin stability and extractability of mangosteen pericarp: A kinetic study. J. Food Eng. 2016, 178, 12–19. [Google Scholar] [CrossRef]
- John, K.M.; Bhagwat, A.A.; Luthria, D.L. Swarm motility inhibitory and antioxidant activities of pomegranate peel processed under three drying conditions. Food Chem. 2017, 235, 145–153. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia Arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Xu, H.; Liu, X.; He, W.; Yuan, F.; Hou, Z.; Gao, Y. Identification of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant capacities by HPLC–ABTS+ assay. Food Res. Int. 2011, 44, 1161–1167. [Google Scholar] [CrossRef]
- Abid, M.; Yaich, H.; Cheikhrouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant properties and phenolic profile characterization by LC–MS/MS of selected Tunisian pomegranate peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Nowicka, P.; Munera-Picazo, S.; Hernández, F.; Carbonell-Barrachina, Á.A.; Wojdyło, A. Identification and quantification of major derivatives of ellagic acid and antioxidant properties of thinning and ripe Spanish pomegranates. J. Funct. Foods 2015, 12, 354–364. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and Comprehensive Evaluation of (Poly)phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, C.; Stander, M.; Grobbelaar, M.; Colling, J.; Kossmann, J.; Hills, P.; Makunga, N. LC–MS-based metabolomics assists with quality assessment and traceability of wild and cultivated plants of Sutherlandia frutescens (Fabaceae). S. Afr. J. Bot. 2012, 82, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shwartz, E.; Glazer, I.; Bar-Ya’Akov, I.; Matityahu, I.; Bar-Ilan, I.; Holland, D.; Amir, R. Changes in chemical constituents during the maturation and ripening of two commercially important pomegranate accessions. Food Chem. 2009, 115, 965–973. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Aradhya, S.M. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 2005, 93, 319–324. [Google Scholar] [CrossRef]
- Medlicott, A.P.; Bhogal, M.; Reynolds, S.B. Changes in peel pigmentation during ripening of mango fruit (Mangifera indica var. Tommy Atkins). Ann. Appl. Biol. 1986, 109, 651–656. [Google Scholar] [CrossRef]
- Bureau, S.; Renard, C.M.; Reich, M.; Ginies, C.; Audergon, J.-M. Change in anthocyanin concentrations in red apricot fruits during ripening. LWT 2009, 42, 372–377. [Google Scholar] [CrossRef]
- Rivera-López, J.; Ordorica-Falomir, C.; Wesche-Ebeling, P. Changes in anthocyanin concentration in Lychee (Litchi chinensis Sonn.) pericarp during maturation. Food Chem. 1999, 65, 195–200. [Google Scholar] [CrossRef]
- Awad, A.M.; de Jager, A. Formation of flavonoids, especially anthocyanin and chlorogenic acid in ‘Jonagold’ apple skin: Influences of growth regulators and fruit maturity. Sci. Hortic. 2002, 93, 257–266. [Google Scholar] [CrossRef]
- Karanjalker, G.R.; Ravishankar, K.; Shivashankara, K.; Dinesh, M.; Roy, T.K.; Rao, D.V.S. A Study on the Expression of Genes Involved in Carotenoids and Anthocyanins During Ripening in Fruit Peel of Green, Yellow, and Red Colored Mango Cultivars. Appl. Biochem. Biotechnol. 2018, 184, 140–154. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Moo-Huchin, M.I.; Estrada-León, R.J.; Cuevas-Glory, L.; Estrada-Mota, I.A.; Ortiz-Vázquez, E.; Betancur-Ancona, D.; Sauri-Duch, E. Antioxidant compounds, antioxidant activity and phenolic content in peel from three tropical fruits from Yucatan, Mexico. Food Chem. 2015, 166, 17–22. [Google Scholar] [CrossRef]
- Sung, D.-Y.; Kaplan, F.; Lee, K.-J.; Guy, C.L. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003, 8, 179–187. [Google Scholar] [CrossRef]
- Pucciariello, C.; Banti, V.; Perata, P. Plant physiology and biochemistry ROS signaling as common element in low oxygen and heat stresses. Plant Physiol. Biochem. 2012, 59, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Zheng, W. Effect of Plant Growth Temperature on Antioxidant Capacity in Strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef]
- Opara, L.U.; Al-Ani, M.R.; Al-Shuaibi, Y.S. Physico-chemical Properties, Vitamin C Content, and Antimicrobial Properties of Pomegranate Fruit (Punica granatum L.). Food Bioprocess. Technol. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Mali, A.B.; Khedkar, K.; Lele, S.S. Effect of Gamma Irradiation on Total Phenolic Content and in Vitro Antioxidant Activity of Pomegranate (Punica granatum L.) Peels. Food Nutr. Sci. 2011, 2, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Khan, M.R.; Shabbir, M.A.; Rahman, K.U. Comparison of antioxidative potential and punicalagin content of pomegranate peels. J. Anim. Plant Sci. 2017, 27, 522–527. [Google Scholar]

| Harvest Maturity | Treatment | Extract Yield (%) | TPC | TTC | TFC | TAC | Vit C | DPPH | ABTS | FRAP | PPO | POD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unripe | Unblanched | 28.1 ± 0.6 c | 12.1 ± 0.1 b | 0.9 ± 0.1 b | 0.8 ± 0.1 d | 0.09 ± 0.00 b | 38.3 ± 0.3 e | 279.1 ± 5.6 c | 721.8 ± 1.2 c | 541.1 ± 89.2 b | 0.5 ± 0.0 b | 3.8 ± 0.0 b |
| Blanched | 31.3 ± 0.6 ab | 14.0 ± 0.0 a | 1.0 ± 0.1 a | 1.1 ± 0.1 c | 0.10 ± 0.00 a | 31.0 ± 0.1 f | 359.2 ± 0.0 a | 912.2 ± 0.0 a | 802.5 ± 4.3 a | 0.2 ± 0.1 e | 1.5 ± 0.0 d | |
| Ripe | Unblanched | 29.5 ± 0.8 bc | 10.6 ± 0.2 d | 0.8 ± 0.0 c | 1.2 ± 0.0 c | 0.07 ± 0.00 e | 45.2 ± 0.1 d | 243.9± 2.4 d | 718.8 ± 2.4 c | 478.0 ± 74.0 c | 0.4 ± 0.0 c | 3.0 ± 0.0 c |
| Blanched | 33.8 ± 1.4 a | 12.2 ± 0.1 b | 1.1 ± 0.1 a | 1.5 ± 0.0 b | 0.08 ± 0.00 c | 53.9 ± 0.5 c | 319.2 ± 4.2 b | 778.8 ± 2.4 b | 525.3 ± 15.8 b | 0.3 ± 0.0 d | 1.5 ± 0.0 d | |
| Over Ripe | Unblanched | 26.8 ± 1.0 d | 9.5 ± 0.0 e | 0.6 ± 0.0 d | 1.4± 0.1 b | 0.07 ± 0.00 e | 77.7 ± 0.2 a | 208.8 ± 2.4 e | 612.2 ± 0.0 d | 370.1 ± 3.6 d | 0.6 ± 0.0 a | 4.5 ± 0.0 a |
| Blanched | 30.9 ± 0.3 b | 11.6 ± 0.0 c | 0.9 ± 0.1 b | 1.8 ± 0.0 a | 0.08 ± 0.00 d | 70.5 ± 0.1 b | 316.7 ± 2.4 b | 614.7 ± 0.6 d | 447.7 ± 98.8 c | 0.5 ± 0.0 b | 1.5 ± 0.0 d |
| Harvest Maturity/Treatment | ||||||
|---|---|---|---|---|---|---|
| Bioactive Compounds | Unripe | Ripe | Over Ripe | |||
| Unblanched | Blanched | Unblanched | Blanched | Unblanched | Blanched | |
| Punicalin α and β | 185 ± 3 d | 414 ± 6 a | 228 ± 5 c | 342 ± 9 b | 237 ± 4 c | 436 ± 16 a |
| α-Punicalagin | 598 ± 13 b | 618 ± 12 b | 562 ± 13 c | 610 ± 11 b | 523 ± 10 d | 659 ± 19 a |
| β-Punicalagin | 696 ± 18 a | 678 ± 18 b | 592 ± 33 d | 644 ± 35 c | 514 ± 19 d | 641 ± 38 b |
| Ellagic acid | 308 ± 18 bc | 279 ± 16 c | 324 ± 22 b | 297 ± 34 bc | 306 ± 17 bc | 363 ± 36 a |
| Catechin | 99 ± 2 b | 151 ± 6 a | 55 ± 4 d | 84 ± 4 c | 93 ± 3 c | 80 ± 3 c |
| Epicatechin | 12 ± 1 c | 227 ± 1 a | 123 ± 1 c | 10 ± 1 c | 20 ± 1 a | 16 ± 2 b |
| Gallic acid | 139 ± 2 c | 85 ± 1 d | 13 ± 4 c | 163 ± 4 b | 165 ± 6 b | 200 ± 9 a |
| Harvest Maturity | Treatment | Gram Negative | Gram Positive | ||
|---|---|---|---|---|---|
| Escherichia coli | Klebsiella pneumonia | Staphylococcus aureus | Bacillus subtilis | ||
| Unripe | Unblanched | 310 | 310 | 310 | 310 |
| Blanched | 160 | 160 | 160 | 160 | |
| Ripe | Unblanched | 310 | 310 | 310 | 310 |
| Blanched | 160 | 160 | 160 | 160 | |
| Over Ripe | Unblanched | 310 | 310 | 310 | 310 |
| Blanched | 160 | 160 | 160 | 160 | |
| Streptomycin (µg/mL) | 1.6 | 1.6 | 0.8 | 1.6 | |
| Solvent Control (70% Ethanol) | - | - | - | - | |
| EY | TPC | TTC | TFC | TAC | Vit C | DPPH | FRAP | PPO | POD | Pα&β | αPun | βPun | EA | Cat | GA | E. c | K. p | S.a | B. s | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EY | 1 | |||||||||||||||||||
| TPC | 0.61 | 1 | ||||||||||||||||||
| TTC | 0.83 | 0.88 | 1 | |||||||||||||||||
| TFC | 0.29 | 0.92 | 0.70 | 1 | ||||||||||||||||
| TAC | 0.39 | −0.23 | −0.08 | −0.40 | 1 | |||||||||||||||
| Vit C | −0.24 | −0.74 | −0.61 | −0.70 | 0.74 | 1 | ||||||||||||||
| DPPH | 0.77 | 0.93 | 0.88 | 0.80 | 0.11 | −0.50 | 1 | |||||||||||||
| FRAP | 0.24 | 0.27 | −0.18 | −0.39 | 0.97 | 0.75 | 0.07 | 1 | ||||||||||||
| PPO | −0.55 | 0.02 | −0.16 | 0.26 | −0.90 | −0.46 | −0.31 | −0.90 | 1 | |||||||||||
| POD | −0.86 | −0.52 | −0.57 | −0.24 | −0.64 | 0.01 | −0.74 | −0.54 | 0.72 | 1 | ||||||||||
| Pα&β | 0.67 | 0.54 | 0.48 | 0.39 | 0.65 | 0.07 | 0.78 | 0.62 | −0.69 | −0.93 | 1 | |||||||||
| αPun | 0.65 | 0.69 | 0.71 | 0.56 | 0.34 | −0.22 | 0.85 | 0.38 | −0.61 | −0.65 | 0.74 | 1 | ||||||||
| βPun | 0.48 | 0.87 | 0.84 | 0.83 | −0.33 | −0.73 | 0.80 | −0.30 | 0.01 | −0.24 | 0.29 | 0.76 | 1 | |||||||
| EA | −0.09 | −0.41 | −0.32 | −0.45 | 0.59 | 0.54 | −0.17 | 0.74 | −0.72 | −0.07 | 0.17 | 0.34 | −0.13 | 1 | ||||||
| Cat | 0.09 | 0.71 | 0.39 | 0.85 | −0.31 | −0.45 | 0.58 | −0.32 | 0.39 | −0.21 | 0.39 | 0.22 | 0.42 | −0.64 | 1 | |||||
| GA | −0.01 | −0.54 | −0.28 | −0.57 | 0.73 | 0.84 | −0.27 | 0.76 | −0.64 | −0.04 | 0.09 | 0.16 | −0.31 | 0.75 | −0.60 | 1 | ||||
| E. c | −0.85 | −0.67 | −0.71 | −0.47 | −0.56 | 0.06 | −0.87 | −0.47 | 0.62 | 0.93 | −0.94 | −0.79 | −0.45 | −0.01 | −0.39 | −0.08 | 1 | |||
| K. p | −0.85 | −0.67 | −0.71 | −0.47 | −0.56 | 0.06 | −0.87 | −0.47 | 0.62 | 0.93 | −0.94 | −0.79 | −0.45 | −0.01 | −0.39 | −0.08 | 1.00 | 1 | ||
| S. a | −0.85 | −0.67 | −0.71 | −0.47 | −0.56 | 0.06 | −0.87 | −0.47 | 0.62 | 0.93 | −0.94 | −0.79 | −0.45 | −0.01 | −0.39 | −0.08 | 1.00 | 1.00 | 1 | |
| B. s | −0.85 | −0.67 | −0.71 | −0.47 | −0.56 | 0.06 | −0.87 | −0.47 | 0.62 | 0.93 | −0.94 | −0.79 | −0.45 | −0.01 | −0.39 | −0.08 | 1.00 | 1.00 | 1.00 | 1 |
| No. | Tentative ID | Retention Time | M-H | MSE Fragment Ions | UV Max | Elemental Formula | References |
|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||
| Hydroxybenzoic acids | |||||||
| 1 | Gallic acid * | 4.72 | 169.0146 | 169.014, 125.025, 124.017 | 270,259 | C7H5O5 | Standard |
| 2 | Ellagic acid * | 14.24 | 300.9969 | 213.597, 137.983, 49.474 | 254,364 | C14H5O8 | Standard |
| 3 | Ellagic acid hexoside | 11.06 | 463.0539 | 463.053, 300.989, 165.021,114.6995 | - | C20H15O13 | [41] |
| 4 | Ellagic acid pentoside | 13.59 | 433.0307 | 433.038, 303.7598, 300.995, 299.997, 201.556, 126.237 | 254,361 | C19H13O12 | [41,42] |
| Organic acids | |||||||
| 5 | Citric acid * | 2.52 | 191.0198 | 191.0198, 173.008, 111.008,87.008, 67.017 | - | C5H7O7 | Standard |
| Flavonoids | |||||||
| Flavanols | |||||||
| 6 | (+)-Catechin * | 8.31 | 289.0733 | 289.071, 245.082, 203.072, 109.028 | C15H13O6 | Standard | |
| 7 | (−)-Epicatechin * | 10.19 | 289.0733 | 289.071, 245.082, 203.072, 109.028 | C15H13O6 | Standard | |
| Flavones | |||||||
| 8 | Apigenin-7-O-glucoside Flavonols | 11.07 | 431.1906 | 161.041, 153.091 | 253, 361 | C20H20O10 | [42] |
| 9 | Kaempferol-3-O-rutinoside | 15.95 | 593.1494 | 593.142, 523.421, 440.063, 316.023, 300.998, 285.033, 211.911, 125.025, 101.031, 80.779 | 275,360 | C27H29O15 | [43] |
| 10 | Punicalin α and β * | 4.985 | 781.0506 | 781.022, 779.000,783.055, 784.065 | 270, 259 | C34H21O22 | Std |
| 11 | α Punicalagin * | 6.46 | 1083.0547 | 1083.060, 781.035, 600.986, 541.027, 300.997 | 258,378 | C48H27O30 | Std |
| 12 | β Punicalagin * | 7.69 | 1083.0558 | 1083.060, 781.035, 600.986, 541.027, 300.997 | 258,378 | C48H27O30 | Std |
| 13 | Punicalagin isomer | 5.305 | 1083.0547 | 1083.060, 781.035, 600.986, 541.027, 300.997 | 258,378 | C48H27O30 | [41,42] |
| 14 | Punicalagin derivative | 6.44 | 541.0341 | 541.027, 300.997 | 258,378 | - | [44] |
| 15 | Galloyl- hexahydroxydiphenol-dehydrohexahydroxydiphenol-hexoside | 6.83 | 951.0479 | 951.042, 907.087, 820.125, 783.062, 300.997, 275.021, 249.035, 102.241 | 255, 230 | C52 H23O19 | [42,44] |
| 16 | Galloyl- hexahydroxydiphenol -dehydrohexahydroxydiphenol-hexoside (Granatin B) | 12.43 | 951.071 | 951.044, 933.079, 915.9995, 763.081, 614.803, 464.044, 341.011, 302.006, 300.999, 273.002, 169.013, 1233.011 | 273 | C52H23O19 | [42,44] |
| 17 | Granatin A | 8.34 | 799.0454 | 799.0454, 800.0641, 802.0781 | C34H23O23 | [45,46] | |
| 18 | Digalloyl- hexa hydroxydiphenol-gluconic acid (punigluconin) | 8.60 | 801.0732 | 801.073, 781.3245 | - | C41H21O18 | [46] |
| 19 | Digalloyl- hexahydroxydiphenol-hexoside (Pedunculagin II)) | 9.37 | 785.0775 | 785.021, 781.795, 635.099, 483.122, 419.050, 345.086, 301.995, 300.9982, 275.032, 165.021, 125.132 | 264,370 | C34H25O22 | [41,42,45] |
| 20 | Galloyl-hexahydroxydiphenol-hexoside | 9.76 | 633.0686 | 633.075, 597.082, 464.051, 301.9998, 275.0219, 125.024 | 260,360 | C27H22O18 | [44,47] |
| 21 | Digalloyl-gallagyl-hexoside | 9.99 | 1085.0889 | 933.081, 783.088, 633.0696, 540.536, 450.989, 301.996, 300.997, 275.014, | 257,370 | C48H29O30 | [41,47] |
| 22 | Bis-hexahydroxydiphenol-hexoside (pedunculagin I) | 8.83 | 783.0746 | 783.567, 635.087, 541.022, 453.103, 392.036, 291.016, 203.0396 | 260,370 | C34H23O22 | [41,44] |
| Unknown | |||||||
| 23 | Unknown a | 1.58 | 217.0461 | 217.042, 191.0211, 173.006 | - | C12H9O4 | - |
| 24 | Unknown b | 2.37 | 353.0731 | 353.0742, 191.0211, 173.0062 | - | C12H17O12 | - |
| 25 | Unknown c | 12.81 | 539.2146 | 541.038, 492.1699, 405.014, 328.021, 302.001, 300.998, 222.005, 169.011, 52.256 | 254,230 | C26H35O12 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magangana, T.P.; Makunga, N.P.; la Grange, C.; Stander, M.A.; Fawole, O.A.; Opara, U.L. Blanching Pre-Treatment Promotes High Yields, Bioactive Compounds, Antioxidants, Enzyme Inactivation and Antibacterial Activity of ‘Wonderful’ Pomegranate Peel Extracts at Three Different Harvest Maturities. Antioxidants 2021, 10, 1119. https://doi.org/10.3390/antiox10071119
Magangana TP, Makunga NP, la Grange C, Stander MA, Fawole OA, Opara UL. Blanching Pre-Treatment Promotes High Yields, Bioactive Compounds, Antioxidants, Enzyme Inactivation and Antibacterial Activity of ‘Wonderful’ Pomegranate Peel Extracts at Three Different Harvest Maturities. Antioxidants. 2021; 10(7):1119. https://doi.org/10.3390/antiox10071119
Chicago/Turabian StyleMagangana, Tandokazi Pamela, Nokwanda P. Makunga, Chris la Grange, Maria A. Stander, Olaniyi Amos Fawole, and Umezuruike Linus Opara. 2021. "Blanching Pre-Treatment Promotes High Yields, Bioactive Compounds, Antioxidants, Enzyme Inactivation and Antibacterial Activity of ‘Wonderful’ Pomegranate Peel Extracts at Three Different Harvest Maturities" Antioxidants 10, no. 7: 1119. https://doi.org/10.3390/antiox10071119
APA StyleMagangana, T. P., Makunga, N. P., la Grange, C., Stander, M. A., Fawole, O. A., & Opara, U. L. (2021). Blanching Pre-Treatment Promotes High Yields, Bioactive Compounds, Antioxidants, Enzyme Inactivation and Antibacterial Activity of ‘Wonderful’ Pomegranate Peel Extracts at Three Different Harvest Maturities. Antioxidants, 10(7), 1119. https://doi.org/10.3390/antiox10071119










